ABSTRACT
We identified relapsing fever (RF) Borrelia in 1.45% (145/10426) of the ticks and 1.40% (40/2850) of the wild mammals in a field investigation in China. Three RF Borrelia species, including human-pathogenic Borrelia miyamotoi, Borrelia persica and unclassified Babesia sp. were determined. Main species determined from ticks was B. miyamotoi (44.14%), followed by the unclassified Borrelia sp. (42.76%), and Borrelia theileri (13.10%). In wild mammals, main species found was B. persica (57.50%), followed by the unclassified Borrelia sp. (40.00%), and B. miyamotoi (2.50%). We determined B. theileri and B. persica in China for the first time. The coexistence of RF Borrelia species in one tick species in a given region was observed, with the most frequent coexistence seen for B. miyamotoi and the unclassified Borrelia sp. in Dermacentor silvarum, Haemaphysalis japonica, Haemaphysalis longicornis, and Ixodes persulcatuss respectively. The wide distribution and high variety of RF Borrelia in China pose a potential threat to public health.
KEYWORDS:
Dear Editor,
Relapsing fever (RF) is a zoonosis caused by the relapsing fever group spirochetes of the genus Borrelia that have been distributed throughout Africa, Central Asia, North America and Southern Europe [Citation1]. Typically, RF Borrelia is transmitted by soft ticks, with the exception of the human louse-borne Borrelia recurrentis, Borrelia lonestari and Borrelia miyamotoi harboured by hard ticks [Citation2–4]. Initially distinguished by geography and vector types, the RF Borrelia used to be arbitrarily grouped into the Old World (Palearctic-Afrotropic ecozone) and the New World (Nearctic ecozone) Borreliae [Citation2]. With recently increasing report of novel species, such as Candidatus Borrelia javanense, in total 27 RF Borrelia species spirochetes have been identified to date [Citation5]. At least three RF Borrelia species have been documented in China, including the identification of B. miyamoti in Heilongjiang; Candidatus Borrelia javanense in ticks in Guangxi; Candidatus Borrelia fainii in bats in Hubei and Shandong according to the recent studies [Citation5–8]. Generally, reports on human disease are few and the studies were limited to the northeastern region. The current study was designed to determine the molecular evidence of RF Borrelia in mammal hosts and ticks in a wide region in China, further exploring the molecular diversity in relation to the vector and host species.
From 2017 to 2021, host-seeking ticks were collected by flagging over vegetation, and wild small mammals were captured by snap traps from 11 provinces in six eco-climate regions in China [Citation9] ((A)). The species of tick and small mammals were identified by morphology and further confirmed by sequencing of mitochondrial 16S ribosomal DNA (16S rDNA) and cytochrome b gene respectively (Table S1). Genomic DNA was extracted from ticks and mammal tissues using AllPrep DNA/RNA mini kit (Qiagen, Germany), which was screened for RF Borrelia using a touchdown PCR targeting the 353-bp 16S rDNA gene (rrs) [Citation10] (Table S2). The positive samples were further characterized by amplifying and sequencing rrs, flagellin (flaB), and glycerophospho diester phosphodiesterase (glpQ) genes [Citation6,Citation7,Citation10–13] (Table S2). The prevalence (95% confidence intervals) was calculated by maximum likelihood estimation using the program PooledInfRate. Phylogenetic tree was established using the maximum-likelihood method with the GTRGAMMA model in RAxML. Bootstrap values were calculated with 1000 replicates. The sequences generated in this study were submitted to GenBank under the accession numbers ON059648∼ON059658, ON209364∼ON209371, ON365959∼ON365960, ON059603-ON059643, ON060662∼ON060671, ON113496∼ON113497, ON184022∼ON184029, ON361163∼ ON361170 ON148112∼ON148132 (Table S3). The study protocol was approved by the Ethics Committee of Beijing Institute of Microbiology and Epidemiology.
