ABSTRACT
African swine fever virus (ASFV) infection is a major public and socioeconomic concern that has a serious impact on the global swine industry. Unfortunately, there are currently no commercially available vaccines or antiviral agents that are both safe and effective against ASFV. In the study, we use primary porcine alveolar macrophages to screen a kinase inhibitor library for anti-ASFV compounds. Six candidate compounds that inhibited ASFV infection with inhibition of > 90% were identified, among which brincidofovir exhibited optimal inhibitory effects on ASFV. Brincidofovir reduces ASFV replication in a dose-dependent manner (IC50 = 2.76 nM) without cytotoxicity (CC50 = 58 μM). It possesses the ability to reduce viral titres and inhibit viral structural protein expression. Time-of-addition assays suggest that the compound interferes with the post-invasion stage of the viral infection cycle. In pig challenge experiments, brincidofovir was indicated to protect pigs against ASFV-induced lethality by decreasing the viral load in organs and peripheral blood, while it alleviated the histopathological changes associated with ASFV infection. Furthermore, brincidofovir also decreased viral shedding in pigs with ASFV infection. Our data together demonstrate that brincidofovir may serve as a potentially effective agent for the prevention and control of ASFV infection, whereas further investigations are still required.
Introduction
African swine fever virus (ASFV) is a member of the Asfarviridae family, an enveloped icosahedral deoxyvirus, and causes severe and fatal haemorrhagic viral disease in domestic pigs and wild boars, with mortality rates approaching 100% for virulent isolates [Citation1,Citation2]. The ASFV genome is a linear double-stranded DNA that is approximately 170–193 kb long and contains 151–167 open reading frames [Citation3]. Differences in the gene numbers and genome lengths of ASFV variations are mainly caused by gains or losses of open reading frames from multigene families. In total, 23 different genotypes have been identified based on the gene sequence that encodes the major capsid protein p72 (B646L gene) [Citation4]. Unfortunately, no commercially available vaccines or antiviral therapies have been proven safe and effective for the prevention or control of African swine fever (ASF). Therefore, current control strategies against ASF primarily rely on quarantine and culling in the affected areas.
ASF was first described in Kenya in the early 1900s [Citation5]. Subsequently, ASFV spread to the savannah areas of eastern and southern Africa through sylvatic circulation in the common warthog (Phacochoerus africanus) and soft-bodies ticks in the genus Ornithodoros, which do not become ill after infection [Citation6]. ASF has undergone two transcontinental transmissions resulting in outbreaks across multiple European countries, including Spain, Portugal, Georgia and Russia. An ASF outbreak was reported for the first time in China in 2018. It has now spread to other Southeast Asian countries. As China is the largest pig producer worldwide, the disease has had significant economic consequences, with an estimated direct cost of over $130 billion [Citation7,Citation8].
As more and more countries are affected by ASFV, the demand for the prevention or control of ASF is growing rapidly. There has been some significant progress in the development of the vaccine, ASFV-G-ΔI177L has been highly efficacious in challenge studies using parental ASFV-G, and the vaccine has been commercialized in Vietnam [Citation9–11]. However, its long-term efficacy and safety have not been established and the cross-protective ability of the vaccine needs to be further evaluated [Citation12–16]. The availability of cell lines and optimization of culture conditions for vaccine scale-up remain the key constraint for ASFV vaccine development [Citation17,Citation18]. An approach that is increasingly gaining interest is the development of antiviral drugs, and various compounds have been reported to inhibit ASFV, including microtubule-stabilizing agents [Citation19], lauryl gallate [Citation20], stilbenes resveratrol, and oxyresveratrol [Citation21]. These compounds have been experimentally demonstrated to inhibit ASFV in vitro, but few compounds have shown inhibiting or blocking effects on disease progression or mortality in vivo, restricting the clinical application of these agents.
The antiviral activity of brincidofovir against dsDNA viruses has been extensively characterized in vitro using various cell culture systems and in vivo through multiple animal models [Citation22]. Brincidofovir has previously demonstrated the ability to suppress viral DNA replication by interfering with the function of viral DNA polymerase and destabilizing viral DNA [Citation23,Citation24]. Additionally, clinical trials have shown that brincidofovir can effectively reduce adenoviral loads in peripheral blood, leading to significant reductions in complications and mortality rates [Citation25]. Brincidofovir has also exhibited broad-spectrum antiviral activity against various members of the herpesviridae family (cytomegalovirus, herpes simplex virus, and varicella-zoster virus), papillomavirus, polyomavirus, and orthopoxviruses [Citation26–30].
In this study, we screened a kinase inhibitor library of 298 small molecules against ASFV to identify compounds that possess antiviral activity during infection of primary porcine alveolar macrophages (PAMs). We showed for the first time the inhibitory effect of brincidofovir on ASFV infection in vitro and in vivo. Brincidofovir effectively suppressed ASFV infection in PAMs without cytotoxicity, and this brincidofovir disturbed the post-invasion stage of ASFV life cycle. Its antiviral efficacy in vivo was determined using Chinese Bama minipigs with ASFV infection, and brincidofovir not only reduced the mortality and tissues viral loads in the ASFV-challenged piglets, but also largely shortened the viral shedding time after the infection. These results strongly suggest that brincidofovir may have promising clinical application prospects for the prevention and treatment of ASFV infection in the pig industry.
Materials and methods
Facility and ethics statements
All experiments with live ASFV were conducted within the Animal Biosafety level 3 (ABSL-3) laboratory at Huazhong Agricultural University and were approved by the Ministry of Agriculture and Rural Affairs of the People’s Republic of China. This study was conducted in accordance with the guidelines of the Guide for the Care and Use of Laboratory Animals of the Ministry of Science and Technology of the People’s Republic of China.
