Smallpox is one of the most known representatives of the Poxviridae family which includes members responsible for poxvirus infections. Despite its eradication, other pathogenic poxviruses infections are still considered threats to human health due to the great potential for human-to-human transmission risk, as well as the risk of re-emerging and/or spread of these pathogenic poxviruses from animal to humans or the infection that may involve novel hosts, that are still currently not known [Citation1]. Some zoonotic orthopoxviruses, such as monkeypox (mpox) virus and cowpox virus still continue to infect humans and animals, leading to a growing concern about any possible outbreak in light of the broad-ranged geographical distribution of poxviruses worldwide [Citation2]. The re-emergence and increase of human mpox cases worldwide from 2022, the atypical cutaneous lesions, the possibility of different routes of transmission, also the atypical clinical presentations of mpox were shown [Citation3,Citation4]. The prompt response of several countries further highlights the importance of a monitoring and management policy to contrast the mpox epidemic, adopting an active surveillance of mpox, which represents a rare but constant threat for human health, along with other poxviruses infections [Citation5]. In this regards, other members belonging to different genera of the Poxviridae family are potentially neglected. Here, we described a zoonotic infection sustained by orf virus occurred through a clear transmission chain from sheep to sheep and from sheep to human.
A 60-year-old otherwise healthy shepherd man from northern China was presented with a solitary cutaneous lesion on his left radiocarpal joint which persisted for a week before the first dermatological clinical evaluation. A rapid evolvement history of the cutaneous lesion was reported that presented as papules at onset, followed by blood blister, rupture, erosion, and exudation within a week after the onset. He noticed that his sheep developed scabby cutaneous lesions around the mouth about three weeks ago. Any systemic symptoms including fever, lymphadenopathy, and chronic systemic disease history were denied. The physical examination showed central ulceration and a bleeding solitary cutaneous lesion involving the left radiocarpal joint of a diameter of about 1.2 * 0.8 cm ((A)). The neighbours reported the sheep (sheep 3,4) she raised began to suffer from cutaneous lesions about two weeks after the onset of the lesion in the sheep raised by the patient and denied that she had a history of the cutaneous lesion ((B,C)). A skin biopsy specimen of the patient was sent for further histologic analysis. The histological findings displayed partial necrosis, intracellular and intercellular oedema in the epidermis and intraepidermal blistering, and a diffuse oedema within the dermis accompanied by dense inflammatory cells infiltration composed of the neutrophils, lymphocytes, and histiocytes, which were pointed using orange arrows. In addition, the cytoplasmic eosinophilic inclusion bodies in the epidermis were also found ((D,E)). A suspect of virus infection was suggested based on the clinical and histological findings, and a skin swab sample was collected from a cutaneous lesion area and analysed by metagenomic next-generation sequencing (mNGS). Orf virus sequences were identified at a high abundance at 97.66%, of which the accession number of raw data has been provided (Supplemental Table S1). In addition, the transmission electron microscopy (TEM) analysis at higher magnifications revealed the presence of typical enveloped virions ((F)). Therefore, the clinical-pathological, molecular, histological, and TEM findings were consistent with a diagnosis of orf virus infection.
Figure 1. Clinical, histological, TEM findings, and the diagram of the transmission relation chain and course. (A) Physical examination showed central ulceration and a bleeding solitary cutaneous lesion involving the left radiocarpal joint in a diameter of about 1.2 * 0.8 cm with peripheral erythema. (B and C) Similar and typical scabby cutaneous lesions around the mouth in orf virus-infected sheep raised by neighbours were found during further tracking. (D and E) The histological findings displayed partial necrosis, intracellular and intercellular oedema in the epidermis and intraepidermal blistering, diffuse oedema existed in the dermis with dense inflammatory cells infiltration including the neutrophils, lymphocytes and histiocytes into the dermis, also the cytoplasmic eosinophilic inclusion bodies in the epidermis were found. (Haematoxylin-Eosin staining, ×100, ×400). (F) Virus detection by transmission electron microscopy (TEM) analysis at higher magnifications showed typical enveloped virions. Scale bar indicates 50 nm. (G) The three weeks ago when the patient seeking dermatological evaluation, approximately 30 sheep raised by the patient in sheepfold A almost at the same time presented scabby cutaneous lesions around the mouth, also the sheep of neihbours raised in sheepfold B who shared the same pasture also reported an extreme similar history of the sheep presenting cutaneous lesions around the mouth when two weeks after the onset of the lesion in the sheep raised by the patient.