Figure 1. The distribution and genetic characterization of relapsing fever Borrelia in ticks and wild small mammals in China. (A) Geographic distribution of Relapsing Fever (RF) Borrelia species in China. The collection site for the sampling and test of RF Borrelia. Eco-climate regions were used for the geographic description, with six of them sampled: Northeastern China, Inner Mongolia-Xinjiang, Northern China, Central China, Southwest China and Southern China. The colour of the circles indicates positive rate of RF Borrelia in ticks; the colour of circle outlines represents tick species; the size of circle represents the number of ticks collected in the area. The colour of the squares indicates positive rate of RF Borrelia in wild small mammals; the colour of square outlines represents families of wild small mammals; the size of square represents the number of wild small mammals collected in the area. (B) The RF Borrelia species determined in ticks and wild small mammals. Phylogenetic analysis of RF Borrelia species based on rrs (C), flaB (D) and glpQ (E) genes. The tree was constructed by using the maximum-likelihood method with the GTRGAMMA model in RAxML. Bootstrap values were calculated with 1000 replicates. Scale bar indicates the degree of divergence represented by a given length of branch. RF Borrelia species determined in the current study were shown in colour. Sequences from ticks and wild small mammals were labelled with circle and square respectively. Chord diagrams between RF Borrelia species and tick species (F) and wild small mammals (G) in five eco-climate regions in China.
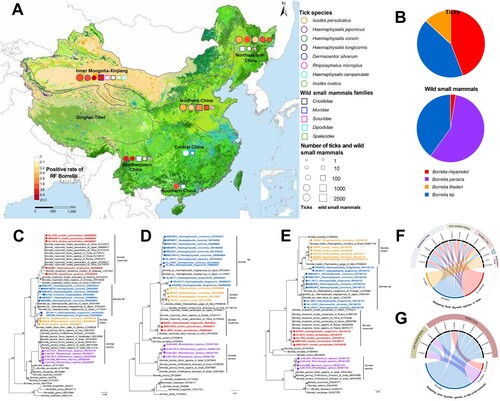
A total of 10,426 ticks that belonged to 4 genus and 8 species were collected, which were grouped into 4268 pools by species and locations for further tests ((A)). In total, positive detection of RF Borrelia was determined in 1.45% (1.23–1.74%) of the ticks of 7 species, including 4.76% of Ixodes ovatus, 3.42% of Ixodes persulcatus, 2.81% of Haemaphysalis japonica, 2.13% of Haemaphysalis concinna, 1.74% of Rhipicephalus microplus, 0.85% of Dermacentor silvarum, and 0.63% of Haemaphysalis longicornis (Table S4). The positive detection was obtained from five of six sampled eco-climate regions, with the highest rate observed in Southwestern China (5.58%), followed by Inner Mongolia-Xinjiang (2.37%), Southern China (2.19%), Northeastern China (1.20%), and Northern China (0.59%) (Table S4).
Totally 2850 wild small mammals that belonged to 50 species in 5 families were tested using spleen samples (A). Altogether, 40 out of 2850 (1.40%) mammals of 11 species were positive for RF Borrelia, including in 25.68% (19/74) of Rhombomys opimus, 9.52% (4/42) of Meriones libycus, 4.76% (7/147) of Niviventer niviventer, 4.55% (1/22) of Niviventer confucianus, 3.57% (1/28) of Cricetulus longicaudatus, 2.70% (2/74) of Myodes rufocanus, 1.39% (1/72) of Apodemus draco, 1.10% (2/181) of Meriones unguiculatus, 0.41% (1/244) of Apodemus agrarius, 0.24% (1/423) of Rattus tanezumi, and 0.22% (1/457) of Mus musculus (Table S5). The positive detection was obtained in two eco-climate regions, i.e. Inner Mongolia-Xinjiang (3.64%) and Northern China (1.01%) (Table S5).