Cell culture, viruses, and compounds
Primary PAMs were collected from 21 to 28-day-old pigs and the cells were maintained in 10% foetal bovine serum–Roswell Park Memorial Institute 1640 medium (Gibco, Massachusetts, USA) at 37°C with 5% CO2. In this study, the genotype II ASFV virulent isolate wild boar/SNJ/2020 (GenBank ID: OL622042) was used. The virus titre of ASFV was measured using a hemadsorption (HAD) assay of PAMs, calculated by the Reed and Muench method, and expressed as HAD50/mL. A kinase inhibitor library of 298 compounds was purchased from MCE (New Jersey, USA) dissolved in DMSO (or mannitol solution). The large packets of brincidofovir were provided from National Engineering Research Center for the Emergency Drug (Beijing, China). Brincidofovir was dissolved in mannitol solution at 20 mM as a stock solution and diluted further in cell culture medium as required.
Cytotoxicity
The cytotoxicity of the compounds in PAMs was evaluated using the CCK8 assay. Confluent cells in 96-well cell culture plates (seeding density of 1.5 × 105 cells/well) were incubated with different concentrations of compounds for 72 h at 37°C in 5% CO2. The medium was removed after incubation, and the cells were washed with sterile phosphate-buffered saline before adding the CCK-8 solution (Vazyme, Nanjing, China). Finally, the cells were incubated at 37°C for another 2 h and subjected to colorimetric measurements using a microplate reader at 450 nm.
Quantitative PCR
ASFV genomic DNA was extracted from the culture supernatant and cell lysates, tissue homogenates, swabs, and EDTA-treated whole peripheral blood using a TIANamp Genomic DNA Kit (TIANGEN, Beijing, China). The B646L gene of ASFV was quantitatively determined using a QuantStudio 3 system (Applied Biosystems, Massachusetts, USA) according to T/CVMA 5-2018. The amplification was conducted in a 10-µL reaction mixture containing 1 µL genomic DNA, 1 µL forward primer (5′-ATAGAGATACAGCTCTTCCAG-3′), 1 µL reverse primer (5′-GTATGTAAGAGCTGCAGAAC-3′), 0.3 µL probe (5′-FAM-TATCGATAAGATTGAT-MGB-3′), 0.2 µL ROX Reference Dye II, and 2.5 µL sterilized water. The quantitative real-time PCR (qPCR) conditions were 95°C for 3 min, followed by 40 cycles of 95°C for 15 s and 52°C for 10 s, and a final step of 60°C for 35 s. The viral genome copies in these samples were determined by the ASFV B646L gene numbers and calculated based on a standard curve.
HAD assay
Primary PAMs were seeded into 96-well plates. Virus dilutions were performed using a macrophage medium and titrated in triplicate using 10 × dilutions. The ASFV titre was determined by the HAD of the erythrocytes around the infected cells. HAD was observed for 7 days, and virus titres (50% HAD doses, HAD50) were calculated using the Reed and Muench method [Citation31,Citation32].
Virus growth assay
Primary PAMs were infected with ASFV at a multiplicity of infection (MOI) of 0.1. The culture supernatant and cell lysates were collected at different time points post-infection. Viral genomic DNA was extracted and virus titres determined by detecting viral B646L gene copy numbers using qPCR.
Western blot
Primary PAMs were plated in six-well plates and infected with ASFV at an MOI of 0.1. At 48 h post-infection, cell lysates were collected, subjected to 12% sodium dodecyl-sulphate polyacrylamide gel electrophoresis under denaturing conditions, and transferred to a polyvinylidene difluoride membrane. The membranes were incubated with specific primary antibodies and the appropriate secondary antibodies conjugated with horseradish peroxidase. The blots were detected using a chemiluminescence kit (Bio-Rad, California, USA) on a ChemiDoc XRS + system (Bio-Rad, California, USA).
Immunofluorescence assay
Primary PAMs were grown in 96-well plates and infected with ASFV. At 72 h post-infection, the cells were fixed in 4% paraformaldehyde for 20 min, permeabilized in 0.1% (w/v) Triton-100 for 10 min at room temperature, and then incubated with specific ASFV p30 primary antibody (prepared in our laboratory) for 1 h at 37°C. The cells were washed three times with PBS and stained with fluorescein isothiocyanate (FITC)-conjugated Goat Anti-Porcine IgG secondary antibody (Southern Biotech, Alabama, USA) for another 1 h, followed by three washes with PBS. The fluorescent signal was observed with a fluorescence microscopy (EVOS FL Auto, Thermo Scientific, Massachusetts, USA), and the fluorescence value was determined by using a multifunctional microplate reader (Tecan Spark 10M, TECAN, Switzerland) and fluorescence intensity was analyzed by SparkControlTM (TECAN, Switzerland).
Animal experiments
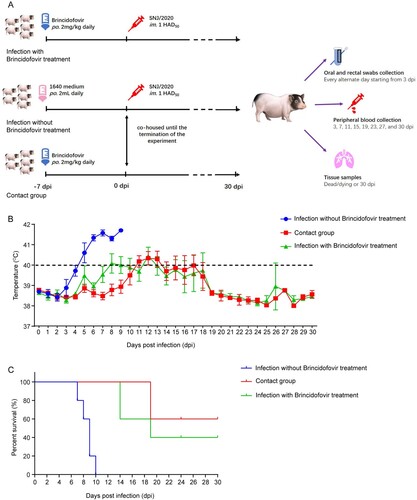
Animal experiments were performed at the Animal Biosafety Level 3 facility at Huazhong Agricultural University, under the protocol approved by the Animal Ethics Committee (HZAUSW-2022-0021). Chinese Bama minipigs (35 days old) were randomly divided into three groups. As shown in (A), pigs were randomly assigned to the control group (infection without treatment, n = 5) or the two treatment groups (infection with treatment and contact group, n = 5 in every group). The pigs in the treatment groups were administered brincidofovir orally at a dosage of 2 mg/kg body weight. The dosing was repeated every 24 h for seven days prior to virus challenge and continued until the end of the experiment. The control group and treatment group with infection were infected intramuscularly (i.m.) with 1 HAD50 SNJ/2020. Throughout the duration of the experiment, the contact group was co-housed with the control group to assess the protective efficacy of brincidofovir against contact transmission in pigs. The rectal temperature of the pigs was monitored daily, and clinical signs and survival were recorded during the study. In addition, blood and oral and rectal swabs were collected for virus copy detection. Necropsy was performed in a timely manner for dead and dying pigs. At 30 days post-infection (dpi), all surviving pigs were euthanized and necropsied. Tissue samples, including heart, liver, spleen, lung, kidney, tonsil, and submaxillary lymph node samples, were collected from all pigs for histopathological examination and viral load analysis.