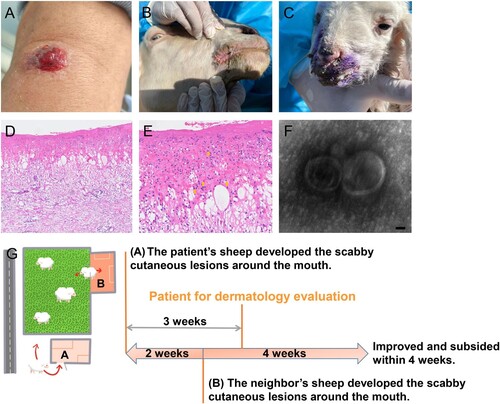
To identify the source of infection in the patient, we traced the potential source of the pathogen to the sheep the patient raised. Within the 30 sheep raised by the patient, the scabby cutaneous lesions around the mouth were observed about three weeks ago before the clinical onset of the patient, and gradually improved and subsided following the care of the patient. Skin swab samples of the mouth from two sheep were obtained (Sheep 1,2). In addition, the sheep of neighbours sharing the same pasture also reported an extremely similar clinical history with typical scabby of the sheep presenting cutaneous lesions around the mouth, that became evident about two weeks after the onset of the lesion in the sheep of the patient. Skin swab samples were also collected from these two sheep (Sheep 3,4). The presence of the orf virus was confirmed from all these samples by mNGS. The reads numbers related to the orf virus from sheep 1, sheep 2, sheep 3, and sheep 4 showed relative abundance rates at 15.79%, 28.12%, 93.89%, and 97.56%, respectively (Supplemental Table S1). Together, these findings proved a clear transmission chain of orf virus from sheep to sheep and from sheep to humans, which was summarized in (G).
Discussion
Besides the eradicated human orthopoxviruses such as smallpox virus, as well as monkeypox virus, the other members from different genera of the Poxviridae family are also pathogenic for human [Citation6]. In addition to the human mpox infection that is currently an ongoing outbreak, the pathogenic potential for humans of other poxviruses should also be emphasized. Orf virus is classified within the Parapoxvirus genus of the Poxviridae family, and it is an important zoonotic causative agent threatening sheep, goats, and humans. Orf virus has the hardy characteristic that can remain viable in the environment for several months, or even potentially years, and thus any direct contact between sheep or goats, or indirect contact with orf virus-contaminated like pastures, buildings can cause transmission between sheep or goats. The transmission routes to humans usually involved contact between a damaged skin and the virus from infected animals as well as exposure to contaminated equipment, with a global risk of exposure for both the animals and humans. The typical scabby cutaneous lesions that are mostly localized around the lips, muzzle, and mouth are typically observed in infected sheep or goats, while the ulcerative lesions or nodules on the hands are usually developed in orf virus-infected human [Citation7]. There is no standardized treatment strategy currently in place, and mostly it is presented as a self-limiting infection [Citation8,Citation9]. The preventive vaccination strategies and protective equipment such as gloves are an important way to prevent orf virus infection, as the infections do not generate long-lasting immunity in both hosts [Citation10–14]. However, this infection in animals or humans has been extensively neglected for decades due to a self-limiting disease course [Citation15].
A clear transmission relation chain of orf virus from sheep to sheep, and from sheep to humans has been demonstrated in the present report. The pastures contaminated by orf virus should be considered as an important route of transmission between the patient’s and neighbour’s sheep, while the main route for patients to be infected by orf virus is caused by a direct contact with infected sheep. The limitation of this study is that we have not isolated the virus and failed to obtain the result of viral genome assembly, which will give more accurate information of the orf strain we have identified. The human mpox outbreak in many countries is gradually growing attention within the field of poxvirus infection. However, other members classified in different genera of the same family are still a public health threat. Because the clinical manifestations of human infection with poxviruses often displayed similar skin lesions, the importance of pathogenic evidence should be constantly emphasized during the current ongoing epidemic of human mpox and also should promote the development of novel tools or kits for a rapid diagnosis. It is important also to be alert for other pathogenic poxviruses, which may represent a potential threat to human and should be included in the differential diagnosis along with mpox, especially in the context of human mpox epidemic. Indeed, a more active mpox surveillance may increase the possibility of detection of other pathogenic poxviruses infection events.