Based on rrs, flaB, and glpQ genes, RF Borrelia genospecies were determined from ticks, with B. miyamotoi most frequently seen, taking 44.14% of the positive detection (B), which was obtained in a wide region in Northeastern China, Inner Mongolia-Xinjiang, and Northern China. For the first time in China, we determined the presence of Borrelia theileri in R. microplus and I. ovatus, which had 99% identity to B. theileri detected in cattle from Zambia (GenBank: LC656239). The unclassified Borrelia sp. which were related to B. theileri and clustered with the Borrelia species detected in Japanese H. japonica (GenBank: AB897891) in a separate lineage was determined (C-E). The unclassified Borrelia sp. was identified in H. concinna (n = 48), H. longicornis (n = 6), I. persulcatus (n = 4), H. japonica (n = 2), and D. silvarum (n = 2) in Inner Mongolia-Xinjiang, Northeastern and Northern China, respectively.
In the 40 positive wild small mammals, the main species of RF Borrelia found was B. persica (57.50%), followed by the unclassified Borrelia sp. that resembled the one detected from the ticks (40.00%), B. miyamotoi (2.50%) (B). We detected B. miyamotoi from 0.41% (1/244) of the A. agrarius, representing its first identification in rodents. We detected Borrelia persica in R. opimus (25.68%, 19/74) and M. libycus (9.52%, 4/42) in China for the first time. The positive detection for the unclassified Borrelia sp. was obtained from 8 species (N. niviventer, A. draco, C. longicaudatus, M. unguiculatus, M. musculus, R. tanezumi, M. rufocanus, N. confucianus) of 2 families (Muridae and Cricetidae) of Rodentia order which were sampled from Inner Mongolia-Xinjiang and Northern China (Table S5).
Here we demonstrated a wide distribution and high variety of RF Borrelia in China. Notably two of them, B. miyamotoi and B. persica were human-pathogenic RF Borrelia species. Except for B. miyamotoi, another known Borrelia species, B. theileri, was identified in ticks. We also determined unclassified Borrelia sp. which were widely distributed among ticks in three eco-climate regions in China.
Historically, the RF Borrelia was considered to follow a cospeciation hypothesis that indicated only one RF Borrelia species could be found in a particular host and vector in a given geographic area [Citation14]. Here we demonstrated coexistence of RF Borrelia species in one tick species in a given region, with the most frequent coexistence as B. miyamotoi and the unclassified Borrelia sp. in D. silvarum, H. japonica, H. longicornis, and I. persulcatus respectively (F, Table S6), all suggesting that the previous geographical distribution studies were not comprehensive.
So far, the reservoir hosts for RF Borrelia remained rarely investigated, except for the molecular evidence in rodents and birds in Netherlands [Citation15], bat in China [Citation5]. Our study demonstrated a high diversity of RF Borrelia in wild small mammals, yet with only low prevalence in most of the species. Unlike ticks, we determined no coexistence of RF Borrelia species in one single mammal species (G, Table S7). Their role in the transmission of RF Borrelia warrants further investigation.
The study was subject to one major limitation that no isolation of RF Borrelia species was achieved, due to their fastidious nature and specialized growth requirement. Recently, multilocus sequence analysis (MLSA) has been accepted as a taxonomic instrument for bacterial species assignment, based on which Borrelia bavariensis was proposed as an independent species [Citation16,Citation17]. The species of the unclassified Borrelia sp. identified in our study warrants future species classification using isolation and MLSA as previously described for B. miyamotoi [Citation18–20]. On the other hand, the current study was mainly focused on the epidemiological investigation, disclosing a wide distribution and high variety of RF Borrelia in both tick and wild small mammals with wide distribution in China. We believe the current findings might be valuable for the early warning and prevention of relapsing fever disease, not only in China but also in countries where similar ecological habitat was harboured.
Supplemental Material
Download MS Word (93.2 KB)Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Trape J-F, Diatta G, Arnathau C, et al. The epidemiology and geographic distribution of relapsing fever borreliosis in West and North Africa, with a review of the Ornithodoros erraticus complex (Acari: Ixodida). PLoS One. 2013;8(11):e78473.
- Liveris D, Hanincová K, Schwartz I. Borreliae. In: I de Filippis, ML McKee, editors. Molecular typing in bacterial infections. Totowa, NJ: Humana Press; 2013. p. 353–369.
