ABSTRACT
The zoonotic bacteria, Brucella canis, is becoming the leading cause of canine brucellosis in Europe. In dogs, it causes reproductive problems as well as non-specific lameness or discospondilitis. In humans, B. canis can be origin of chronic debilitating conditions characteristic to its genus such as undulant fever, splenomegaly, and lymphadenopathy. Although B. canis shows some pathogenic characteristics similar to B. abortus and B. melitensis, it lacks surface O-polysaccharide, like nonzoonotic B. ovis. This review shows that host–B. canis interactions are still poorly understood, with many knowledge and capability gaps, causing relatively poor sensitivity and specificity of existing diagnostic tools. Currently, there is no vaccine for this rough Brucella species. Besides, antimicrobial therapy does not guarantee bacterial elimination, and infection relapses are frequently reported, increasing the risks of antibiotic resistance development. B. canis has been detected in dogs in almost all European countries which increased human exposure, but currently there is no systematic surveillance. Moreover, B. canis caused brucellosis is not included in Animal Health Law, and therefore there is no legal framework to tackle this emerging infectious disease. To map out the diagnostic strategies, identify risks for human infections and propose management scheme for infected pet and kennel dogs, we present current understanding of canine B. canis caused brucellosis, outline major knowledge gaps and propose future steps. To address and highlight challenges veterinary and public health services encounter in Europe, we developed two B. canis infection scenarios: of a single household pet and of a kennel dog in larger group.
Introduction
Pet animals currently occupy an important position in our society. In the European Union, the number of dogs is estimated to be 61 million [Citation1] and the dog population is constantly increasing. Around the World, canine brucellosis has been mainly caused by Brucella canis, while in Europe, until 2000s dog infections were caused occasionally by B. melitensis, B. suis, or B. abortus. Until then B. canis was only identified after being diagnosed as causative agent in imported dogs. B. canis is an important cause of infertility in dogs and has been recognized as the cause of significant economic losses in kennels with infected dogs. Canine brucellosis, due to B. canis, is characterized by one or more of the following clinical signs: miscarriage or absorption in the pregnancy, orchitis, epididymitis, endocarditis, uveitis, and discospondylitis. In humans, B. canis can cause undulant fever, splenomegaly, lymphadenopathy, etc., and if untreated endocarditis and disease of central nervous system. Therefore, canine brucellosis due to B. canis as a public health threat should be considered within the context of the relationship between dogs and humans, especially when close contact with children or immunocompromised people is present [Citation2,Citation3], and as an occupational hazard for breeding kennel personnel, veterinarians and veterinary nurses, as well as dog owners.
Brucella can infect, survive, and replicate in various host cells, with the higher affinity to the cells of the reproductive and immune systems [Citation4]. For intracellular infection, Brucella have evolved multiple strategies to evade immune response by modulating innate and the adaptive immune systems, as well as autophagy and apoptosis mechanisms [Citation5]. As it has rough lipopolysaccharide, B. canis has variable success entering macrophages or monocytes, while thorough preserved PI3-kinase is much more successful entering the dendritic and epithelial cells, explaining bacterial replication and inflammation of placenta [Citation6]. With a rough LPS on the surface, B. canis triggers TLR-2 and TLR-4 which could potentially explain initial immune response detectable by indirect diagnostic tools, but still low concentration of bacteria for the direct isolation. B. ovis also has rough LPS. Though, so far there have been no proof that B. ovis based vaccines can protect dogs against B. canis infection. Once in the cell, B. canis preserved most of the type IV secretion system (T4SS) characteristics present in highly pathogenic Brucella species, to modify the cellular compartments, and delay the apoptosis signalling [Citation7]. Slow metabolism and replication, absence of functional gadBC gene (glutamic acid decarboxylase gene for the resistance to low pH values) and alteration of apoptosis would explain prolonged localization of B. canis within cells, often within sequestered tissues. The prolonged intracellular cycle could explain lengthy antibiotic therapy, low success rates of the treatment and relapses in infected dogs. Since stealthiness mechanisms, used by other pathogenic Brucella spp., such as production of PAMPs, Toll/Interleukin-1 Receptor (TIR) domain-containing proteins, or positively charged core sugars, still have not been identified in B. canis it is unclear how it shuts both TLR-2 and TLR-4 signalling, maintaining stealthiness [Citation8]. Yet, upon experimental infection of DH82 canine macrophages, TLR-3, -7, and -8 are activated in time-dependent manner with the TLR-7 being the most expressed, but not TLR-2 nor TLR-4 [Citation8]. Interestingly, IFN-gamma, IL-4 nor IL-2 were not expressed in significant concentrations, similarly to another experimental infection of mice when B. canis was compared to B. melitensis and B. abortus. This would explain lower levels of pro-inflammatory cytokines secreted when macrophages are infected with B. canis, compared to B. abortus, but also higher replication rates in dendritic and epithelial cells compared to latter Brucella species [Citation8]. Moreover, just like B. abortus and B. suis, B. canis possess all the mechanisms to hinder maturation of dendritic cells and alter adaptive immune response, which could explain how, infected dogs can transmit the disease even without developing the disease . B. canis is zoonotic, however, the virulence of the bacterium to humans is not fully elucidated either [Citation9]. However, many of these deductions yet have to be tested and there are still many unknowns regarding the B. canis, especially as an emerging potential public health threat in Europe. Unequivocal diagnosis depends on the detection of bacteria. In the case of subclinical canine brucellosis, the biopsies of predilected tissues are unjustified, while diagnosis from blood is challenging both with indirect and direct methods. The imperfect diagnostic accuracy of serological tests coupled with and intermittent bacteraemia make culture from blood an unreliable isolation and diagnostic method.
Another challenging factor, relatively new from the point of view of infectious animal disease professionals, is dealing with a largely private, non-commercial, layperson group of owners when compared to farmers and with limited legal options for competent authorities. Compared to other diseases like rabies, the legal options to control movements and trade are limited or unclear for B. canis infected animals. Moreover, effective communication to all stakeholders involved is challenging, especially with respect to a zoonotic infection in companion animals and for dogs which may be infectious without showing clinical signs.
The main goal of this paper is to give a state-of-the-art update on the European situation with regard to B. canis, to present current knowledge gaps and with two different scenarios demonstrate limitations in proposing guidelines to address the emergence and the disease spread.
Country overviews
The presence of B. canis infection has been demonstrated in different countries of the world with the isolation of the etiological agent, while in others its presence has been suspected based on the serological response. Canine brucellosis has been reported as endemic in Central and South America, in Asia, and in the Southern USA [Citation10]. In endemic countries, canine brucellosis due to B. canis is especially common among stray dogs, and in shelter or in commercial breeding kennels [Citation11]. When the disease is introduced into a kennel, it spreads rapidly [Citation11–13].
Reports of B. canis infections in Europe currently largely reflect the occurrence of clinical symptoms either in dogs or humans. There has been no systematic or cross-sectional study performed in any European country to evaluate the prevalence of this disease. The lack of data and surveillance programmes does not currently allow for an exact understanding of countries where the disease might be considered endemic. Since 2017, increasing numbers of sporadic cases, clusters, or outbreaks have been reported in different EU countries, including Switzerland, Ukraine, the Netherlands, the UK, and Turkey [Citation12,Citation14–18] (). Retrospective, laboratory data collected from samples submitted in a non-systemic manner between 2016 and 2022, from 20 countries (mainly from Western Europe), indicate that 3.7% (61/1657) of samples subjected to laboratory veterinary diagnosis for brucellosis testing led to the detection of Brucella spp. DNA, with positive samples coming mainly from Spain (11.1% of the samples submitted), Poland (6.7% of the samples submitted), and more rarely from Italy and France [Citation14]. However, these results should be interpreted with caution, since in this study commercial PCR kit for Brucella spp. detection was used, which has not yet been validated by competent authorities. Moreover, the non-systematic sample submission, with very uneven representation of different countries, means that it is not possible to use the results for any formal estimation of prevalence. Currently, pre- or post-import testing of dogs for B. canis is not required in Europe, so laboratory detection usually follows clinical disease manifestations, if the veterinarian is sufficiently informed and aware of it. Moreover, there is no mandatory testing scheme in any EU country. For policy makers, there is indeed a considerable difference between (risk of) emergence, outbreak, or endemic situation. This impacts the risk assessment in terms of dissemination, frequency, perennity of the disease and drives management decision making, as well as the level of any policy engagement. Recent data highlighted movements of dogs between kennels and countries as a major source of disease circulation, given the absence of sanitary control before and after travel. Movements of dogs between countries and online trade have increased during the last years, especially during the COVID pandemics. Recent risk assessments from the UK, France, and Finland [Citation2,Citation3] have led to regulations changes in these countries (such as control of imported dogs or making cases reportable), since recommendations are decided on a country level. Additionally, canine brucellosis is not a notifiable disease for the World Organization for Animal Health (WOAH, founded as OIE) and EU countries, which negatively impacts on the reporting of positive cases, whereas in the UK now, positive laboratory results for B. canis have been made reportable.
Table 1. European incidents of Brucella canis infection that have been identified (note many cases may go unidentified or unreported).
In Western European countries, B. canis has historically not been regarded as endemic, as defined by the constant presence and/or usual prevalence of a disease or infectious agent in a population within a geographic area. However, there is no clear definition when a country is considered endemic for canine brucellosis. In the European Union, laboratory testing is only done in animals travelling to countries that mandate a set of blood tests (including B. canis) for all pet dogs entering their territories, such as Australia, New Zealand, and South Africa, or after a veterinary request following clinical disease (non-specific bacterial culture investigations or specific B. canis serological testing). Serologically positive reactions are not systematically reported to competent authorities. However, some studies carried out in the last decade showed that this bacterial infection may be present even if no strains have been isolated. For instance, in 2013/2014, a study including 62 dogs from different kennels in the Lisbon region was carried out, and 6 animals (9.7%) were diagnosed as positive in both serological (Rapid Slide Agglutination Test – RSAT, 2 mercapto-ethanol RSAT – RSAT-ME and Complement Fixation Tests – CFT with rough antigen) and molecular tests (Ferreira AC, personal communication). More recently, in 2018/2019, a follow-up was performed in two kennels with epidemiologically linked dogs. The study included 19 animals, 16 adults (tested twice at 3 months interval) and 3 young dogs (tested once). Nine out of 16 adult dogs (56.2%) were positive in serological and/or molecular tests. The clinical investigations identified one dog with cervical spondylosis and two females with history of abortion and embryonic resorption at day 45 of pregnancy. The three puppies were serologically negative but PCR positive for Brucella spp. (Ferreira AC, personal communication).
In France, canine B. canis cases are on the rise as well. During the last 2 years more than 900 dogs have been reported to be at high exposure risk, from more than 250 owners throughout the country. Out of all tested animals, 170 cases had positive indirect diagnostic result from which strain isolation was attempted (C. Ponsart & A. Ferreira Vicente, personal communication) The infection was confirmed in 31 dogs by strain isolation ().
In Italy, a major B. canis brucellosis outbreak was first reported in 2020 in a large commercial breeding kennel [Citation12] (). In the first round of serological tests of 598 animals, 269 (46.1%) tested serologically positive. In the second round of laboratory tests performed 4–5 weeks later, the number of tested dogs that were serologically positive was 241 out of 683 sampled (35.3%), while the number of dogs from which a strain was isolated was 68 out of 683 tested (10.0%). Before this outbreak, the only report recorded in Italy was a presumptive B. canis infection in a dog with chronic prostatitis and discospondylitis, detected by PCR [Citation22] (). The study also highlighted the need to increase the B. canis genome database for molecular epidemiological surveillance, as well as the need to better control the dog movements and testing for canine brucellosis, especially for animals commercialized for breeding purposes.
In the UK, until recently, there had been only sporadic cases of B. canis identified, linked to pet trade with Eastern Europe [Citation15,Citation21] (). However, a rapidly increasing number of suspect canine brucellosis due to B. canis have been reported by private veterinarians and/or veterinary laboratories, following clinical suspicion and/or laboratory identification (bacteriology, serology, or PCR) since July 2020 [Citation3]. As of February 2020 until the end of 2022, more than 100 epidemiologically distinct cases of canine brucellosis (confirmed and probable based on laboratory, clinical and epidemiological investigations), including one large household cluster in England with evidence of dog-to-dog transmission, have been reported [Citation3] (). New cases continue to be reported on a regular basis – mainly related to importation or overseas travel (J. McGiven, personal communication). A review of domestic dog commercial import data by the UK government found that by the end of November 2020, commercial dog imports from Romania had increased in that year alone by 51% compared to 2019, with 29,348 dogs brought into the country [Citation3].