Statistical analysis
Data are presented as mean ± standard error of the mean from at least three replicates. T-tests were performed using GraphPad Prism 7.0 (GraphPad Software Inc., California, USA). The abbreviation “ns” indicates no significant difference (P > 0.05); ∗ indicates a significant difference (P < 0.05); ∗∗ indicates a highly significant difference (P < 0.01); and ∗∗∗ indicates an extremely significant difference (P < 0.001).
Results
Screening of a kinase inhibitor library for anti-ASFV compounds
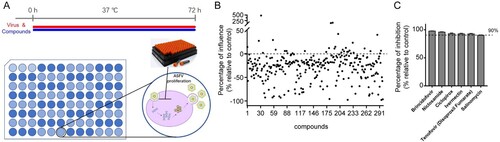
To select potential compounds against ASFV, we selected the PAMs for the screening, PAMs is the target cells of ASFV, as the cell type supports high levels of infection [Citation33,Citation34]. Here, we screened a library of 298 kinase inhibitor that targeting pathogenic microorganisms and immunology/inflammation-related enzymes ((A)). We used viral genome copies to characterize virus replication during the screening process. The genome copies were determined by qPCR of the ASFV B646L gene and calculated based on a standard curve. Result of the screening is depicted in (B). Compounds (10 μM) that exerted a > 90% inhibition against ASFV were defined as prime candidates; based on this criterion, 6 compounds (2.01%) were selected ((C)).
Figure 1. Screening of antiviral compounds against ASFV using the kinase inhibitor library. (A) Assay scheme: PAMs were treated with compounds and ASFV (MOI = 0.1) and incubated for 72 h. The culture supernatant and cell lysates were collected to determine viral genome copies by qPCR of the ASFV B646L gene and calculated based on a standard curve. (B) Screening of 298 kinase inhibitors for primary candidates that inhibit ASFV infection. Each dot represents the percent influence achieved with each compound at a concentration of 10 μM, which was calculated compared to that of the DMSO-treated control group. (C) These compounds of inhibition > 90% were shown in the bar graph. Data represented three independent experiments with three technical replicates (shown as mean ± SEM).
Brincidofovir robustly inhibits ASFV with low cytotoxicity
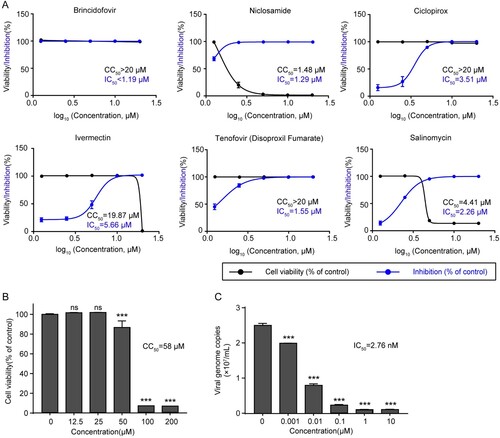
Six potential anti-ASFV compounds were identified in the primary screening and their cytotoxicity and inhibitory effects were further determined. The results showed that Brincidofovir, Tenofovir (Disoproxil Fumarate), and Ciclopirox were all able to inhibit ASFV replication with varying degrees of cytotoxicity ((A)). Among these compounds, brincidofovir exhibited optimal inhibitory effects on ASFV in PAMs. The CCK-8 assay results indicated that brincidofovir had very low cytotoxicity to cells, showing more than 80% of cell viability when receiving brincidofovir at less than 50 μM, with a 50% cytotoxic concentration (CC50) of 58 μM ((B)). Meanwhile, it exhibited a robust dose-dependent inhibitory effect with a 50% inhibitory concentration (IC50) of approximately 2.76 nM ((C)). Specifically, brincidofovir significantly inhibited viral replication at a concentration of 0.001 μM. The selective index (CC50/IC50) of this compound was 21014.5, suggesting the great potential as a promising anti-ASFV drug.
Figure 2. Dose-response curves and toxicity of the candidate compounds for inhibition of ASFV infection. (A) PAMs were treated with different concentrations of candidate compounds (20, 10, 5, 2.5, and 1.25 μM) and infected with ASFV (MOI = 0.1). Data showed the ratio of inhibition in ASFV-infected cells (blue) and the ratio of cell viability in uninfected cells (black). (B) The cytotoxicity of brincidofovir in PAMs was analyzed by CCK-8 assay. PAMs were treated with different concentrations (200, 100, 50, 25, and 12.5 μM) of brincidofovir, and the relative viability of PAMs without brincidofovir treatment (0 μM) was set to 100%. (C) PAMs were treated with ASFV (MOI = 0.1) and brincidofovir of different concentrations (10, 1, 0.1, 0.01, 0.001, and 0 μM) and incubated for 72 h. The culture supernatant and cell lysates were collected to determine viral genome copies by qPCR of the ASFV B646L gene and calculated based on a standard curve. Data represented three independent experiments with three technical replicates (shown as mean ± SEM), and T-tests was performed. ns, not significant (P > 0.05); ***, P < 0.001.