Supplemental Material
Download MS Excel (11.9 KB)Acknowledgements
We thanked Xu Li from Harvard Medical School, Hang Xu from Fuwai Hospital, National Clinical Research Center for Cardiovascular Diseases, National Center for Cardiovascular Diseases, Chinese Academy of Medical Sciences and Peking Union Medical College for their kind supports for this work, and Dr. Lei Jia and Lin Li from Beijing Institute of Microbiology and Epidemiology for the help with TEM experiments. Dr. Mingyue Chen from National 111 Center for Cellular Regulation and Molecular Pharmaceutics, Key Laboratory of Fermentation Engineering, Hubei University of Technology for the help with bioinformatics analysis.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- McFadden G. Poxvirus tropism. Nat Rev Microbiol. 2005 Mar;3(3):201–213. doi:10.1038/nrmicro1099
- Silva NIO, de Oliveira JS, Kroon EG, et al. Here, there, and everywhere: the wide host range and geographic distribution of zoonotic orthopoxviruses. Viruses. 2020 Dec 30;13(1):43. doi:10.3390/v13010043
- Yao K. The diversity of clinical manifestations of human monkeypox should be emphasized in practice. Pediatr Investig. 2022 Sep;6(3):224–225. doi:10.1002/ped4.12349
- Mitjà O, Ogoina D, Titanji BK, et al. Monkeypox. Lancet. 2023 Jan 7;401(10370):60–74. doi:10.1016/S0140-6736(22)02075-X
- Patil DY, George S, Sahay RR, et al. A case of human buffalopox in Malappuram, India: the role of mpox surveillance in 2022. J Med Virol. 2023 Feb 15;95(2):e28580. doi:10.1002/jmv.28580
- Essbauer S, Pfeffer M, Meyer H. Zoonotic poxviruses. Vet Microbiol. 2010 Jan 27;140(3-4):229–236. doi:10.1016/j.vetmic.2009.08.026
- Andreani J, Fongue J, Bou Khalil JY, et al. Human infection with Orf virus and description of its whole genome, France, 2017. Emerg Infect Dis. 2019 Dec;25(12):2197–2204. doi:10.3201/eid2512.181513
- Kromer C, Bierschenk C, Czerny CP, et al. Orf (ecthyma contagiosum) in a sheep and a shepherd. Lancet Infect Dis. 2018 Jan;18(1):122. doi:10.1016/S1473-3099(17)30317-1
- Tobler C, Ritter-Schenk C, Zimmermann P. Orf virus infection: ecthyma contagiosum. J Pediatr. 2022 Apr;243:236–237. doi:10.1016/j.jpeds.2021.11.067
- CDC. Orf virus (sore mouth infection). Centers for Disease Control and Prevention; [cited 2023 Feb 20]. Available from: https://www.cdc.gov/poxvirus/orf-virus/
- Bergqvist C, Kurban M, Abbas O. Orf virus infection. Rev Med Virol. 2017 Jul;27(4). doi:10.1002/rmv.1932
- Demiraslan H, Dinc G, Doganay M. An overview of ORF virus infection in humans and animals. Recent Pat Anti-Infect Drug Discovery. 2017;12(1):21–30. doi:10.2174/1574891X12666170602080301
- Jia X, Yan H, Mu Q. An ulcerated nodule on the forearm of a shepherd. CMAJ. 2023 Feb 27;195(8):E304. doi:10.1503/cmaj.221135
- Liu Y, Li X, Yan H, et al. A case of orf virus infection in the human: other zoonotic poxvirus infection events should not be ignored during the mpox epidemic. Travel Med Infect Dis. 2023 May 12;53:102588. doi:10.1016/j.tmaid.2023.102588
- Bala JA, Balakrishnan KN, Abdullah AA, et al. The re-emerging of orf virus infection: a call for surveillance, vaccination and effective control measures. Microb Pathog. 2018 Jul;120:55–63. doi:10.1016/j.micpath.2018.04.057