Investigations conducted in the Netherlands in 18 seropositive and epidemiologically linked dogs showed that many of these animals had been imported from Eastern European countries, in which, according to cases identified in dogs imported into Western Europe, canine brucellosis could be endemic (). Most animals suffered from lameness and neck or back pain (14 animals, 78%); while discospondylitis was diagnosed in 11 dogs [Citation17].
In Sweden, B. canis was first detected in 2011 in a female dog with reproductive disturbances. The dog had been mated in Poland to a Serbian and to a Polish dog [Citation19]. A second known cluster was detected in dogs mated to a dog imported from Spain [Citation20]. A third confirmed cluster occurred in 2020: a male dog, imported from the Netherlands and serologically positive for B. canis had mated a female dog imported from Mexico. The female dog aborted, but was never tested for brucellosis and was put to sleep (Lahti E, personal communication).
In Germany, abovementioned non-systemic study found no positive dogs out of 386 tested by molecular tests (PCR) [Citation14]. Additional clinical investigations identified four young dogs (aged 7–30 months), imported from Moldova, Romania, and North Macedonia, with discospondylitis in Germany, of which B. canis could be isolated in three cases ().
Human B. canis caused brucellosis is infrequently reported in the scientific literature from endemic countries, but given the generally mild and non-specific nature of human infections and the lack of awareness and validated tests for B. canis in humans, cases are probably underreported globally [Citation3]. However, some cases can be severe, especially in more vulnerable individuals [Citation23]. Recently the first human cases of B. canis caused brucellosis have been reported in the 2021 in the Netherlands, and in 2022 in the UK, both related to people with significant exposure to canine reproductive excrements [Citation24].
Gaps analyses and recommendations
Several gaps have been identified in the surveillance and understanding the epidemiological status of B. canis.
Epidemiological situation, and risk factors for persistence and dissemination of canine brucellosis caused by B. canis in Europe are mostly unknown, including prevalence.
The questions faced by countries wishing to define disease status include:
choice of sampling and testing strategies;
definition of criteria, to determine the sanitary situation at country, establishment and individual scales;
choice of tools to notify and report the disease and some forethought about the approach to individual positive cases could also be valuable.
Is it cost-effective to control movements in order to limit the dissemination of the disease? Define a consensus protocol before introducing dogs into the EU, although current data indicate that in countries in the Eastern edge of the EU and Americas may already have endemic B. canis and more strict surveillance would be necessary.
How to assess the disease risk at EU level both for humans and animals? A lack of knowledge about the virulence for humans was identified as a key evidence gap in the UK [Citation3].
Recommendations
To fill the surveillance gaps, representative epidemiological surveys in Europe would be required to assess the prevalence of the disease within a defined confidence interval. To choose the best sampling strategy, the number of human and canine clinical cases as well as results of tested dogs, epidemiologically linked with confirmed cases, would have to be taken into account. Furthermore, epidemiological questionnaires would ensure data collection for the analysis of risk factors. With this knowledge, endemic countries would have the evidence needed to consider control programmes to prevent further dissemination of the disease. This could include mandatory notifications, movement restrictions, and control measures in positive kennels. Since transport of dogs to and between EU countries, but not within country, is mandatory to report, it would be possible to trace back the routes and include it in the risk management schemes. Centralized data collection could be used for intelligent tracing and, with enough cases, forecasting, to prevent disease dissemination and better develop management strategies. A multivariate risk factors analysis could give insights into whether restriction of movement can restrain the spread of the disease, in which conditions and what are the risks for public health.
Diagnostics
Clinical diagnostic
Well-known and described manifestations of B. canis infection in dogs are discospondylitis, reproductive disorders, back pain, unilateral, or bilateral lymph node enlargement, as well as prostatitis and epididymitis in males, and late abortion in pregnant females. Most live-born puppies die within a few hours or days; those that survive normally show a generalized increase in lymph node volume, which is the main symptom until they reach sexual maturity [Citation11]. In some dogs with chronic B. canis infection, the onset of recurrent anterior uveitis with corneal oedema has also been described [Citation25] as well as polyarthritis [Citation11].
Isolation and identification of B. canis
B. canis is a small aerobic Gram-negative facultative intracellular bacterium with a rough cell wall antigen [Citation10]. The unequivocal diagnosis of B. canis infection in dogs is only possible via the isolation and identification of the bacterium itself from material derived from the animal in question. The diagnostic sensitivity of this approach is dependent on a variety of factors such as the sample type, the stage of infection, the handling of the sample and the bacteriological methods employed. Classical bacteriological methods for Brucella culture are effective at detecting the target if viable cells are present in the material [Citation26]. Automated culturing, such as VITEK-2 can also identify cultures as Brucella spp. [Citation27], but results should be confirmed with additional techniques.
The confirmation of bacterial genus identity can be done by mainstream MALDI-ToF [Citation28] – noting that it is imperative that the correct database is in place; – by 16S rRNA sequencing [Citation29], or by Brucella spp. PCR [Citation30]. Laboratories that suspect the growth of a Brucella isolate should take appropriate precautions with respect to any further manipulation to protect human health and prevent accidental infections. The speciation of Brucella isolates can be done by classical bio-typing and/or by various molecular methods including PCRs such as SNP based PCR typing [Citation13] and multiplex “Bruce ladder” PCR [Citation31] or by HRM PCR [Citation32].
The optimal material for recovery of B. canis from an infected animal is fresh abortion or parturition material. This is due to the number of bacterial cells which may be up to 1010/ml [Citation33]. Vaginal swabs from females that have recently given birth or aborted may also yield positive results for up to 5 weeks [Citation17,Citation34], but sensitivity will decrease substantially over time as bacterial numbers decline [Citation34]. The culture of urine and semen samples from infected males may yield good sensitivity especially when clinical signs related to the reproductive organs are observed [Citation34].
In many cases, however, these samples are not available and bacteriology on blood samples is the only practical option. Blood samples should be collected into sodium citrate to avoid clotting and to prevent the inhibition of bacterial growth [Citation26]. Although not optimal, targeted Brucella culture from blood is more commonly used for dogs than for livestock. This may be most effective if the infection is relatively recent and because of B. canis recurrent bacteraemia. Maximizing the sensitivity of this approach involves liquid culture with periodic sub-culture onto solid media. Estimates of sensitivity for this approach and the duration of bacteraemia are mixed and much of the data are derived from experimental studies conducted between 1960s and 1980s [Citation3,Citation17] rather than from natural infections. These studies demonstrate that, under experimental circumstances, blood culture is sensitive and bacteraemia prolonged. More recently, the sensitivity of blood culture was assessed from an investigation of the first reported outbreak of B. canis in a kennel in Italy [Citation12]. In this example, from a total of 683 kennel dogs sampled, 68 (9.6%) were B. canis positive by culture from whole blood, whereas 241 (35.3%) were serologically positive (of which 64 were blood culture positive, 26.5%). Broth techniques with regular subculture onto solid media were applied. The diagnostic specificity of the serological method used (mSAT) was reported as 96.5%.
Quantifying the diagnostic sensitivity of bacterial culture on whole blood samples from natural infections is challenging, as this method is considered the “gold standard” against which the sensitivity of all other methods is calculated. This is because for now, only bacterial culture shows 100% specificity and is the only method to demonstrate the presence of live and potentially infectious bacteria. It is the best method for the diagnosis of early infection in dogs that have not received antibiotic treatment. Unfortunately, a negative result does not rule out the infection, since the bacteria temporarily may not be present in the cultured sample [Citation33]. This view is supported by data from studies where blood culture was undertaken in a population of dogs at high risk of infection, on the basis of epidemiology, clinical signs and serology (even if the precise infection status of each animal is not definitively known). A study of samples from 298 dogs, considered to be at high risk of infection, identified 74% to be serologically positive by RSAT (using a B. canis M-antigen – see below for further comment), but only 19% to be blood culture positive (although it was not specified if broth or solid culture was used, the former being more sensitive) [Citation35]. In the same study, B. canis was isolated in 16.4% of the 73 urine samples cultured (all from males).
Overall, the data suggest that bacterial culture from blood, semen [Citation33] or vaginal swabs [Citation34] is a useful but limited diagnostic tool, as it is not sufficiently reliable to determine the infection status of individual animals unless the results are positive. The application of this approach is valuable for confirmation on a “pack” or “kennel” level, where serology could be used to determine the infection status of an individual animal. Culture also has considerable value in enabling in depth molecular epidemiological approaches to be enacted.
Molecular detection of B. canis
A series of studies by Lara Keid’s group have demonstrated that the application of conventional PCR to whole blood samples can match and, in some cases, exceed the diagnostic efficacy of bacterial culture[Citation33,Citation34,Citation36]. However, in some circumstances PCR had a distinct edge by detecting both live and dead cells [Citation33]. This portfolio of work includes the investigation of primers targeted to IS711 and to ribosomal DNA using different extraction methods. Such methodological details are important, as are specific laboratory factors such as cleanliness of PCR workflows and these provide a wide opportunity for variation between methods that affects diagnostic performance. This whole process is integral to the PCR approach and validated diagnostic specificity and sensitivity values are specific only to the particular application and are not transferable to others.
In the investigation of the outbreak in the kennel in Italy [Citation12], a commercial PCR kit (Brucella genus, Genesig®, Advanced Kit) was used. Unfortunately, the target and primer sequences are not disclosed by the manufacturer, nor a commercial extraction method to obtain DNA samples from blood. In total, 32 of 683 samples were PCR positive (4.7%), approximately half the number that were culture positive although just over half the PCR positive samples were from culture negative samples from blood. To the authors’ best knowledge, this is the only published study on B. canis to have generated useful validation data on a real-time PCR approach from naturally infected dogs.
In another study [Citation35], diagnostic sensitivity (DSn) of PCR was as high as 69.8%, diagnostic specificity (DSp 95.6%), using primers from the 16S–23S interspacer region [Citation33]. However, this value was derived from just 122 of the 297 samples used to generate the DSn value for culture and it is not clear how this subsample was selected. Results from the same samples using PCR derived from the IS711 element had significantly inferior DSn, 22.8% (DSp 99.7%) a result that was incorrectly attributed to B. canis having a low copy number of the targeted region. B. canis has been reported to have six IS711 copies per genome [Citation30,Citation37], which is significantly greater than the single copies available for most other targets (although there will also be multiple copies of 16s targets per cell), and PCR (especially real time PCR) based on alternative primers derived from this target have been reported as having superior sensitivity [Citation30].
As with bacterial culture, the success of PCR depends on the homogeneity of bacterial distribution in the sampled tissue. The samples are often homogenized and one quantity used for bacterial culturing, while other part, often smaller in volume or mass, is used for PCR. Even if the primers, probes and PCR conditions themselves are rooted in solid science, all these factors impact DSn. Therefore, we have to rely on indirect detection of specific antibodies, to fill the diagnostic gap when it is important to minimize false negatives.
Indirect detection methods
Since B. canis isolation may be compromised by intermittent bacteraemia, serological methods have a central role in the diagnosis of canine brucellosis. Unfortunately, the sero-diagnostic landscape for B. canis is still relatively complicated. Sensitivities and specificities of serological tests vary greatly. As a rough Brucella spp., B. canis does not share the outer O-polysaccharide (OPS) – the primary sero-diagnostic antigen used for detection of infection with smooth species, associated with livestock. Three major questions arise on this topic: (1) there is no consensus on a specific strain of B. canis from which to derive diagnostic antigens (or even if this species strains are necessary); (2) which specific antigens are most important or which are the best platforms for them to be applied to; (3) no international scientific community consensus on serological testing strategies according to the stage of infection. There is no WOAH chapter to cover B. canis and no international standard anti-B. canis serum (although an EU standard is available to members) to promote laboratory harmonization and maintain minimum analytical standards. Canine brucellosis is not notifiable to the WOAH, but with increased international movements of dogs and this disease becoming more common [Citation3], guidance on best practice would be welcome.
Most reviews on B. canis contain a summary of sero-diagnosis. However, the plethora of antigens and platforms makes it difficult to pigeonhole any one approach and many reviews themselves rely on secondary data. A chief example of where misunderstandings arise is the attribution of the specificity shortcomings of antigens derived with M+ (mucoid) strains – and the tests using them – to antigens derived with M- (less mucoid) strains as the latter are much more specific [Citation11, p.198] albeit potentially a little less sensitive. Platforms may be the same, for example RSAT (Rapid Slide Agglutination Test) or TAT (Tube Agglutination Test)/SAT (Serum Agglutination Test) but with different antigens the diagnostic properties are very different. Given the nature of the infection, B. canis public health risks and the emotional distress caused to pet owners when results are positive it is imperative that these tests reach an acceptable level of diagnostic competency.