Effect of brincidofovir on viral titre and protein synthesis in PAMs
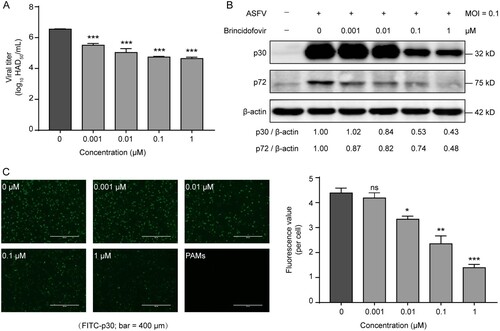
To further evaluate the inhibitory activities of brincidofovir against ASFV, we investigated the effects of the compound on viral titre and viral structural protein expression. The HAD assay showed that treatment with compounds at the non-toxic concentration (0.001, 0.01, 0.1, and 1 μM) resulted in dose-dependent reductions in the viral titre with magnitudes of 1–2 logs ((A)). Using western blotting analysis, we also evaluated the expression of an early (p30) and a late (p72) viral structural protein in the presence of brincidofovir at different concentrations. The results showed that the generation of both viral proteins was significantly inhibited by treatment with multiple concentrations of brincidofovir ((B)). Notably, the presence of brincidofovir at even low concentrations (0.001 and 0.01 μM) could affect the synthesis of the late protein p72 ((B)). We also assessed the effect of the compound on viral protein (p30) expression by using immunofluorescence. Following brincidofovir treatment, the fluorescence value, which reflected viral protein expression, was significantly reduced in a dose-dependent manner relative to that of the control ((C)). Together, these data confirm that brincidofovir significantly inhibits the viral titre and the early and late structural proteins expression of ASFV in target cells.
Figure 3. Inhibitory effect of brincidofovir on African swine fever virus (ASFV) replication. PAMs were treated with different concentrations (1, 0.1, 0.01, 0.001 and 0 μM) of brincidofovir and infected with ASFV at a multiplicity of infection of 0.1 (MOI = 0.1). (A) The culture supernatant and cell lysates were collected together at 72 hpi to determine viral titre by HAD assay. (B) The cell lysates were collected at 24 hpi to evaluate the expression of ASFV p30 and p72 proteins in the presence of different doses of brincidofovir by western blot. (C) Immunofluorescence assay was used to determine the antiviral activity of brincidofovir at different doses against ASFV in PAMs at 72 hpi. FITC-conjugated (green) secondary antibody was used to visualize ASFV p30. The fluorescence value was determined by using a multifunctional microplate reader and fluorescence intensity was analyzed and normalized to the DAPI. Data represented three independent experiments with three technical replicates (shown as mean ± SEM), and T-tests was performed. ns, not significant (P > 0.05); *, P < 0.05; **, P < 0.01; ***, P < 0.001.
Brincidofovir inhibits ASFV infection during viral DNA replication without directly inactivating the virus
We investigated whether brincidofovir exerted its antiviral effect by directly inactivating the virus. Brincidofovir (0.01 and 0.001 μM) was incubated with ASFV directly for 1 h at 37°C. The mixture was then diluted 20-fold to non-inhibitory concentrations (0.0005 and 0.00005 μM) and added to PAMs for 72 h. The viral copies from the supernatants and cell lysates were detected by qPCR. As shown in (A), the viral copies were not affected by pre-incubation with brincidofovir, indicating that brincidofovir does not inactivate the virus directly.
Figure 4. Time-of-addition analysis of the antiviral activity of brincidofovir. (A) Schematic illustration of the virucidal assay (top). Brincidofovir (0.01 and 0.001 μM) and ASFV (MOI = 0.1) were mixed and incubated at 37°C for 1 h and then diluted 20-fold before adding to PAMs. The possible inactivating effect of brincidofovir on viral genome copies was determined by qPCR assay at 72 hpi (bottom). (B) Schematic illustration of the primary time-of-addition experiment. PAMs were treated with brincidofovir (0.01 μM) and ASFV (MOI = 0.1) at different times as indicated, and the culture supernatant and cell lysates were collected at 72 hpi to determine viral genome copies by qPCR of the ASFV B646L gene and calculated based on a standard curve. (C) The effects of brincidofovir added at different stages were shown as viral genome copies. (D) Schematic illustration of the second time-of-addition experiment. PAMs were infected with ASFV at an MOI of 0.1 and treated with brincidofovir (0.01 μM) at different time points (1, 4, 8, 12, 16, and 24 hpi). The viral genome copies were determined by qPCR at 72 hpi. (E) The effects of brincidofovir added at different time points on the viral genome copies. Data represented three independent experiments with three technical replicates (shown as mean ± SEM), and T-tests was performed. ns, not significant (P > 0.05), ***, P < 0.001.