- Kahlig P, Paris DH, Neumayr A. Louse-borne relapsing fever–a systematic review and analysis of the literature: part 1-epidemiology and diagnostic aspects. PLoS Negl Trop Dis. 2021;15(3):e0008564.
- Lee JK, Smith WC, McIntosh C, et al. Detection of a Borrelia species in questing gulf coast ticks, Amblyomma maculatum. Ticks Tick Borne Dis. 2014;5(4):449–452.
- Li ZM, Xiao X, Zhou CM, et al. Human-pathogenic relapsing fever Borrelia found in bats from central China phylogenetically clustered together with relapsing fever Borreliae reported in the new world. PLoS Negl Trop Dis. 2021;15(3):e0009113.
- Jiang B-G, Jia N, Jiang J-F, et al. Borrelia miyamotoi infections in humans and ticks, Northeastern China. Emerg Infect Dis. 2018;24(2):236–241.
- Jiang B-G, Wu A-Q, Jiang J-F, et al. Molecular detection of novel Borrelia species, Candidatus Borrelia javanense, in Amblyomma javanense ticks from pangolins. Pathogens. 2021;10(6):728.
- Han H-J, Liu J-W, Wen H-L, et al. Pathogenic new world relapsing fever Borrelia in a Myotis Bat, eastern China, 2015. Emerg Infect Dis. 2020;26(12):3083–3085.
- Xing Y, Zhou L, Zhang Y, et al. Geographical patterns based on faunal types of breeding birds and mammals in China. Integr Zool. 2008;3(4):280–289.
- Hovius JWR, de Wever B, Sohne M, et al. A case of meningoencephalitis by the relapsing fever spirochaete Borrelia miyamotoi in Europe. Lancet. 2013;382(9892):658.
- Picken RN. Polymerase chain reaction primers and probes derived from flagellin gene sequences for specific detection of the agents of Lyme disease and North American relapsing fever. J Clin Microbiol. 1992;30(1):99–114.
- Takano A, Fujita H, Kadosaka T, et al. Characterization of reptile-associated Borrelia sp. in the vector tick, Amblyomma geoemydae, and its association with Lyme disease and relapsing fever Borrelia spp. Environ Microbiol Rep. 2011;3(5):632–637.
- Safdie G, Farrah IY, Yahia R, et al. Molecular characterization of Borrelia persica, the agent of tick borne relapsing fever in Israel and the Palestinian authority. PLoS One. 2010;5(11):e14105.
- Felsenfeld O. Borreliae, human relapsing fever, and parasite-vector-host relationships. Bacteriol Rev. 1965;29:46–74.
- Wagemakers A, Jahfari S, de Wever B, et al. Borrelia miyamotoi in vectors and hosts in The Netherlands. Ticks Tick Borne Dis. 2017;8(3):370–374.
- Stackebrandt E, Frederiksen W, Garrity GM, et al. Report of the ad hoc committee for the re-evaluation of the species definition in bacteriology. Int J Syst Evol Microbiol. 2002;52(Pt 3):1043–1047.
- Margos G, Wilske B, Sing A, et al. Borrelia bavariensis sp. nov. is widely distributed in Europe and Asia. Int J Syst Evol Microbiol. 2013;63(Pt 11):4284–4288.
- Replogle AJ, Sexton C, Young J, et al. Isolation of Borrelia miyamotoi and other Borreliae using a modified BSK medium. Sci Rep. 2021;11(1):1926.
- Koetsveld J, Kolyasnikova NM, Wagemakers A, et al. Development and optimization of an in vitro cultivation protocol allows for isolation of Borrelia miyamotoi from patients with hard tick-borne relapsing fever. Clin Microbiol Infect. 2017;23(7):480–484.
- Takano A, Toyomane K, Konnai S, et al. Tick surveillance for relapsing fever spirochete Borrelia miyamotoi in Hokkaido, Japan. PLoS One. 2014;9(8):e104532.