The variety of different antigen and platform types have been summarized in . Different platforms include: RSAT (with or without 2-mercaptoethanol (2ME)), TAT/SAT (with or without 2ME) and microtiter plate versions – mSAT, AGID (Agar Gel Immuno-Diffusion test), CFT (Complement Fixation Test), LFIA, IFA, and iELISA. These platforms detect different antibody isotypes with varying sensitivity as shown in the central column of . Different antigens (applied to one or more of the above platforms) include whole cell (RSAT and TAT/SAT) and cytoplasmic and cell wall antigens (AGID) from B. canis M+, B. canis M–, or B. ovis. For ELISA, the antigens evaluated include cytoplasmic and cell wall antigens but also recombinant proteins and rLPS extracts. Antigens used for immuno-comb, IFA as well as antigens used for most of the LFIAs on the market are not described.
Table 2. Summary of available indirect serologic tests and their properties.
The diagnostic performance of each platform is also summarized in . In some cases (e.g. TAT/SAT), it is not possible to provide an estimate of DSn or DSp as the published data does not enable a single point value for each. However, a qualitative assessment has been presented which concludes that the test is at best moderate for one parameter whilst being poor for the other (which depends on the application of 2ME) and has lower efficacy with samples from chronically infected animals [Citation26], most likely due to the inferior agglutinating properties of IgG compared to pentameric IgM. The available validation data on this platform is currently limited to the use of M+ [Citation26,Citation43] antigens although the test has been implemented with M– antigens (McGiven, personal communication).
There have been several studies conducted using the RSAT platform () with the DSp and DSn (in particular) variations between 70%–100% and 83%–100%, respectively. However, the impression emerges that the use of M– B. canis antigen is superior and that the addition of 2ME has a substantial negative effect on DSn. The AGID platform has been lauded for its superior DSp but the bottom range for the DSn is disturbingly low although there is considerable variation in antigen types applied. The CFT, as used for B. ovis, has also been repurposed for B. canis, however, validation data is very limited, and anti-complimentary reactions have been described as frequent.
More modern techniques such as Immuno-comb and IFA have been developed. However, validation or even descriptive data on these are very limited, and it is therefore hard to evaluate their performance and thus difficult to recommend. More recently, many ELISAs and also LFIAs (Lateral Flow Immunoassay) have been developed. Whereas the precisions about the diagnostic antigen used for LFIAs are missing for many commercial tests (with the exception of Chemtest), the ELISAs have been better described in this regard ().
A summary of the evaluation for each of the sero-diagnostic methods is presented in and . It should be emphasized that it is impossible to present a complete picture within a summary as, for example, the selection criteria used to select the samples for validation has a big impact on the outcome. For example, the DSn and DSp data for the Chemtest ELISA [Citation49] looks extremely strong but it should be noted that the data were generated using serum panels that were already serologically negative (DSp) or positive (DSn) in RSAT and AGID. Generating data that is totally unbiased is extremely difficult, so it is important that authors are clear what the qualifying criteria are for the validation sample sets. The Chemtest ELISA does deserve particular attention as it is one of the few published assays that are commercially available (at the time of writing).
Table 3. Detectability results by platform using the European Union Dog Brucellosis Standard Serum (EUDBSS).
The advent of Lateral Flow Immunoassays is a positive development as there is a clear and beneficial application for portable testing, for example in veterinary practice, breeding kennels, importers/exporters, and rescue charities. LFIAs may also have a place as a screening assay, whereby positive samples should be sent to a laboratory for further confirmatory testing. includes the four LFIA products for which validation data has been published.
In the absence of OPS (and therefore sLPS), it may be asking too much of B. canis sero-diagnostics to match the sensitivity and specificity of the RBT (Rose Bengal Test) and sLPS-based iELISAs used for the serodiagnosis of brucellosis in livestock [Citation52]. shows the detectability of the EU standard serum (EUDBSS) between the platforms. The application of the best current approaches for the detection of B. canis infection still leaves uncomfortable levels of uncertainty (especially in particular groups) with respect to false-positive and false-negative results. No method should be expected to be perfect. A strategy to detect acute or chronic B. canis infection is advantageous and therefore combining an agglutination assay (IgM) with an iELISA or LFIA (IgG) and/or mSAT has merit as long as the diagnostic parameters are acceptable. In this regard, the use of M+ antigens and 2ME should be avoided as the former generates an unnecessary level of false positives and the latter too many false negatives. AGID assays have high specificity but, as with use of 2ME, diagnostic sensitivity is too low for use on an individual animal basis.
The interpretation of the results should be accompanied by an understanding of the epidemiological context, for example – in combination with other risk factors (clinical symptoms, travel history, transmission opportunity). Even treatment is an extreme response (cost, duration, side effects). For example, the impact of an incorrect diagnosis and improper action can have far greater consequences for breeding dogs than for neutered animals. Dogs that are in regular contact with young children or immunocompromised persons may also present a greater risk to human health if not handled correctly.
Gaps analysis and recommendations
There are no fully effective confirmatory diagnostic tests for B. canis infection in dogs:
The diagnostic sensitivity of blood culture for B. canis in naturally infected dogs is not defined and may be too low for confirmation of infection in individual dogs.
There are no commercially available diagnostically validated PCR tests for B. canis (including PCR on blood samples) and sensitivity of blood PCR may be too low for confirmation of infection in individual dogs.
There are few commercially available, well validated and diagnostically accurate serological tests for B. canis infection in dogs:
There are many lateral flow tests commercially available, but few have passed validation for diagnostic sensitivity and specificity by competent authorities.
Validated ELISA tests have become more available, but choice remains very limited irrespective of antigens types.
It is not clear if there is sufficient strength in the market demand to promote commercial interest in developing this sector.
The comparative effectiveness of diagnostic methods for different post-infection time points, dogs of different ages, especially puppies, and breeds, is not well known and the differences may be significant.
There is a lack of materials available for the standardization and harmonization of diagnostic methods between laboratories:
There is poor availability of referent sera with the strong provenance from confirmed clinical cases, required to support good validation.
There are no globally defined reagents for B. canis anti-sera or standards, for diagnostic protocols or antigen source and production (in contrast to the case for B. abortus, B. melitensis, B. suis and B. ovis as described WOAH terrestrial manual).
There are no validated and diagnostically accurate serological tests for B. canis infection in humans, nor awareness that existing tools, used for smooth Brucella associated with livestock ones are not appropriate for this etiological agent.
Recommendations
International Reference Laboratories for brucellosis should generate guidance and reagents that support the development and adoption of best practice diagnostic methods.
The description of diagnostic methods and production of International Serum Standards (and how to use them) will provide a focal point around which best practice can be described and harmonized. This will enable understanding of the various methods, so that better test choices can be made, and results have greater inter-laboratory and international meaning. The development of a central serum panel accessible for test developers to aid in the validation of methods is a laudable aim but unlikely to be feasible in practice owing to limitations in volumes of available sera, challenges in establishing provenance and possibly also commercial value of such sera.
Efforts should be made to communicate the risks posed by B. canis to strengthen the marketplace and incentivize the development of superior tests and the strengthening of the commercial diagnostic sector. Canine brucellosis is a neglected branch of a neglected disease (brucellosis) and the incentive for commercial companies to develop and validate improved tests have been relatively low as the value to the market has also been relatively small. This might be changing as pet ownership has increased [Citation1] and the infection also appears to have spread further in dog population and into new geographical areas. The communication of this increased threat, particularly to those with strong interests in controlling it (owners, vets, breeders, etc.) may increase demand, stimulate more commercial interest which may lead to better availability of good commercially available tests. Part of this communication should be the message that validation data is an important aspect of test selection.
An emphasis should be placed on the effective and transparent validation of applied methods so that appropriate tests are used and the uncertainties around their results are known and can be accounted for. This would enable the choice of the most appropriate tests and testing strategies to use and to understand their diagnostic limitations. It would also help those seeking to develop surveillance programmes or movement controls to consider the value and limitations that such approaches may have in terms of generating reliable data.
Effective diagnostic tests for humans should be developed, or – at least – the efficacy of existing serodiagnostic tests used for dogs when applied to human sera should be better established. These tests could then be used to more confidently identify cases of human infection and support studies into the threat B. canis poses to humans. There is a lack of evidence about the pathogenicity of B. canis for humans. A clearer understanding of this would clarify the risks presented by the disease and enable a better assessment of the level of resource that should be assigned to control. Better diagnostic tests for humans would provide an important tool to enable this gap to be filled.
Transmission routes
B. canis can be transmitted by venereal routes, as well as via contact with body fluids through ingestion or via contact with broken skin or the mucosa [Citation53]. If cohabitating, a dog with canine brucellosis can transmit the disease to healthy dogs within a few weeks to months [Citation11]. Genital secretions from dogs infected with B. canis contain high bacterial loads, and dog-to-dog transmission primarily occurs during breeding or after contact with uterine discharges, semen, and aborted material. B. canis shedding in semen is highest during the first 8 weeks after infection, but intermittent shedding can last for years [Citation53]. Puppies can be infected by intrauterine vertical transmission or by drinking milk of infected females [Citation53]. Other body fluids including saliva, nasal, and ocular secretions and urine may also contain bacteria, although in lower loads, rendering contact with these fluids a possible transmission route as well.
Although the bacterial load in urine (up to 106 bacteria/ml [Citation17]) is lower than in genital discharges (up to 1010 bacteria [Citation11]), the risk of exposure to urine may be greater and therefore it does potentially play a major role in transmission. After becoming infected, the urinary bacterial load increases, and it is highest after 1 to 4–6 months after infection [Citation11]. In general, urine from male dogs contain higher B. canis loads than from females [Citation17]. Infected neutered animals cannot show reproductive signs, but a recent case study showed that both intact and neutered dogs shed B. canis in urine (31% and 33%, respectively) [Citation17], suggesting that neutered dogs can also transmit the disease via urine. This is due to the localization of the bacteria in the prostate of males. However, the presence of canine urine in various environmental conditions, whether bacteria survive in those conditions, and even more importantly, for how long urine stays infectious, remains to be elucidated. This is of utmost importance especially for the risk management and future recommendations on measures to be taken to stop the spread of the disease.
It has been suggested that B. canis in urine emerges from prostatic secretions of adult males [Citation11], but recent evidence revealed a pantropic distribution of the bacterium and its presence was confirmed in the kidneys as well [Citation54]. A number of cases have been described in which puppies were involved in the transmission of canine brucellosis [Citation23,Citation34]. Based on the pantropic distribution of the bacterium in neonates and the absence of abortion and mating in these puppies, some studies hypothesize that young puppies can spread the infection indirectly via urine, faeces, or skin [Citation11]. Hence, indirect transmission by both young and adult neutered animals can play a more prominent role than previously thought.
Transmission via indirect routes depends largely on the persistence of the bacterium in the environment, since it should be able to invade the host after remaining extracellular for prolonged time periods. Brucella species have been described to survive in water, food, soil, pastures, and manure for up to 8 months, although this is dependent on humidity, exposure to sunlight, the availability of organic matter and temperature [Citation55]. Survival is longer at low temperatures. B. microti has been isolated from soil samples 7 years after primary identification, indicating that soil might function as a constant source of infection [Citation29]. Despite some historic data, currently there are still many unknowns around how B. canis survives in various environmental conditions (humidity, soil temperatures, types of soil, etc.). Further it is unclear to what extent, if any, the absence of OPS from the outer membrane of B. canis impacts environmental survival (and susceptibility to disinfection) in comparison to smooth Brucella spp.
Due to the role that fomites may have in the transmission of B. canis, preventive measures can help in minimizing the risk of spread. For example, disinfection procedures, separate feeding/drinking bowls, the use of personal protective equipment, separate areas for animals around delivery, can minimize the transmission of B. canis infections. If an infection is suspected, the biosecurity measures between areas should be implemented (different clothing, boot protection, etc.). However, not all dog owners are aware or trained in application of these preventive measures. To raise awareness, improve the public health protection and prevent further spread of the disease, depending on the epidemiological situation, commercial breeders, charity kennels, and adoption centres workers, as well as private owners should be informed and thought about preventive measures.
Gaps analysis and recommendations
Further investigations into the epidemiological significance of non-reproductive contact routes of transmission between dogs are necessary.
The epidemiological significance of environmental contamination and potential infection has not been analysed. Levels of B. canis in urine, stability of bacteria in the environment, and the susceptibility of B. canis to disinfectants (does the mucoid surface provide protective effects).
The effect of gender and neutering on the infectiousness and susceptibility of dogs.
How can management of dogs prevent (minimize) risk of transmission? How should owners be incentivized to take action to benefit public health and larger dog population?
Do puppies and asymptomatic dogs transmit the disease?
Recommendations
Currently, as soon as dogs are diagnosed with B. canis, one recommendation is to neuter them, if they have not been already, to decrease excretion of the bacteria and to avoid further reproductive contacts. However appropriate mitigations must be in place to protect the surgical team, due to the exposure risk this activity may present. Additional longitudinal studies would be necessary to evaluate the risks of non-reproductive contact as well as the effects of neutering and gender, on further dissemination of the disease under varied scenarios with respect to housing and animal lifestyle. Since these animals would still pose the risks for further dissemination and public health, based on current data, the extent of these risks cannot be estimated. Therefore, initial laboratory trials on bacterial (both non- and mucoid strains) survival and/or infectivity in various conditions (environmental, cellular infection models, etc.) would be of utmost importance to determine the restrictions these dogs would be subjected to, which would greatly improve risk management decisions as well.