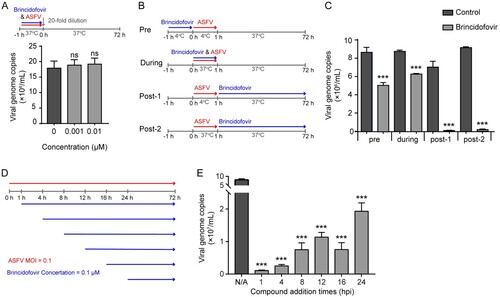
To determine which stage of the viral replication cycle is targeted by brincidofovir, we performed a time-of-addition assay in PAMs. Cells were infected with ASFV (MOI = 0.1), and brincidofovir was added at 0.1 μM at different times during the experiment ((B)). As shown in (C), antiviral brincidofovir activity was observed at all stages of virus infection. Noticeably, highly significant inhibition of ASFV replication was demonstrated when the brincidofovir was added 1 h after initial viral incubation at either 4 or 37°C (post-1 and post-2) ((C)). These results suggest that brincidofovir affects the virus during the post-entry stage. To further explore the action phase of the compound, we refined the timing of compound addition, and added the brincidofovir to ASFV-infected PAMs at six time points, followed by viral quantification at 72 hpi using qPCR ((D)). As shown, the data demonstrates that brincidofovir effectively inhibits viral replication within 1 h of administration. A longer duration of drug action during the viral replication cycle would result in a more significant inhibitory effect ((E)). Given that the virion enters late endosomes within one hour of infection, ASFV completes genome release and initiates replication in these organelles [Citation33,Citation35], we therefore propose that brincidofovir may affect the post-entry stage of ASFV and subsequent viral replication.
Brincidofovir significantly improves the survival rate of ASFV infection
We subsequently evaluated the antiviral activity of brincidofovir in vivo in Chinese Bama minipigs ((A)). As shown in (C), pigs infected with ASFV without brincidofovir treatment displayed a 100% mortality rate. Specifically, pigs exclusively challenged with ASFV began to develop fever at 3 dpi ((B)) and eventually died within 10 dpi ((C)). In contrast, challenged pigs receiving brincidofovir pre-treatment exhibited only slight fluctuations in body temperature and a significantly increased survival rate (40%) during the challenge. The contact pigs exhibited similar body temperature fluctuations and a 60% survival rate during the observation period ((B and C)). Notably, the body temperatures of all pigs receiving brincidofovir treatment increased only moderately and did not exceed 41°C. Moreover, the surviving brincidofovir-treated pigs did not show any typical clinical signs except for a transient depression. However, pigs without brincidofovir treatment exhibited a fast elevation in body temperature, approaching 42°C, before death.
Figure 5. Inhibitory activities of brincidofovir against ASFV in vivo. (A) Schematic illustration of the animal experiment. The animal experiment was performed as the schematic diagram showed. Chinese Bama minipigs (35 days old) were randomly divided into three groups. Pigs were randomly assigned to the control group (infection without treatment, n = 5) or the two treatment groups (infection with treatment and contact group, n = 5 in each group). The pigs in the treatment groups were orally dosed with brincidofovir at 2 mg/kg body weight. The pigs were dosed every 24 h for 7 days before the virus challenge until the termination of the experiment. The control group and infection with treatment group were infected intramuscularly (i.m.) with 1 HAD50 SNJ/2020. From the first day of infection until the termination of the experiment, the contact group was co-housed with the control group, to evaluate whether brincidofovir could protect pigs from succumbing to contact transmission. The rectal temperature of the pigs was monitored daily, and clinical signs and survival were recorded during the study. Peripheral blood was collected for viral genome copies detection. Oral and rectal swabs were collected for shedding detection. Necropsy was performed in a timely manner for dead and dying pigs. All surviving pigs were euthanized and necropsied at 30 days post-infection (dpi). Tissue samples were collected from all pigs for histopathological examination and viral load analysis, including heart, liver, spleen, lung, kidney, tonsil, and submaxillary lymph node. (B) The rectal temperature of all surviving pigs in each group was monitored for 30 days after ASFV infection. (C) Survival of pigs in each group was recorded for 30 days after ASFV infection. Data were shown as Kaplan-Meier survival curves (n = 5 for each group).
Brincidofovir can significantly reduce ASFV viremia and virus shedding in pigs
Next, we monitored the dynamic changes in viral load and shedding in peripheral blood samples, throat swabs, and rectal swabs to evaluate the potential antiviral effects of brincidofovir in vivo. Whole peripheral blood was collected from all surviving pigs at 3, 7, 11, 15, 19, 23, 27, and 30 dpi, and throat and rectal swabs were collected every other day starting from 3 dpi. qPCR detection of the ASFV B646L gene copies in the peripheral blood samples showed that a high level of viremia began in the infected pigs from 3 dpi and reached a peak at about 7 dpi. In contrast, pigs receiving brincidofovir exhibited relatively lower viremia at 7 dpi, and the viral genome copies in the surviving treated pigs decreased significantly over the time. Moreover, viremia was not observed in the contact pig samples during the initial 7 days; they began to develop a low level of viremia at 11 dpi. Viremia levels in the surviving contact pigs also decreased significantly over the time ((A)).
Figure 6. Viremia and virus shedding of ASFV-infected pigs affected by brincidofovir. (A) Peripheral whole blood was collected from all surviving pigs at 3, 7, 11, 15, 19, 23, 27, and 30 dpi. Throat swabs (B) and rectal swabs (C) were collected and suspended in 1 mL PBS every alternate day. The viral genomic DNA was extracted from the swab suspension and viral genome copies in these samples were determined by qPCR of the ASFV B646L gene and calculated based on a standard curve. Each point represented the data from one pig.
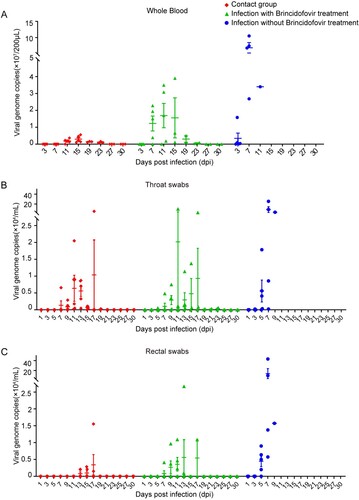
Viral shedding was also dynamically monitored using viral B646L gene copies in the throat and rectal swabs. As shown in (B and C), in the ASFV-challenged group, high numbers of viral genome copies were synchronously detected in both swabs from 5 dpi until death. In contrast, the throat swabs of pigs receiving brincidofovir treatment showed temporary viral shedding after 5 days, the median viral shedding period was 6 days, and the viral copies in the throat and rectal swabs of the surviving pigs decreased significantly until they were undetectable. Likewise, the viral copies in the throat swabs of the contact pigs showed a temporary fluctuation (duration 3–7 days) after 7 days and then decreased to undetectable levels. More importantly, viral shedding was never detected in the rectal swabs of the surviving contact pigs. Together, these results demonstrate that compound brincidofovir can effectively suppress viremia, reduce the level of viral shedding, and shorten the shedding duration in ASFV infection.