The immediate actions to mitigate further spread of disease and aid in raising public/professional awareness would be:
Awareness material preparation for owners/handlers/transporters as well as veterinarian clinicians, veterinary staff, and diagnostic laboratories.
Organize more profession specific panels for veterinarians and diagnostic laboratories on:
new information and current epidemiology;
new diagnostic strategies;
possible new treatment recommendations.
Risks for human health
Humans can acquire B. canis infection through direct contact with fluids or tissues of infected dogs, especially via genital secretions, aborted and parturition materials, urine, or blood of infected dogs [Citation56] (). Usual clinical symptoms correspond to ones caused by other zoonotic Brucella species may be chronic including fever (sometimes undulant), chills, headaches, sweats, joint pain, peripheral lymphadenopathies, and splenomegaly, while in severe cases it can cause the endocarditis and affect central nervous system [Citation56]. Damaged skin, mucous, or respiratory membranes are presumed points of infection. The infection should be suspected in patients with compatible symptoms, negative serology with B. abortus antigen [Citation57], and a history of contact with dogs. Contact with a joint fluid of infected dogs, obstetric surgery, neutering, and semen collection can pose a risk of professional exposure to B. canis [Citation16,Citation45]. Even M-strains used for antigen production, of low virulence for dogs, can cause clinical brucellosis in laboratory workers a few days after exposure [Citation58], the case indicating the dose-dependent manner of infection. It is evident that the lack of knowledge about brucellosis, in addition to direct contact with animal fluids, and failure to comply with the use of personal protective equipment and biosafety standards, increase the probability of infection [Citation59]. In 2012, one B. canis infection case of a child and dog in New York, exposed 31 laboratory workers to isolated culture [Citation23]. Five job-related groups are considered at high risk of B. canis-caused brucellosis: rural workers living in close contact with stray/farm dogs, kennel owners and workers, veterinarians and veterinary assistants, laboratory workers and hunters. Since canine companions have a special place in many households and cultures, their presence within the households exposes the most vulnerable categories (i.e. children, elderly or immunocompromised persons) to B. canis infection.
Figure 1. Currently known B. canis transmission routes for human infections. It has been proven that humans can be infected by B. canis through direct contact with bodily discharges of infected dogs, their bodily discharges or through accidental laboratory exposure. Other possible sources of infection could be contact with wild canids. In the same time, the survival of B. canis in the environment has not been tested (although it has been shown that other members of the Brucella genus can survive in the environment for up to eight months under favourable conditions), and this cannot be excluded as possible source for human infection. Proven sources of human infection are linked by full line arrows, while possible sources are presented by dotted line arrows.
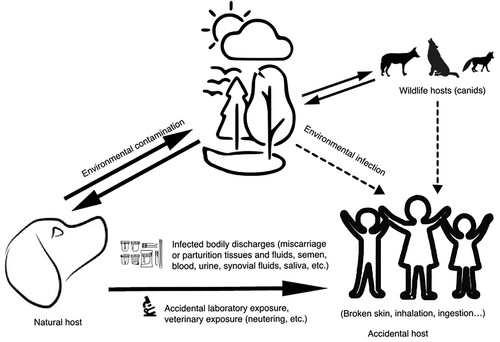
Human B. canis infections have been described in Europe, North and South America [Citation56,Citation60,Citation61], Hawaii [Citation62], Japan [Citation63], China [Citation64], and Malaysia [Citation65]. In Europe, only recently, human cases of B. canis-caused brucellosis were reported in the Netherlands – a 55-year-old kennel owner without non-specific clinical symptoms [Citation24]; in Germany – a 30-year-old female patient with recurrent fever and so-called granulomatous hepatitis and splenomegaly [Citation66]; and in the UK – a 61-year-old woman who contracted an infection with clinical symptoms from an imported dog, leading to hospitalization for more than 2 weeks [Citation67].
The first human cases of B. canis infection were described from Texas four decades apart, where 48-year-old man had an intermittent fever and bacteraemia over a 4-month period [Citation68]. The youngest ever recorded case was of a 17-month-old child, also from Texas [Citation69]. In humans, the symptoms of B. canis caused brucellosis vary depending on age and immune status. In children with inherited diseases such as sickle cell anaemia, B. canis can cause arthritis, lymphadenopathy or septic arthritis, or preconditioned with other types of inflammations causes mycotic aneurysms [Citation70]. In previously healthy children, B. canis may cause a plethora of symptoms ranging from fever and dyspnoea [Citation23], to cardiac inflammations aneurysms of extremities, calvarial osteomyelitis, epidural abscess, pleural effusions, and pulmonary nodules [Citation70]. In adults, symptoms can be so mild that the infection can go undiagnosed even for several decades [Citation9,Citation60]. Similarly, a presumptive case of B. canis causing endocarditis was described in Israel [Citation71]. In immunocompromised patients, B. canis infection can cause serious consequences [Citation56,Citation60]. Fortunately, after timely diagnosis and antibiotic therapy, in children, healthy or HIV-infected adults it seems that B. canis caused brucellosis does not influence general health conditions, nor has long-term impacts [Citation56].
Based on current data, without reliable diagnostic tools, it is estimated that B. canis has a low zoonotic risk [Citation2]. Given the few reported cases, rare development of unspecific clinical symptoms, very low mortality rate [Citation2,Citation3,Citation64,Citation66], and resolution of the disease with antibiotics, the absence of reliable diagnostic tools for B. canis in humans, leads to a curtailed estimation of overall prevalence. However, the virulence of B. canis in humans is untested. Systematic monitoring of canine cases and human brucellosis could clarify the number of exposures that result in infection, both clinical and subclinical, indicating an urgent need for the development of more reliable diagnostic tools.
The absence of cross-reactivity between antibodies to B. canis and other Brucella spp. makes it complicated to adapt new, specific indirect diagnostic tests. Moreover, neither sensitivity nor limits of detection for any of the current direct or indirect methods have been analysed for diagnostics of human B. canis infections. Since approved diagnostic tests for human B. canis serology are missing, several studies used tests developed for dogs. These results show that in humans, infection or exposure may be higher than previously thought. However, given the already limited standardization of sero-diagnostic tests for dogs, their application on human samples should be interpreted with caution [Citation10]. Very few public health laboratories are aware that specific tests are needed to correctly diagnose human B. canis infection, and therefore they need support from respective National Reference Laboratories (NRLs).
Therefore, a One Health approach is necessary to address the need to update information on canine and human seroprevalence worldwide, elucidate the infectivity of B. canis for humans, and determine the most appropriate treatment and prevention strategies. In recent years, the worldwide growth of large urban populations has created a new set of global health challenges, to which B. canis can be added. Changes in the urban environment due to the expansion of slum communities have resulted in increased dog populations, where uncontrolled breeding is common. There is currently no surveillance plan for canine brucellosis in humans at the EU level. Only individual cases in some countries are tested and reported. To fill the gaps in surveillance, epidemiological surveys would be needed to assess the prevalence of the disease. This would greatly improve reporting of cases, especially since recent studies, using Bayesian models estimate actual global rates of brucellosis to be closer to 2 million cases annually, rather than the 500,000 reported previously [Citation72].
Gaps analysis and recommendations
Poor diagnostic tools for human infection (and poor reporting), related also to the reporting and diagnostics of canine cases.
Lack of awareness among clinicians is important as patient history (contact with dog/litter) can be instrumental to get to the right diagnosis when symptoms are non-specific.
Level of virulence and pathogenicity of B. canis for humans are poorly defined.
Routes of infection and their relative risk not clearly defined.
Lack of surveillance or monitoring scheme for people, especially for professional exposure.
What are conditions associated with highest zoonotic risks?
Recommendations
The identification of specific B. canis or rough Brucella antigenic markers is urgently needed, to develop new indirect immunologic tests for humans and improve diagnostics. Many diagnostic laboratories nowadays use either MALDI-TOF or VITEK approaches to streamline, shorten, and assure quality control, of direct bacterial pathogen identification. To have the accurate identification to at least genus, if not species, level, enlarged libraries should be developed for MALDI-TOF, which would also decrease the number of errors of indirect diagnostics due to cross-reactivity with other bacteria (see section on Diagnostics). With reliable diagnostic tools, the prevalence of B. canis caused brucellosis in humans can be more precisely estimated, as well as the prevalence of infected but healthy individuals. Furthermore, validated diagnostic tools and awareness raising among clinicians would include B. canis in differential diagnosis, although nonspecific symptoms make it complicated, and therefore under reported without means to assess the risks. Long-term follow-up of confirmed canine clinical cases and investigation of epidemiological links would improve the evaluation of B. canis pathogenicity to humans and zoonotic risks: of horizontal transmission; of continuous bacteria shedding and their infectivity rate; as well as of handling the infected waste.
If not timely diagnosed or left untreated B. canis infection in humans may have long-lasting neural and cardiac symptoms, recurrent fevers, joint pain, fatigue, etc. Beside evident patient well-being, late B. canis diagnosis in humans may have economic consequences through decreased work capabilities, while follow-ups and long antibiotic treatment can put the additional costs to health systems.
Treatment and prophylaxis
No vaccines are commercially available for B. canis and antimicrobial treatment of infected animals, when applied, is seen as an alternative to removal of animals and usually combined with animal sterilization. Cross-protection with other anti-Brucella vaccines, used for cattle and small ruminants, has been reported, but not considered practical, since it presents a risk of vaccine strain shedding in a domestic environment with current live vaccines retaining a degree of virulence for humans [Citation73,Citation74]. The treatment of infected dogs is a debated issue as antimicrobial therapy does not guarantee B. canis elimination, with relapses of infection frequently reported in addition to the risks related to the development of antimicrobial resistance [Citation53,Citation75]. However, many owners consider their dogs as family members and have strong emotional bonds with them, which may lead to careful evaluation of sanitary measures (i.e. euthanasia versus antimicrobial treatment) being applied to B. canis infected animals and attempts to treat may represent the first choice for owners. This is even more exacerbated by the fact that there are few legal means to impose the management of infected dogs.
Data from past studies on in vitro efficacy of different antimicrobials against B. canis suggest that tetracyclines, aminoglycosides, and fluoroquinolones possess bactericidal activity against B. canis and combination therapy is more effective than treatment using one antimicrobial group [Citation75]. Unfortunately, this in vitro susceptibility does not always reflect the efficacy of treatment in vivo.
Data from the few in vivo studies on antimicrobial efficacy for B. canis infection have been recently reviewed [Citation53,Citation75,Citation76]. Therapeutic protocols based on a combination of tetracycline-based molecules, administered orally every day for 1 or 2 months, and aminoglycosides (dihydrostreptomycin, streptomycin, or gentamicin), administered parenterally for 7 days in the first 1–2 weeks of therapy and thereafter every 3–4 weeks were associated with high percentages of recovery with bacteriological and serological negativity of treated animals. However, a retrospective review from 30 dogs treated between 2017 and 2022 in the USA concluded that although poly-antimicrobial treatment is most effective, relapses and persistent infections frequently occur and 3 of these 30 dogs were therefore maintained on antibiotics indefinitely [Citation77].
Due to the potential toxicity of aminoglycosides, alternative protocols based on enrofloxacin monotherapy have been proposed [Citation78]. Animals treated with 5 mg/kg of enrofloxacin orally every 12 h for 30 days with additional treatment of female animals during oestrual and luteal phases resulted in regression of clinical signs, absence of bacterial detection in treated animals and offspring and reduction of antibody titres.
Data from these in vivo studies are difficult to compare as experimental settings (i.e. sex and age of animals, stage of infection and therapeutic protocols administered) differed as well as criteria applied to define recovery (i.e. bacteriological vs serological negativity). Nevertheless, these data indicate that in some circumstances antimicrobial treatment is effective in clearing the infection and reducing the spread of bacteria and the combination of antimicrobial treatment of infected animals with sterilization would reduce the risks of disease spreading, especially in females. Less encouraging data are reported for males as B. canis persists in the prostate and other lymphoid tissues [Citation11,Citation17,Citation34].
One of the major concerns related to antimicrobial treatment of B. canis infected animals is relapses of infection. The frequency of relapses is generally considered high, however, precise data are difficult to obtain because of several variables that may influence the efficacy of treatment, such as the stage of the infection (acute versus chronic), bacterial localization, individual susceptibility to B. canis infection and immune competence, sex, and age. Furthermore, information on efficacy of antimicrobial treatments in the long term are scant due to the difficulties of constant monitoring of animals over the years. Moreover, when the relapses are recorded, the severity of the clinical symptoms is equal to the acute infection.