Brincidofovir largely reduced ASFV-caused tissue damage and viral load
We further investigated the protective effects of brincidofovir on ASFV-induced tissue damage and viral loads in different tissue samples, including heart, liver, spleen, lung, kidney, tonsil, and submaxillary lymph node samples. As shown in (A), the viral loads in the different tissues of the pigs exclusively challenged with ASFV were much higher than those of the groups that received brincidofovir treatment. The viral loads of surviving pig tissues in the contact group were almost undetectable, and only relatively low viral loads were detected in the tissues of surviving pigs with brincidofovir treatment. Notably, the viral loads in these tissues were much lower in all surviving pigs than in diseased pigs.
Figure 7. Effects of brincidofovir treatment on tissues viral load, pathological and histopathological changes in ASFV-infected pigs. Necropsy was performed in dead or dying pigs, and tissue samples were collected. All surviving pigs were euthanized at 30 dpi, and tissues were collected. (A) The viral loading in tissues were determined by qPCR of the ASFV B646L gene and calculated based on a standard curve. Each point represented a pig. (B) The tissue lesions of the pigs in the control group (without brincidofovir treatment) and two brincidofovir-treated groups (surviving pigs). (C) Tissue samples from each group were sectioned (H&E) and histopathologically analyzed (Scale bar is 100 μm).
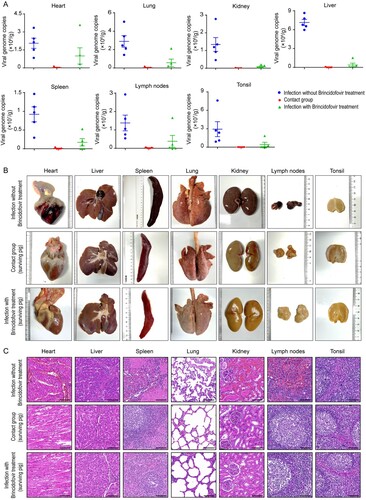
Severe hydropericardium, haemorrhage/oedema of the gall bladder, pulmonary oedema, haemorrhage of the kidney and lymph nodes, and splenomegaly were clearly observed in the ASFV-challenged pigs without brincidofovir treatment. Tissue damage was also observed in the dead or dying pigs in the brincidofovir-treated and contact groups, but it was much milder (). Noticeably, all the surviving pigs in these two groups showed only minor tissue damage, including slight haemorrhage of the heart and lymph nodes, and splenomegaly was not observed in the surviving pigs ((B)).
Table 1. The gross lesion in ASFV-infected pigs affected by brincidofovir.
In addition, histopathological analysis showed that the pigs exclusively infected with ASFV exhibited typical pathological changes in multiple tissues ((C)). Specifically, the ASFV infection group showed obvious myocardial fibre arrangement disorder and bleeding. The structure of the liver cord was disordered, the liver cells were swollen and necrotic, and the inflammatory cells were infiltrated. The spleen showed pathological changes indicative of acute splenitis; the splenic pulp was congested, the splenic corpuscles were almost disappeared, the red pulp structure was unclear, and the splenic trabecular structure was loose. The alveolar walls were thickened and the alveoli contained multiple eosinophilic exudates, red blood cells, and numerous inflammatory cells. The renal medulla was heavily congested, the renal tubules and capillary lumens were dilated, and eosinophilic components were observed in the lumen. The lymphatic tissue structure was nearly destroyed with significant congestion and bleeding. In contrast, these lesions were largely alleviated in surviving pigs of two treatment groups (infection with treatment and contact group), which showed almost normal histological morphologies ((C)). Brincidofovir did not show any side effects in pigs based on their daily states and weights.
Together, these data suggest that brincidofovir holds great potential as a viable candidate for the prevention and management of ASFV infection in the absence of safe commercial vaccines or antiviral agents.
Discussion
ASFV causes severe and fatal haemorrhagic disease in domestic pigs and wild boars, resulting in the rapid death of almost all infected animals [Citation1]. Current ASF control methods largely rely on the quarantine and culling of infected pigs in affected areas; however, the transmission of ASFV is often not completely blocked, and pig farmers are eagerly anticipating the availability of effective and safe vaccines and antiviral drugs against ASFV [Citation4,Citation36]. Here, we screened a kinase inhibitor library and identified candidate compound, brincidofovir. For the first time, we have demonstrated the potential of brincidofovir as a robust anti-ASFV agent; it showed anti-ASFV activity even at low molar concentrations (IC50 = 2.76 nM). The optimal protective effect of brincidofovir on ASFV was also confirmed in the Chinese Bama minipigs model. Orally delivered brincidofovir significantly improves the survival rate of ASFV infected, and reduced viral loads in the peripheral blood and tissues in ASFV infected pigs significantly.
As the cytopathic effect of ASFV infection in PAMs are difficult to observe and must rely on fresh porcine erythrocytes for virus titre determination [Citation37,Citation38], we chose to quantify the virus in terms of viral gene copy number during the screening process for reasons of stability of results and animal welfare, but the changes in virus titre caused by the compounds were investigated during the validation process.
Brincidofovir inhibited the post-entry stage of ASFV and viral replication in this study. During the multiplication cycle of ASFV, the virion enters the late endosomes within one hour post-infection, after which ASFV completes genome release and initiates replication in the same compartment [Citation33,Citation35]. When Brincidofovir was added at 1 h after ASFV infection, ASFV was maximally inhibited. Therefore, brincidofovir may affect the post-entry stage of ASFV and the subsequent viral replication [Citation26,Citation27,Citation29,Citation30,Citation39]. Brincidofovir was previously reported to suppress viral DNA replication by interfering with the function of viral DNA polymerase and destabilizing viral DNA [Citation23,Citation24]. We have molecularly docked the binding activity of brincidofovir to ASFV DNA polymerase X (AsfvPolX), result showed that brincidofovir competitively binds to the 42ARG domain of viral DNA polymerases through covalent bonds. Although we have demonstrated the binding activity of brincidofovir to AsfvPolX by molecularly docked, the loss of function of AsfvPolX is not sufficient to exert such a strong inhibitory effect on virus replication [Citation40,Citation41], which suggested that brincidofovir inhibited replication of ASFV through a different mechanism or pathway. We will further investigate the inhibitory effect of brincidofovir on ASFV through more biological experiments.
Several studies have shown that brincidofovir has in vitro or in vivo activity against adenovirus, herpesviridae, papillomavirus, polyomavirus, and orthopoxviruses [Citation26,Citation27,Citation29,Citation30,Citation39]. In this study, brincidofovir treatment showed powerful antiviral activity in animal experiments. We found that continuous brincidofovir administration at 2 mg/kg substantially improved the survival rate in pigs from ASFV infection, reduced ASFV viral loads in the peripheral blood and tissues, and delayed the onset or progression of the disease. In addition, the compound could shorten the viral shedding time, which is conducive to controlling the viral spread.
Previous studies have documented the adverse reactions of brincidofovir, mainly focusing on gastrointestinal damage, although that damage is reversible [Citation42]. We monitored the gastrointestinal health of all compound-treated piglets during the trial and found no constipation, diarrhoea or loss of appetite. In our other study (results not shown), one pigs (1/5) treated with 4 mg/kg body weight in daily showed an adverse effect of loss of appetite and progressive wasting. As the side effects of brincidofovir in pigs are still not fully understood, this will depend on more systematic studies on the safety of the compound.
In conclusion, for the first time we report and characterize the promising inhibitory activity of brincidofovir against ASFV infection in vitro and in vivo. However, the inhibitory activity of brincidofovir against ASFV has had limited effectiveness, which could potentially be used in combination with other drugs to improve the efficacy of the antiviral effect. These findings may provide new ideas and approaches for the prevention and control of ASF, which are highly valuable in the current context where there are no safe commercial vaccines or antiviral drugs available against ASFV.
Supplemental Material
Download MS Excel (29.2 KB)Acknowledgements
We sincerely appreciate Academician and Prof. Song Li and Prof. Wu Zhong (National Engineering Research Center for the Emergency Drug, Beijing, China) for providing the compound. We thank Dr. Dengguo Wei (Huazhong Agricultural University, Wuhan, China) for providing technical support in the AutoDock experiments. S.G. and X.W. conceived and designed the experiments and drafted the manuscript. S.G., Y.Z., Z.L. carried out the experiments; Technical assistance for the data analysis were performed by D.W., H.L., L.L., Q.C., D.Y., Q.L., H.G., and S.M.; X.W. and H.C. provided technical and administrative support. All authors have read and approved the final manuscript.
Data availability statement
The authors confirm that the data supporting the findings of this study are available within the article.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Galindo I, Alonso C. African swine fever virus: A review. Viruses. 2017;9:103.
- Wang N, Zhao D, Wang J, et al. Architecture of African swine fever virus and implications for viral assembly. Science. 2019;366:640–644.
- Wang G, Xie M, Wu W, et al. Structures and functional diversities of ASFV proteins. Viruses. 2021;13:2124.
- Sanchez-Cordon PJ, Montoya M, Reis AL, et al. African swine fever: A re-emerging viral disease threatening the global pig industry. Vet J. 2018;233:41–48.
- Montgomery E. On a form of swine fever occurring in British East Africa (Kenya Colony). J Comp Pathol Therap. 1921;34:159–191.
- Craig AF, Schade-Weskott ML, Harris HJ, et al. Extension of sylvatic circulation of African swine fever virus in extralimital warthogs in South Africa. Front Vet Sci. 2021;8:746129.
- Qu H, Ge S, Zhang Y, et al. A systematic review of genotypes and serogroups of African swine fever virus. Virus Genes. 2022;58:77–87.
- Hanh TX, Phuong LTT, Huy NQ, et al. African swine fever virus vaccine candidate ASFV-G-DI177L efficiently protects European and native pig breeds against circulating Vietnamese field strain. Transbound Emerg Dis. 2021;69:e497–e504.
- Borca MV, Ramirez-Medina E, Silva E, et al. ASFV-G-ΔI177L as an effective oral nasal vaccine against the Eurasia strain of Africa swine fever. Viruses. 2021;13:765.
- Tran XH, Phuong LTT, Huy NQ, et al. Evaluation of the safety profile of the ASFV vaccine candidate ASFV-G-ΔI177L. Viruses. 2022;14:896.
- Gladue DP, Borca MV. Recombinant ASF live attenuated virus strains as experimental vaccine candidates. Viruses. 2022;14:878.
- O'Donnell V, Holinka LG, Sanford B, et al. African swine fever virus Georgia isolate harboring deletions of 9GL and MGF360/505 genes is highly attenuated in swine but does not confer protection against parental virus challenge. Virus Res. 2016;221:8–14.