Economic and management aspects of B. canis infected animals undergoing treatment represent additional issues. Dog owners have to be clearly informed on costs, duration (weeks) and possible inconvenience (relapses and repeated treatments) related to the therapy and the necessary follow-up of laboratory investigations to monitor the efficacy of therapy. Costs of treatment together with long-term management of infected animals need to be considered when the infection occurs in a breeding kennel with tens of individuals involved and outbreak control is based on a no-kill strategy [Citation12,Citation75].
In all instances when dog owners are unwilling to euthanize infected dogs or when a no-kill strategy is adopted, antimicrobial treatment, combined with neutering may represent the only alternatives to euthanasia but some gaps need to be filled. First, criteria to support the decision of treatment versus euthanasia are missing. Second, universally accepted treatment protocols for B. canis infected animals are not available and when antimicrobial therapy is considered, the choice of the antimicrobials and the duration of the treatment remain a decision of veterinary clinicians. The development of guidelines covering these aspects would facilitate decision making for the management of infected animals.
Gaps analysis and recommendations
There is no preventive treatment, and a vaccine against B. canis infection is not available:
Vaccines exist that provide some protection for ruminants against infection with smooth strains of Brucella and of these the vaccine B. melitensis Rev1 has been shown to provide some protection in sheep against infection from the rough species such as B. ovis. However, no vaccine has been developed for dogs or against B. canis. Some candidates have been tested in mice [Citation73,Citation79,Citation80] but no data have yet been published of testing done in dogs.
Were a vaccine to be developed, consideration should be given as to how vaccinated dog would respond in serodiagnostic tests and how any positive results would be interpreted.
Lack of clearly effective options for treating B. canis infection in dogs:
There are no published data that demonstrate a reliably effective curative treatment for dogs (including follow-up and long-term prognosis) and the potential side effects of treatment. Published data suggest frequent re-emergence of infection following cessation of treatment.
There is no clear guidance or consensus on the best (least ineffective) treatment option for dogs.
There is an absence of novel treatment options in the pipeline although a more rigorous assessment of existing options may yield more promising data.
The absence of effective treatment options, if combined with unwelcome control measures, may lead to testing avoidance in suspect cases, risking that disease become under reported.
Lack of knowledge with respect to potential development of antibiotic resistant strains:
Although the Brucellae are less susceptible to acquiring antibiotic resistance than other significant Gram-negative bacteria they can harbour and retain such properties as evidenced by the vaccine strains B. abortus RB51 and B. melitensis Rev1. The treatment of infected dogs may lead to large-scale prolonged antibiotic application, with frequent relapse, which may transmit that strain to other dogs, environment and humans. This is a situation favourable for driving resistance and one that has not previously occurred in the field of brucellosis.
Lack of knowledge about the efficacy of neutering dogs as a means of slowing disease progression or reducing non-reproductive transmission risk:
Neutering dogs evidently removes predilection sites of infection and prevents reproductive transmission. Based on current knowledge, it seems reasonable to consider that the removal of reproductive tissue will impede disease progression and reduce the excretion of B. canis (especially in males), but data to support this are thin.
It seems reasonable to consider that neutering will act synergically with antibiotic treatment to eliminate infection but there is currently no evidence to support it.
There may be some risk for veterinary surgeons that neuter infected dogs but this risk has not been quantitatively assessed, and mitigation steps have not been defined.
Is it an effective and justifiable option to give antibiotic treatment to infected dogs (even those that are outwardly healthy) with the objective of reducing the zoonotic risk to the veterinary surgeon and their team?
The costs and side effects of treatment are not clear, although this will vary between cases, locations, and therapeutic approach:
Treatment is likely to be prolonged and may require dual therapy. More accessible information around the probable cost parameters of treatment may help owners to make earlier and more informed decisions about their dogs.
The side effects of some treatments can be very severe and themselves require additional veterinary care and costs to try and resolve.
There is no clear guidance about the use of antibiotics to try and clear infection from asymptomatic dogs:
Is it justifiable to attempt treatment on a dog that is currently exhibiting signs of chronic B. canis infection (Discospondylitis, Uveitis, etc.), given the limited chance[open-strick]s[close-strick] of full recovery (if any)?
Recommendations
Attempts to develop vaccines should be encouraged particularly if there are an effective means by which vaccinated dogs can be differentiated from infected. A successful vaccine will reduce spread of disease and protect dog and human health.
Efforts to robustly determine the most effective treatment options and define their efficacy should be encouraged. An effective treatment that eliminates the infection in a high proportion of dogs would provide positive options following a diagnosis, improve animal welfare, and reduce zoonotic risk.
Measures should be taken to ensure that antibiotics are used only when necessary and responsibly. If treatment of dogs becomes a more frequent occurrence, then isolated B. canis strains should more regularly be monitored for evidence of antibiotic resistance emergence. This will help prevent the emergence of a greater risk for human and dog health.
Developing evidential knowledge to determine with high confidence if there is a positive effect of neutering on disease progression and non-reproductive transmission would require either experimental or a large quantity of highly detailed case studies.
Having a better understanding of the risks presented by neutered infected for other dogs and humans would enable more effective decisions to be made about the management of such animals and their impact upon sustaining disease.
Communications about B. canis infection should contain clearer information about the potential costs and side effects of antibiotic treatment, to make better and earlier decisions with respect to the management of their dogs. The decisions would also need to factor in the uncertainties that currently exist with the likelihood of treatment success.
Some veterinary consensus about treating asymptomatic dogs to reduce disease transmission and reduce the likelihood of disease emergence would be welcome. Given the existing uncertainties around the efficacy and side effects of treatment and issues relating to antimicrobial stewardship it is difficult to foresee consensus on this issue in the near future.
Control – regulations and legislation
The notification status and the criteria for notification of canine brucellosis vary between countries in Europe. In some countries, the detection of the bacterium in an animal by culture or by molecular methods is reportable, while in others a positive serological response is reportable. Whether the competent authorities perform trace-back investigations of suspected or confirmed cases vary as well. The authorities may recommend control measures to contain an outbreak and prevent further transmission but due to the lack of legal powers the veterinary authorities often encounter difficulties enforcing control measures.
Dogs have to conform to specific animal health requirements to travel and enable importation into the majority of countries of the world. The European Commission Delegated Regulation (EU) 2020/692 to describe the conditions companion animals (dogs, cat, and ferrets) have to meet to grant the importation (2020). Accordingly, the following conditions must be met when dogs are imported from authorized non-EU countries (Commission Implementing Regulation (EU) 2021/404). These animals must be from registered establishments under the control of the competent authority, with a system to maintain and to keep up-to-date animal health records, receiving regular animal health visits and not subject to any ban on animal health grounds (including rabies). Also, dogs must be marked by universal transponder chips. The same regulations are applied for the transport of dogs within the EU member states and described in the regulation 2020/688. Regarding the health status, dogs must be vaccinated against rabies, accompanied by a passport, have a health certificate issued by an official veterinarian and if entering Finland or Malta, be treated against Echinococcus multilocularis. Otherwise, currently there are no other measures in place to prevent the spread of B. canis by dogs in the EU. Only very recently, Iceland and now more and more EU countries have added B. canis free status as a requirement for import of dogs, while the UK recommends testing, but there are no legal powers to enforce this.
The awareness of the importance of applying biosecurity routines may be low or variable among dog breeders. Further, the awareness of the importance of biosecurity measures is likely lower among dog owners than among the dog breeders.
Gaps analysis and recommendations
The EU Animal Health Law does not specifically describe B. canis infections in dogs and there are no specific provisions that have to be followed with respect to: pre/post movement testing, surveillance, pre-breeding (natural or artificial), notification of disease, reporting of positive test results (for now, this is required only by UK law), definition of specific tests or actions following discovery of a case.
There are uncertainties for veterinarians, breeders, and laboratory staff with respect to compliance with Health and Safety at Work legislation as this extends into the acquisition of infection in the workplace. Workplace acquisition of B. canis infection may not only be detrimental to the health of individuals but may also lead to prosecution if reasonable precautions were not taken to avoid infection.
There are differences between countries in the way cases of B. canis infection in dogs are handled.
Some countries may have legislation in place that allows the authorities to intervene in cases where there is a public health threat due to a zoonotic disease.
Outside of the legislative parameters, countries have different approaches to disease identification and control.
Some countries also have different approaches to enforcing actions on dogs against the will of the owner, such as taking samples, following up cases, tracing suspect dogs, treatment, and – potentially – euthanasia. A policy which involves the control or euthanasia of a dog is likely to engage Article 1 Protocol 1 of the European Convention on Human Rights as it would interfere with a person’s rights of ownership over their dog.
The negative impact on people’s mental health should be weighed in decisions made about dog management.
There is a lack of awareness among veterinarians (clinicians and inspectors alike), and dog owners about B. canis, as well as amongst policy makers, to control this zoonosis.
Although levels of knowledge about this disease are increasing this is from a very low base (at least within Europe). Better communication about the disease, the risks it presents (to dogs and humans), and the tools available for control would help to prevent the spread of infections.
Recommendations
Policy makers should consider the need to cover infection with B. canis within legislation to try and stem the spread of this infection and protect animal and human health.
Effective legislation would lead to the reduced spread of the infection and its potential elimination, taking into consideration all animal and human welfare concerns (including mental health). However, it is not evident what controls would be both proportionate and effective.
Policy makers will require clear information about the extent of the existing evidence for this disease. This will include the impact it has on human and animal health and the efficacy of the tools and methods that can be used to control and treat it.
This document aims to provide an effective template against which the available knowledge about B. canis infection can be assessed.
Policy makers will require clear information about the key knowledge and capability gaps to enable them to judge what actions can or should be taken now with respect to any new legislation or whether there are key gaps that need to be addressed before policy decisions can be made.
Feedback from policy makers on the priorities of the knowledge and capability gaps will enable the One Health community to target these, generate the evidence and tools and support policy making.
Two hypothetical cases of B. canis infection and subsequent management, based on present knowledge
Two main scenarios are presented below to cover the two most common cases in Europe currently, suspected/confirmed infected individual pet dog in a household setting, and a kennel situation. The scenarios and subsequent management responses are based on the current knowledge, also highlighting current gaps that urgently have to be resolved. These scenarios may assist legislators/policy makers to consider what actions may be appropriate, proportionate, and effective in controlling disease. The scenarios have been created based on the authors’ experiences from various European countries. However, local authorities should consider further investigation and management steps taking into account the ongoing prevalence of disease, size and density of (local) dog population, level of presented risk, the risk appetite of the owners, veterinarians and authorities involved, as well as legal perimeters at their disposal.
Currently, the presence of B. canis has no impact on the countries’ official brucellosis status according to WOAH as this relates only to B. abortus, B. melitensis, and B. suis, in livestock (B. canis has not been identified as a threat to livestock or brucellosis surveillance systems).
Definitions
A dog with a confirmed B. canis infection – Currently, the isolation of B. canis strain is the only unequivocal proof of infection (with or without clinical symptoms). Dogs with compatible clinical symptoms and at least positive serology or PCR may be considered as infected, beyond reasonable doubt, especially if epidemiological links with infected dogs are highlighted (). Various other situations are observed, in which the probability of infection depends on results and epidemiological situation. An example of guidelines as to how risk factors relate to the probability of infection is described in .
Table 4. Guidelines on determining the dogs’ status regarding the B. canis infection.
A dog with a suspected or probable B. canis infection – B. canis has not been confirmed but some (although not all) risk factors are present (as described in ). The additional uncertainty about the true infection status creates additional management issues.
B. canis positive kennel - at least one confirmed infected dog (strain isolation or PCR positive) or at least two reproductively active animals with confirmed serological positive response.
Scenario 1: individual dog as a house pet
If there is a suspicion of infection with or exposure to B. canis, a dog in the household should be tested for potential infection. Within the same concept, in case of B. canis potential exposure or clinical symptoms, individual dogs should be tested as described in Diagnostics section, taking into account the epidemiological situation regarding the choice of diagnostic protocol.
Diagnostics
Although canine brucellosis is associated with prominent clinical signs, dogs may show no symptoms at all. Bacteriology remains the gold standard to confirm the infection in these animals (see Diagnostics section). However, since sensitivity of bacterial culture from blood is low, and may not be possible, validated serological and Brucella DNA detection methods can be used to complement the diagnosis (). Because bacteraemia is intermittent (currently, the frequency and the extent are not exactly known), and isolation of bacteria or detection of their DNA may have low diagnostic sensitivity. Therefore, serological methods provide the most diagnostically sensitive means to test the status of dogs. However, if serologically positive, multiple criteria have to be taken into account to determine whether the dog can be considered as infected or false positive (). In the case of recent infection, the antibody response may not yet have developed. Therefore, in the event of a negative results a further test, a minimum 6 weeks apart, is needed to properly assess the serological status of the animal (). The possibility of a false-positive result has to be considered as well, although this result has to be interpreted in light of other risk factors. The more risk factors are present, the more likely it is that a serologically positive result is true (the likelihood that a positive result is true is called the Positive Predictive Value and it will vary according diagnostic sensitivity and specificity and the prevalence of the disease in the population being tested, when more risk factors are present the subject dog becomes a member of a population with a higher disease prevalence). Moreover, if the antibody value is high and remains consistent for at least 6 weeks, it can provide further evidence that the dog should be considered as B. canis infected. However, the possibility of coinfections cannot be excluded, although currently there are no real evidences suggesting that some other bacteria may cause false-positive B. canis diagnosis. Hence, repeated sampling may help to exclude a false-positive and -negative reactions, and to follow antibody titre kinetics. If two different tests are used, it may increase diagnostic specificity, but the consequence will be lowered sensitivity, leading to false-negative results (). Moreover, if a quantitative test is used, it would help even further to interpret serological profiles.
Principal issues related to definitive status definition: (1) Does a serologically positive result corresponds to an infected dog (true positive) or uninfected (false-positive serology – cross reaction; low specificity). Does a positive serological test (not being FPSR) signify exposure, infection, infectiousness? (2) Does a negative result correspond to a dog free of B. canis infection? False-negative results may also occur (incubation period, low titters, low sensitivity of existing tests, etc.) and complicate the interpretation of a single negative result.
A positive serological reaction cannot determine when a dog was in contact with B. canis, nor if the infection is still ongoing and to what extent the animal is infectious to others and humans. However, it shows that there is a risk of B. canis presence in the local population, and therefore a potential public health threat. Moreover, local authorities should consider further investigation and management steps taking into account the ongoing number of confirmed B. canis canine cases, estimated prevalence, size and density of (local) dog population, specific epidemiological factors in each country, region or household, as well as legal perimeters at their disposal. Current state of the art forbids us to further elaborate on aforementioned problematics, so until further advances, each serologically positive dog should be considered as a threat for further dissemination, until proven that either the reaction was false positive (these conclusions could be supported by two consecutive negative results). In the case of high antibody titres (both IgG and IgM) – presume acute infection, sampling for direct diagnosis could be recommended, since both diseased and asymptomatic dogs (especially males) are reported to excrete infectious bacteria. Due to the low sensitivity of direct methods, repeated sampling could be recommended to increase the sensitivity of overall approach (although this will be financially costly approach). Nevertheless, there are not enough data to estimate the optimal number of samples needed to have a high probability of isolation, nor how much this would improve the diagnostic sensitivity.
Management of suspected dogs
In case of non-conclusive results, and dogs that remain suspected of probably infected but not confirmed even more difficult judgment about risks will have to be made. The dog can be considered as B. canis positive to avoid the risk of disseminations and to preserve public health, but this decision has to be made in context of present risk factors. Accommodation in endemic countries or contact with infected animals can be considered as risk factors that argue to consider the dog as potential infected.
Based on current knowledge of B. canis transmission routes, pathogenicity and lack of data to estimate infection rates in Europe, the management strategies should be curtailed to set of factors associated with each case or group. Such factors include ongoing number of confirmed B. canis canine and human cases, estimated prevalence, size and density of (local) dog population, specific epidemiological factors in each country, region or household, as well as legal perimeters at disposal. Dogs in non-reproductive contact with an infected animal, i.e. those that share same social spaces, should be considered at risk of infection (). Based on the nature of dogs’ social behaviour, provides potential transmission routes for infection with B. canis, dogs in contact, from other private owners, could have a serological follow-up if epidemiological situation requires; at least two negative tests 4–6 weeks apart. If the disease is endemic in the compartment, dog owners and veterinary clinicians should be informed of the potential risks, management rules, and regulations. The isolation of suspected animals should be recommended, at least in endemic countries, while relevant authorities should assess these risks in areas where the disease is sporadic ().
Figure 2. Overview of the diagnostic management scheme evaluated in this study for B. canis infection in individual dog and/or kennel. In the case of a confirmed B. canis infection in individual dog or kennel, the following workflow aims to provide proper bio-risk and public health management. After all biosecurity measures have been put in place to avoid the spread of infection, a detailed epidemiological investigation must be carried out to identify exposed persons, animals or facilities with which need to be surveyed.
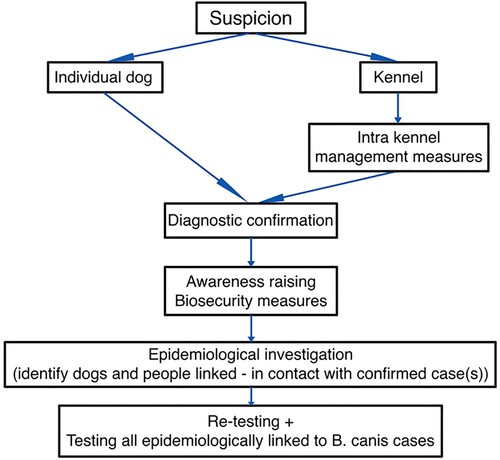
Management of confirmed infected dogs
If a dog is B. canis infected, an epidemiological investigation should be conducted, by competent authorities, to avoid further dissemination and public health risks (see Control – Regulations and Legislation section) (). The epidemiological investigation will identify other dog(s) in close contact with the infected animal, which should be tested. Based on the epidemiological context, decision makers should determine the scale/perimeter of additional testing and measures. For these animals, at least serological tests are required to determine the presence/absence of specific antibodies against B. canis. In the case of a positive test result, the animal should be considered as potentially infected, otherwise a new test will be advised after 4–6 weeks.
Pet dogs may have multiple daily close contacts with animals from other households. These should be considered as contact dogs. The awareness of the need for biosecurity routines is presumably low among private owners (even when symptoms are present) (see Transmission Routes and Risks for Human Health sections). In addition, if semi-environmental exposure via urine (sniffing, licking, aerosols) is important, management becomes more complicated as the contaminated environment may become a direct source of B. canis infection (for more details, see Transmission Routes section).
As discussed in Transmission Routes section, even after neutering, dogs can still excrete infectious bacteria via urine, especially if clinical symptoms are present. Likewise, as described in Treatment and Prophylaxis section, described antimicrobial treatments are aggressive, correct doses still have to be adjusted and side effects can be expected. For now, brucellosis in dogs is considered as a lifelong infection [Citation14]. Therefore, even after treatment and initial recovery, bacteria can remain latent in some tissues, which may cause relapses after changes in the immune status of dogs, including possible re-excretion. Re-excretion of B. canis bares the risks of antimicrobial resistance development, but the data on this topic are scarce (see Treatment and Prophylaxis section). Furthermore, currently there are no vaccines available for B. canis in dogs. Since the risk of relapses of B. canis shedding exist, the care for the infected dog (isolation, travel ban, etc.) as well as waste management system should be defined and maintained during the life of that dog. Therefore, in the absence of effective treatment, the animal’s wellbeing should be considered, especially in case of clinical relapses. Consequently, removal can be proposed. However, regulations in many countries oppose euthanasia as a solution for animals whose lives are not in danger or suffering by this disease is not proven.
In the same time, public health officials should be informed about B. canis incidents so that the appropriate protection and monitoring measures can be proposed in order to protect public health.
Puppies and prepubescent dogs
Puppies and prepubescent dogs (0- to 2-year-old) born of, or in close contact with infected animals should be treated as suspected animals irrespective of serological analysis as this may be inaccurate as their immune response against B. canis is not completely mature. The absence of specific antibodies against B. canis has been reported in infected puppies and young dogs born to infected mothers. However, no serological test can distinguish between maternal and newly acquired anti-B. canis antibodies (neither IgG nor IgM). Finally, no commercial diagnostic test has been validated for puppies. Strain isolation or positive PCR is the only definite proof of ongoing infection in puppies, but the sensitivity of bacteriology and molecular methods still has not been analysed for this age category although the authors have recently been able to isolate B. canis stillbirths or foetuses and also from 6 out of 120 prepubescent animals [Citation76].
Scenario 2: B. canis infected kennels
For B. canis, two major risks in kennels are introduction of new animals and dissemination in the establishment and/or beyond. The number of dogs directly influences the size and space necessary for proper management of the infection risk. All facilities must have the possibility of individual isolation, without contact with the animals already present at the premises – quarantine. If individual isolation is not possible, dogs can be separated in small groups that will be treated as epidemiological units. Considering the known kinetics of B. canis infection in dogs the advised period of quarantine should be at least 6 weeks. Therefore, the complete isolation of dogs during this period should be planned, taking into account the separated social space, walks, toys, feeding and toilet areas, as well as dedicated outdoor space for quarantined dogs.
Diagnostics
Usually, the first reported sign of infection in a breeding kennel is likely to be an abortion(s), in which case the aborted material should be cultured for B. canis, as described in Transmission Routes section. However, in the case of adoption centres for example, or dog hotels, where pregnant bitches are less frequent, isolation is more difficult. In any case, if there is a suspicion of contamination or exposure to B. canis inside a kennel, all dogs should be tested to determine the status of each animal, define epidemiological unit(s) and regroup infected and probably infected animal(s) (). However, negative results should be treated with caution and multiple tests, at least 6 weeks apart should be performed in each epidemiological unit. Depending on the epidemiological situation, competent authorities may require two or three consecutive serologically negative results to consider a dog as B. canis free (). If at least two reproductively active dogs are confirmed as serologically positive (at least two positive samples), the kennel should be considered as infected even without the isolation of the strain. This percentage should be adapted as new epidemiological data in a specific country or region become more readily available.
As described in Transmission Routes section, depending on the breed/origin, the immune system in puppies and prepubescent dogs (0–2 years old) is not yet fully developed and reaction to B. canis infection not completely described. For this reason, it is recommended to verify the serological status of both parents (two negative serological results, 6 weeks apart), 3–6 months before the introduction, until specific tests are available for the offspring. In the event of birth during the quarantine period, it is assumed that the puppy maintains the same serological status as the mother. In the case of puppies and young dogs of unknown origin (stray animals), epidemiological risk of infection should be evaluated first based epidemiological situation in the region/country and then on the group of dogs they spend the most time with.
Management of risks:
Examples of rules before introduction
Two or three negative serological tests at 6 weeks interval (1 before introduction/importation (if possible, to organize a “controlled” test) and/or 1 after arrival, 1 before the end of quarantine) ().
Contacts with other dogs during transport is possible: A 6-week quarantine is recommended, no interaction with dogs of the kennel/no access to collective or social space/urine collected and disposed of/thorough cleaning and disinfection of the quarantine premises/good hygiene when handling quarantined animals and other animals in the kennel are recommended.
As a rule, quarantine should be concluded only when all dogs present 2 successive negative tests at least 6 weeks apart, or infection is proven.
For young dogs/puppies, it is recommended to test both parents within 3–6 months before introduction.
Table 5. Recommendations on B. canis risks mitigations in kennels.
To avoid B. canis introduction into the kennel, each new dog should be quarantined for 6 weeks before mixing with the other dogs (). A dog can be considered as non-infected only after two consecutive negative serological tests at least 6 weeks apart, during the quarantine period (). For now, it is recommended to test the serological status upon arrival of the animal and before the end of the quarantine period. If possible, tests performed before the movement of dog(s) (previous kennel) can be used only to follow the serological status but not to prove the negativity, since the infection can occur during the transport (). Experimental infection models proved that the serological status could change during the 6 weeks post infection. During the quarantine period, the individual dog or group of dogs should not have interaction with other animals present in the kennel, nor have contact with the same playgrounds, feeding and water bowls or equipment. The kennel should be encouraged to apply stringent biosecurity routines: personal protection equipment for the persons working in the quarantine and other parts of the kennel, cleaning, and disinfection of the premises, no external visitors allowed to enter the quarantine (). If the quarantined animals need to be taken to a veterinarian, preventive measures should be taken to avoid possible dissemination(s). Access to the collective social space should be eliminated, and unique space dedicated to quarantined dogs or an approved decontamination process is followed before and after. As a rule, quarantine should be finished only when all dogs present two successive negative serological results. If one or more animals become serologically positive for B. canis, during the quarantine, the 6-week period should be restarted for other dogs (if applicable), after the isolation of individual positive case(s) ().
Rules before sale/adoption
To sell or adopt a dog, a negative serological test seven days in advance can be required to prove the health status (). When a kennel is declared suspected, the sale or adoption of dogs should be stopped/discouraged. Similarly, movements of dogs should be prohibited from facilities with infected/suspected animals, even for seronegative dogs, until the disease has been eradicated or suspicion lifted, and the kennel is declared as B. canis free. However, kennels that test regularly all animals and provide a proof of protective measures employed, could be labelled as B. canis free. In cases of kennels labelled free and the use of appropriately authorized transporters (transport only individual dogs, respect the decontamination measures or strict separation during transport), quarantine in the new kennel would not be necessary. In the same way, for B. canis free kennels, adoption could take place without negative serological proof.