- Monteagudo PL, Lacasta A, López E, et al. BA71ΔCD2: a New recombinant live attenuated African swine fever virus with cross-protective capabilities. J Virol. 2017;91:e0105817.
- Lopez E, van Heerden J, Bosch-Camós L, et al. Live attenuated African swine fever viruses as ideal tools to dissect the mechanisms involved in cross-protection. Viruses. 2020;12:1474.
- Sang H, Miller G, Lokhandwala S, et al. Progress toward development of effective and safe African swine fever virus vaccines. Front Vet Sci. 2020;7:84.
- Wang T, Luo R, Sun Y, et al. Current efforts towards safe and effective live attenuated vaccines against African swine fever: challenges and prospects. Infect Dis Poverty. 2021;10:137.
- Urbano AC, Ferreira F. African swine fever control and prevention: an update on vaccine development. Emerg Microbes Infect. 2022;11:2021–2033.
- Borca MV, Rai A, Ramirez-Medina E, et al. A cell culture-adapted vaccine virus against the current African swine fever virus pandemic strain. J Virol. 2021;95:e0012321.
- Sirakanyan S, Arabyan E, Hakobyan A, et al. A new microtubule-stabilizing agent shows potent antiviral effects against African swine fever virus with no cytotoxicity. Emerg Microbes Infect. 2021;10:783–796.
- Hurtado C, Bustos MJ, Sabina P, et al. Antiviral activity of lauryl gallate against animal viruses. Antivir Ther. 2008;13:909–917.
- Galindo I, Hernaez B, Berna J, et al. Comparative inhibitory activity of the stilbenes resveratrol and oxyresveratrol on African swine fever virus replication. Antiviral Res. 2011;91:57–63.
- Toth K, Spencer JF, Dhar D, et al. Hexadecyloxypropyl-cidofovir, CMX001, prevents adenovirus-induced mortality in a permissive, immunosuppressed animal model. Proc Natl Acad Sci USA. 2008;105:7293–7297.
- Alvarez-Cardona JJ, Whited LK, Chemaly RF. Brincidofovir: understanding its unique profile and potential role against adenovirus and other viral infections. Future Microbiol. 2020;15:389–400.
- Feghoul L, Mercier-Delarue S, Salmona M, et al. Genetic diversity of the human adenovirus species C DNA polymerase. Antiviral Res. 2018;156:1–9.
- Florescu DF, Pergam SA, Neely MN, et al. Safety and efficacy of CMX001 as salvage therapy for severe adenovirus infections in immunocompromised patients. Biol Blood Marrow Transplant. 2012;18:731–738.
- Hartline CB, Gustin KM, Wan WB, et al. Ether lipid-ester prodrugs of acyclic nucleoside phosphonates: activity against adenovirus replication in vitro. J Infect Dis. 2005;191:396–399.
- Williams-Aziz SL, Hartline CB, Harden EA, et al. Comparative activities of lipid esters of cidofovir and cyclic cidofovir against replication of herpesviruses in vitro. Antimicrob Agents Chemother. 2005;49:3724–3733.
- Florescu DF, Keck MA. Development of CMX001 (brincidofovir) for the treatment of serious diseases or conditions caused by dsDNA viruses. Expert Rev Anti Infect Ther. 2014;12:1171–1178.
- Randhawa P, Farasati NA, Shapiro R, et al. Ether lipid ester derivatives of cidofovir inhibit polyomavirus BK replication in vitro. Antimicrob Agents Chemother. 2006;50:1564–1566.
- Kern ER, Hartline C, Harden E, et al. Enhanced inhibition of orthopoxvirus replication in vitro by alkoxyalkyl esters of cidofovir and cyclic cidofovir. Antimicrob Agents Chemother. 2002;46:991–995.
- Carrascosa AL, Bustos MJ, de Leon P. Methods for growing and titrating African swine fever virus: field and laboratory samples. Curr Protoc Cell Biol. 2011;Chapter 26:26.14.21–26.14.25.
- Malogolovkin A, Sereda A. African swine fever virus hemadsorption inhibition assay. Methods Mol Biol. 2022;2503:159–167.
- Gaudreault NN, Madden DW, Wilson WC, et al. African swine fever virus: An emerging DNA arbovirus. Front Vet Sci. 2020;7:215.
- Andrés G. African swine fever virus gets undressed: New insights on the entry pathway. J Virol. 2017;91:e0190616.
- Wang Y, Kang W, Yang W, et al. Structure of African swine fever virus and associated molecular mechanisms underlying infection and immunosuppression: A review. Front Immunol. 2021;12:715582.
- Dixon LK, Sun H, Roberts H. African swine fever. Antiviral Res. 2019;165:34–41.
- Bustos MJ, Nogal ML, Revilla Y, et al. Plaque assay for African swine fever virus on swine macrophages. Arch Virol. 2002;147:1453–1459.
- Arabyan E, Kotsynyan A, Hakobyan A, et al. Antiviral agents against African swine fever virus. Virus Res. 2019;270:197669.
- Lanier R, Trost L, Tippin T, et al. Development of CMX001 for the treatment of poxvirus infections. Viruses. 2010;2:2740–2762.
- Chen Y, Zhang J, Liu H, et al. Unique 5'-P recognition and basis for dG:dGTP misincorporation of ASFV DNA polymerase X. PLoS Biol. 2017;15:e1002599.
- Redrejo-Rodríguez M, Rodríguez JM, Suárez C, et al. Involvement of the reparative DNA polymerase Pol X of African swine fever virus in the maintenance of viral genome stability in vivo. J Virol. 2013;87:9780–9787.
- Painter W, Robertson A, Trost LC, et al. First pharmacokinetic and safety study in humans of the novel lipid antiviral conjugate CMX001, a broad-spectrum oral drug active against double-stranded DNA viruses. Antimicrob Agents Chemother. 2012;56:2726–2734.