As described above (see Treatment and Prophylaxis section), the adoption of a seropositive dog is strongly discouraged in the absence of effective treatment against the disease. However, in case removal is not possible and the adoption route is chosen, it should be strictly supervised to minimize the risk of human and other animal(s) infection(s). The animal should be neutered (with the risks of this procedure appropriately mitigated) and treated, its clinical conditions monitored regularly for the rest of its life and both the organization and the future owner must be informed of the risks and disease management plans.
For all identified dogs in close contact with the infected kennel, serological follow-up should be organized as previously described. If only sporadic cases of B. canis have been detected in the country/region, the quarantine and separation of dogs into groups may not need to be enforced, between two tests, but movement and contact with other dogs outside the kennel should be at least strictly followed, since the risk of infection is generally considered to be lower. In endemic areas, to prevent further dissemination of bacteria, dogs in kennels that had contact with the infected one(s), should also be quarantined, and grouped as described until two successive negative serological tests for each group.
Management of infected or probably infected dogs
The infected or probably infected dogs should be separated from non-infected animals (). Different scenarios are possible: 1 – creation of an independent “epidemiological unit” within the facility (with biosecurity protocols); 2 – isolation outside of the kennel (including adoption with informed consent) ().
Furthermore, both adoption organization(s) and future owners should be informed of a risk, management rules including follow-up testing. They would have to be informed that from current scientific data, the probability to detect a dog as infected or infectious is low, how the serological status of infected animals changes is also unknown, but also whether they harbour the infectious bacteria or not, nor the risks of further bacterial dissemination.
When a kennel is confirmed as B. canis positive, an epidemiological investigation should be conducted to find the source of infection, avoid further dissemination and public health risks. All dogs present in the kennel should be tested individually and positive animal(s) should be separated. For a kennel, with a large number of animals, separation into small sub-groups could be an option. Each group should be followed, until all animals present two negative serological tests, at least 6 weeks apart. All kennels proven to be in epidemiological contact with the infected one, should automatically become suspect and all dogs tested.
Kennel owners should clearly be informed about necessary sterilization, lifelong follow-up minimum once a year, no contact with uninfected dogs as well as specific waste management until further information about this disease in dogs is obtained. At the same time, it would include long and expensive and aggressive antimicrobial therapy.
Follow-up of dogs sold in the at least 6 weeks prior to the first identified case, should be undertaken as well. After the first serological results, it is recommended to split dogs into small groups (depending on the size of the kennels), according to different sanitary status (infected, seropositive, seronegative, unknown, etc.). Furthermore, necessary instructions for owners, veterinarians and visitors should be distributed and clearly communicated. Hygiene procedures for working with infected, non-infected and suspected groups in one facility, including the treatment of common areas, playgrounds, individual cages, food, and waste management should be implemented, and each group/unit should be treated independently (care for negatives first, then positives then change clothes, etc.). Brucella species are sensitive to known disinfectants and regular re-disinfections. The cleaning of organic material is pivotal prior to disinfection. The removal of bacteria from outdoor spaces may be a challenge due to environmental toxicity. Therefore, an effective solution would be for each epidemiological unit to be allocated dedicated outdoor space.
Kennels should keep records on the movement of animals, testing and treatment protocols and results of the testing as well as on the signs of illness, as for rabies. These records would be part of specifications to be considered when labelling the kennel as B. canis free. This certification process has not yet been implemented for canine brucellosis. However, it may be a way to incentivize the kennel owners towards more regular testing and controlling dissemination of this disease.
When a kennel is confirmed as B. canis positive, an epidemiological investigation should be conducted to find the source of infection and to avoid further dissemination and public health risks. All dogs present in the kennel should be tested individually and positive animal(s) should be separated. For a kennel, with a large number of animals, separation of dogs into small sub-groups could be an option. Each group should be followed individually, until all animals present two negative serological tests, at least 6 weeks apart. An epidemiological investigation should be recommended to identify all animals and kennels in contact with the infected facility during the past 6 weeks. All kennels proven to be in epidemiological contact with the infected one, should automatically become suspect until proof of their negativity is provided.
Follow-up of dogs sold in the at least 6 weeks prior to the first identified case, should be undertaken as well. After the first serological results, it is recommended to split dogs into small groups (depending on the size of the kennels), according to different sanitary status (infected, seropositive, seronegative, unknown, etc.). Furthermore, necessary instructions for owners, veterinarians and visitors should be distributed and clearly communicated. Hygiene procedures for working with infected, non-infected and suspected groups in one facility, including the treatment of common areas, playgrounds, individual cages, food, and waste management should be implemented, and each group/unit should be treated independently (care for negatives first, then positives then change clothes, etc.). Brucella species are sensitive to known disinfectants and regular re-disinfections. The cleaning of organic material is pivotal prior to disinfection. The removal of bacteria from outdoor spaces may be a challenge due to environmental toxicity. Therefore, an effective solution would be for each epidemiological unit to be allocated dedicated outdoor space.
Conclusion
Although known throughout the World since the 1980s, B. canis started spreading in Europe only recently with the rise of canine imports from eastern Europe. B. canis causes brucellosis in dogs presented primarily as a reproductive disease, expressed through the disease of reproduction, myo-skeletal and neural tissues. In humans, debilitating disease can become chronic without specific symptoms, characteristic to the infections with other highly zoonotic B. abortus and B. melitensis. However, many of the pathogenic mechanisms B. canis deploys are unknown. Moreover, currently sensitive and specific tools to identify B. canis infection do not exist, and there is no consensus on diagnostic strategy nor availability of reference standards – antigens and antibodies. Therefore, the only unequivocal evidence of infection is the strain isolation, while the negative results cannot exclude the disease. At the same time, there is no vaccine for dogs and current treatment options, combining several antibiotics with neutering, are very long, sometimes even dangerous for animal, often with lifelong relapses. Although B. canis has been diagnosed in almost all European countries, no systemic epidemiological investigation has been published and there are no means to estimate current spread of the infection throughout the continent nor to evaluate the risks for public health. Furthermore, in Europe, there is no legal framework to impose neither diagnostic, nor management options for suspected and infected dogs, exacerbating the risks for further spread of the disease in dogs and for public health. This review shows that new epidemiological and experimental studies are urgently needed to elucidate some of the most important questions such as diagnostics of the B. canis infection, length of infection, bacterial survival in the environment as well as different treatment options and susceptibility of bacteria to development of antibiotic resistance. Furthermore, there is no diagnostic tool validated for puppies, or the test that can determine whether the infection is recent or chronic. Moreover, current control and management options are very generalized, and there are no established protocols to protect public health.
Acknowledgments
Authors would like to thank colleagues: Andrew Frost, Roland Ashford, Sascha Al Dahouk, Kitty Masssen, Rob Dewar, Alessandro Gerada, Manuela Tittarelli, Nicholas Beeching, Ana Pelerito, Dre Kampfraath, Regina Cardoso, Sandra Cavaco, Liliia Kharchevska Weis, Dirk Hofreuter, Henny Osmosigho, and Jeroen Koomen, who all helped identify Brucella canis as emerging threat in European canine population, and structure this manuscript. We would like to thank them for their time and ideas exchanged during regular meetings on this topic.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Day MJ. Cats are not small dogs: is there an immunological explanation for why cats are less affected by arthropod-borne disease than dogs? Parasit Vectors. 2016;9:507. doi:10.1186/s13071-016-1798-5
- HCSP. Avis relatif à la conduite à tenir vis-à-vis de personnes exposées à des animaux contaminés par Brucella canis. publique HC de la santé, editor. 2022; Available from: https://www.hcsp.fr/Explore.cgi/Telecharger?NomFichier=hcspa20220318_cotevividepeexdeanatdebrca.pdf.
- HAIRS members and external experts. Risk review and statement on the risk Brucella canis presents to the UK human population [Internet]. HAIRS; 2021. Available from: https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/960013/20210210_Brucella_canis_statement.pdf.
- Celli J. The intracellular life cycle of Brucella spp. Microbiol Spectr. 2019;7. Available from: https://www.ncbi.nlm.nih.gov/pubmed/30848234.
- Stranahan LW, Arenas-Gamboa AM. When the going gets rough: The significance of Brucella lipopolysaccharide phenotype in host-pathogen interactions. Front Microbiol. 2021;12:713157. doi:10.3389/fmicb.2021.713157
- Fernández AG, Hielpos MS, Ferrero MC, et al. Proinflammatory response of canine trophoblasts to Brucella canis infection. PLOS ONE. 2017;12:e0186561.
- Ahmed W, Zheng K, Liu ZF. Establishment of chronic infection: Brucella’s stealth strategy. Front Cell Infect Microbiol. 2016;6:30. doi:10.3389/fcimb.2016.00030
- Bin Park W, Kim S, Kyung SM, et al. Gene expression of toll-like receptors, cytokines and a nuclear factor and cytokine secretion in DH82 canine macrophage cells infected with Brucella canis. Vet Immunol Immunopathol. 2023;260:110607. doi:10.1016/j.vetimm.2023.110607
- Lucero NE, Corazza R, Almuzara MN, et al. Human Brucella canis outbreak linked to infection in dogs. Epidemiol Infect. 2010;138:280–285. doi:10.1017/S0950268809990525
- Hensel ME, Negron M, Arenas-Gamboa AM. Brucellosis in dogs and public health risk. Emerg Infect Dis. 2018;24:1401–1406. doi:10.3201/eid2408.171171
- Carmichael LE, Joubert JC. Transmission of Brucella canis by contact exposure. Cornell Vet. 1988;78:63–73.
- De Massis F, Sacchini F, Averaimo D, et al. First isolation of Brucella canis from a breeding kennel in Italy. Vet Ital. 2021;57:3.
- Ferreira Vicente A, Girault G, Corde Y, et al. New insights into phylogeography of worldwide Brucella canis isolates by comparative genomics-based approaches: focus on Brazil. BMC Genomics. 2018;19:636. doi:10.1186/s12864-018-5001-6
- Buhmann G, Paul F, Herbst W, et al. Canine Brucellosis: insights into the epidemiologic situation in Europe. Front Vet Sci. 2019;6:151. doi:10.3389/fvets.2019.00151
- Whatmore P, Friggens. Second UK isolation of Brucella canis. Vet Rec. 2017;180:617.
- Egloff S, Schneeberger M, Gobeli S, et al. Brucella canis infection in a young dog with epididymitis and orchitis. Schweiz Arch Tierheilkd. 2018;160:743–748. doi:10.17236/sat00190
- van Dijk MAM, Engelsma MY, Visser VXN, et al. Transboundary spread of Brucella canis through import of infected dogs, The Netherlands, November 2016–December 2018. Emerg Infect Dis. 2021;27:1783–1788. doi:10.3201/eid2707.201238
- Ahmet Murat S, Altuntaş MB, ÖZ Gülserenyildiz, et al. The first-time isolation of Brucella canis from two aborted bitches in a kennel in Turkey. Turk J Vet Anim Sci. 2018;42:3.
- Holst BS, Lofqvist K, Ernholm L, et al. The first case of Brucella canis in Sweden: background, case report and recommendations from a northern European perspective. Acta Vet Scand. 2012;54:18. doi:10.1186/1751-0147-54-18
- Kaden R, Agren J, Ferrari S, et al. Whole-genome sequence of Brucella canis strain SVA13, isolated from an infected dog. Genome Announc. 2014: 2. Available from: https://www.ncbi.nlm.nih.gov/pubmed/25035330.
- Morgan, Rys, Wake, et al. Brucella canis in a dog in the UK. Vet Rec. 2017;180:384–385. doi:10.1136/vr.j2143
- Corrente M, Franchini D, Decaro N, et al. Detection of Brucella canis in a dog in Italy. New Microbiol 2010;33:337–341.
- Dentinger CM, Jacob K, Lee LV, et al. Human Brucella canis infection and subsequent laboratory exposures associated with a puppy, New York city, 2012. Zoonoses Public Health. 2015;62:407–414. doi:10.1111/zph.12163
- Kolwijck E, Lutgens SP, Visser VX, et al. First case of human Brucella canis infection in The Netherlands. Clin Infect Dis. 2022. Available from: https://www.ncbi.nlm.nih.gov/pubmed/35653425.
- Ledbetter EC, Landry MP, Stokol T, et al. Brucella canis endophthalmitis in 3 dogs: clinical features, diagnosis, and treatment. Vet Ophthalmol. 2009;12:183–191. doi:10.1111/j.1463-5224.2009.00690.x
- Alton GG, Jones LM, Angus RD, et al. Techniques for the brucellosis laboratory. Paris: INRA; 1988.
- Elbehiry A, Aldubaib M, Al Rugaie O, et al. Proteomics-based screening and antibiotic resistance assessment of clinical and sub-clinical Brucella species: an evolution of brucellosis infection control. PloS One. 2022;17:e0262551.
- Ferreira L, Vega Castano S, Sanchez-Juanes F, et al. Identification of Brucella by MALDI-TOF mass spectrometry. Fast and reliable identification from agar plates and blood cultures. PLoS One. 2010;5:e14235. doi:10.1371/journal.pone.0014235
- Scholz HC, Pfeffer M, Witte A, et al. Specific detection and differentiation of Ochrobactrum anthropi, Ochrobactrum intermedium and Brucella spp. by a multi-primer PCR that targets the recA gene. J Med Microbiol. 2008;57:64–71. doi:10.1099/jmm.0.47507-0
- Bounaadja L, Albert D, Chénais B, et al. Real-time PCR for identification of Brucella spp.: a comparative study of IS711, bcsp31 and per target genes. Vet Microbiol. 2009;137:156–164. doi:10.1016/j.vetmic.2008.12.023
- Lopez-Goni I, Garcia-Yoldi D, Marin CM, et al. New Bruce-ladder multiplex PCR assay for the biovar typing of Brucella suis and the discrimination of Brucella suis and Brucella canis. Vet Microbiol. 2011;154:152–155. doi:10.1016/j.vetmic.2011.06.035
- Girault G, Perrot L, Mick V, et al. High-resolution melting PCR as rapid genotyping tool for Brucella species. Microorganisms 2022: 10. Available from: https://www.ncbi.nlm.nih.gov/pubmed/35208791.
- Keid LB, Soares RM, Vieira NR, et al. Diagnosis of canine brucellosis: comparison between serological and microbiological tests and a PCR based on primers to 16S-23S rDNA interspacer. Vet Res Commun. 2007;31:951–965. doi:10.1007/s11259-006-0109-6
- Keid LB, Soares RM, Vasconcellos SA, et al. A polymerase chain reaction for detection of Brucella canis in vaginal swabs of naturally infected bitches. Theriogenology. 2007;68:1260–1270. doi:10.1016/j.theriogenology.2007.08.021
- Boeri EJ, Wanke MM, Madariaga MJ, et al. Comparison of four polymerase chain reaction assays for the detection of Brucella spp. in clinical samples from dogs. Vet World. 2018;11:201–208. doi:10.14202/vetworld.2018.201-208
- Batinga MCA, de Lima JTR, Gregori F, et al. Comparative application of IS711-based polymerase chain reaction (PCR) and loop-mediated isothermal amplification (LAMP) for canine brucellosis diagnosis. Mol Cell Probes. 2018;39:1–6. doi:10.1016/j.mcp.2018.02.003
- Ashford R, Brucella W. Mol typing bact infect. de Filippis. Springer International Publishing; 2022. p. 217–245.
- Carmichael LE, Joubert JC. A rapid slide agglutination test for the serodiagnosis of Brucella canis infection that employs a variant (M-) organism as antigen. Cornell Vet. 1987;77:3–12.
- Keid LB, Soares RM, Vasconcellos SA, et al. Comparison of agar gel immunodiffusion test, rapid slide agglutination test, microbiological culture and PCR for the diagnosis of canine brucellosis. Res Vet Sci. 2009;86:22–26. doi:10.1016/j.rvsc.2008.05.012
- Keid LB, Diniz JA, Oliveira TM, et al. Evaluation of an immunochromatographic test to the diagnosis of Canine Brucellosis caused by Brucella canis. Reprod Domest Anim. 2015;50:939–944. doi:10.1111/rda.12612
- Wanke MM, Cairó F, Rossano M, et al. Preliminary study of an immunochromatography test for serological diagnosis of canine brucellosis. Reprod Domest Anim Zuchthyg. 2012;47(Suppl 6):370–372. doi:10.1111/rda.12108
- Escobar GI, Boeri EJ, Ayala SM, et al. The feasibility of using antigens prepared with rough Brucella strains for diagnosis of canine brucellosis. Rev Argent Microbiol. 2010;42:35–40.
- Badakhsh FF, Carmichael LE, Douglass JA. Improved rapid slide agglutination test for presumptive diagnosis of canine brucellosis. J Clin Microbiol. 1982;15:286–289. doi:10.1128/jcm.15.2.286-289.1982
- Zoha SJ, Carmichael LE. Serological responses of dogs to cell wall and internal antigens of Brucella canis (B. canis). Vet Microbiol. 1982;7:35–50. doi:10.1016/0378-1135(82)90004-9
- Lucero NE, Escobar GI, Ayala SM, et al. Sensitivity and specificity of an indirect enzyme-linked immunoassay for the diagnosis of Brucella canis infection in dogs. J Med Microbiol. 2002;51:656–660. doi:10.1099/0022-1317-51-8-656
- de Oliveira MZ, Vale V, Keid L, et al. Validation of an ELISA method for the serological diagnosis of canine brucellosis due to Brucella canis. Res Vet Sci. 2011;90:425–431. doi:10.1016/j.rvsc.2010.07.004
- Baldi PC, Wanke MM, Loza ME, et al. Brucella abortus cytoplasmic proteins used as antigens in an ELISA potentially useful for the diagnosis of canine brucellosis. Vet Microbiol. 1994;41:127–134. doi:10.1016/0378-1135(94)90142-2
- Baldi PC, Wanke MM, Loza ME, et al. Diagnosis of canine brucellosis by detection of serum antibodies against an 18 kDa cytoplasmic protein of Brucella spp. Vet Microbiol. 1997;57:273–281. doi:10.1016/S0378-1135(97)00134-X
- Cortina ME, Novak A, Melli LJ, et al. Development of improved enzyme-based and lateral flow immunoassays for rapid and accurate serodiagnosis of canine brucellosis. Vet Microbiol. 2017;208:174–180. doi:10.1016/j.vetmic.2017.08.005
- Mateu-de-Antonio EM, Martín M, Soler M. Use of indirect enzyme-linked immunosorbent assay with hot saline solution extracts of a variant (M-) strain of Brucella canis for diagnosis of brucellosis in dogs. Am J Vet Res. 1993;54:1043–1046.
- Kim JW, Lee YJ, Han MY, et al. Evaluation of immunochromatographic assay for serodiagnosis of Brucella canis. J Vet Med Sci. 2007;69:1103–1107. doi:10.1292/jvms.69.1103
- Ducrotoy MJ, Muñoz PM, Conde-Álvarez R, et al. A systematic review of current immunological tests for the diagnosis of cattle brucellosis. Prev Vet Med. 2018;151:57–72. doi:10.1016/j.prevetmed.2018.01.005
- Santos RL, Souza TD, Mol JPS, et al. Canine brucellosis: an update. Front Vet Sci. 2021;8:594291. doi:10.3389/fvets.2021.594291
- de Souza TD, de Carvalho TF, Mol J, et al. Tissue distribution and cell tropism of Brucella canis in naturally infected canine foetuses and neonates. Sci Rep. 2018;8:7203. doi:10.1038/s41598-018-25651-x
- Falenski A, Mayer-Scholl A, Filter M, et al. Survival of Brucella spp. in mineral water, milk and yogurt. Int J Food Microbiol. 2011;145:326–330. doi:10.1016/j.ijfoodmicro.2010.11.033
- Lawaczeck E, Toporek J, Cwikla J, et al. Brucella canis in a HIV-infected patient. Zoonoses Public Health. 2011;58:150–152. doi:10.1111/j.1863-2378.2010.01334.x
- Lucero NE, Escobar GI, Ayala SM, et al. Diagnosis of human brucellosis caused by Brucella canis. J Med Microbiol. 2005;54:457–461. doi:10.1099/jmm.0.45927-0
- Wallach JC, Giambartolomei GH, Baldi PC, et al. Human infection with M- strain of Brucella canis. Emerg Infect Dis. 2004;10:146–148. doi:10.3201/eid1001.020622
- Pereira CR, Cotrim de Almeida JVF, Cardoso de Oliveira IR, et al. Occupational exposure to Brucella spp.: a systematic review and meta-analysis. PLoS Negl Trop Dis. 2020;14:e0008164.
- Ahmed-Bentley J, Roman S, Mirzanejad Y, et al. Laboratory exposures from an unsuspected case of human infection with Brucella canis. Emerg Infect Dis. 2021;27:2489–2491. doi:10.3201/eid2709.204701
- Viana MVC, Wattam AR, Govil Batra D, et al. Genome sequences of three Brucella canis strains isolated from humans and a dog. Genome Announc. 2017;5:e01688–16.
- Lum MK, Pien FD, Sasaki DM. Human Brucella canis infection in Hawaii. Hawaii Med J. 1985;44:66–68.
- Nomura A, Imaoka K, Imanishi H, et al. Human Brucella canis infections diagnosed by blood culture. Emerg Infect Dis. 2010;16:1183–1185. doi:10.3201/eid1607.090209
- Piao D, Wang H, Di D, et al. MLVA and LPS characteristics of Brucella canis isolated from humans and dogs in Zhejiang, China. Front Vet Sci. 2017;4:223. doi:10.3389/fvets.2017.00223
- Mohamed Zahidi J, Ahmad N, Tay BY, et al. Genome sequences of Brucella melitensis, isolated from blood samples of brucellosis patients in Malaysia. Genome Announc. 2017;5. Available from: https://www.ncbi.nlm.nih.gov/pubmed/28774972.
- Schoenemann J, Lutticken R, Scheibner E. [Brucella canis infection in man]. Dtsch Med Wochenschr. 1986;111:20–22. doi:10.1055/s-2008-1068393
- King J. Heartbroken woman forced to put five dogs down after contracting rare disease. METRO UK [Internet] 2022 Aug 14. Available from: https://metro.co.uk/2022/08/14/woman-forced-to-put-five-dogs-down-after-contracting-rare-disease-17180788/.
- Blankenship RM, Sanford JP. Brucella canis. A cause of undulant fever. Am J Med. 1975;59:424–426. doi:10.1016/0002-9343(75)90402-7
- Tosi MF, Nelson TJ. Brucella canis infection in a 17-month-old child successfully treated with moxalactam. J Pediatr. 1982;101:725–727. doi:10.1016/S0022-3476(82)80301-6
- McKee MA, Ballard JL. Mycotic aneurysms of the tibioperoneal arteries. Ann Vasc Surg. 1999;13:188–190. doi:10.1007/s100169900240
- Ying W, Nguyen MQ, Jahre JA. Brucella canis endocarditis: case report. Clin Infect Dis. 1999;29:1593–1594. doi:10.1086/313545
- Laine C, Johnson V, Scott H, et al. Human brucellosis: first calculated estimate of global annual incidence. Brucell 2022 Int Res conf.; Terramo, Italy: IZSAM; 2022.
- Eckstein C, Mol JPS, Costa FB, et al. Brucella ovis mutant in ABC transporter protects against Brucella canis infection in mice and it is safe for dogs. PLoS One. 2020;15:e0231893. doi:10.1371/journal.pone.0231893
- Kim WK, Moon JY, Cho JS, et al. Brucella abortus lysed cells using GI24 induce robust immune response and provide effective protection in Beagles. Pathog Dis. 2018;76. Available from: https://www.ncbi.nlm.nih.gov/pubmed/29272378.
- Hollett RB. Canine brucellosis: outbreaks and compliance. Theriogenology. 2006;66:575–587. doi:10.1016/j.theriogenology.2006.04.011
- De Massis F, Sacchini F, Petrini A, et al. Canine brucellosis due to Brucella canis: description of the disease and control measures. Vet Ital. 2022;58:5–23.
- Guarino C, Franklin-Guild R, Goodrich E, et al. Antibody response over time correlated with treatment outcome in 30 dogs naturally infected with Brucella canis (2017–2022). Am J Vet Res. 2023;1:1–7. doi:10.2460/ajvr.23.01.0014
- Wanke MM, Delpino MV, Baldi PC. Use of enrofloxacin in the treatment of canine brucellosis in a dog kennel (clinical trial). Theriogenology. 2006;66:1573–1578. doi:10.1016/j.theriogenology.2006.01.034
- Liu Y, Sun J, Peng X, et al. Deletion of the LuxR-type regulator VjbR in Brucella canis affects expression of type IV secretion system and bacterial virulence, and the mutant strain confers protection against Brucella canis challenge in mice. Microb Pathog. 2020;139:103865. doi:10.1016/j.micpath.2019.103865
- Stranahan LW, Chaki SP, Garcia-Gonzalez DG, et al. Evaluation of the efficacy of the Brucella canis RM6/66 DeltavjbR vaccine candidate for protection against B. canis infection in mice. mSphere. 2020;5. Available from: https://www.ncbi.nlm.nih.gov/pubmed/32434839.
