ABSTRACT
Immature feathers are known replication sites for high pathogenicity avian influenza viruses (HPAIVs) in poultry. However, it is unclear whether feathers play an active role in viral transmission. This study aims to investigate the contribution of the feather epithelium to the dissemination of clade 2.3.4.4b goose/Guangdong/1996 lineage H5 HPAIVs in the environment, based on natural and experimental infections of domestic mule and Muscovy ducks. During the 2016–2022 outbreaks, H5 HPAIVs exhibited persistent and marked feather epitheliotropism in naturally infected commercial ducks. Infection of the feather epithelium resulted in epithelial necrosis and disruption, as well as the production and environmental shedding of infectious virions. Viral and feather antigens colocalized in dust samples obtained from poultry barns housing naturally infected birds. In summary, the feather epithelium contributes to viral replication, and it is a likely source of environmental infectious material. This underestimated excretion route could greatly impact the ecology of HPAIVs, facilitating airborne and preening-related infections within a flock, and promoting prolonged viral infectivity and long-distance viral transmission between poultry farms.
Introduction
High pathogenicity avian influenza viruses (HPAIVs) of clade 2.3.4.4b goose/Guangdong/1996 (Gs/GD) H5 lineage have greatly impacted both the poultry industry and wild bird populations during the last few years, resulting in large-scale culling of infected flocks and mass mortality of endangered species worldwide [Citation1,Citation2]. Ducks, in particular, play a crucial role in the transmission of HPAIVs due to the shedding of large amounts of infectious material during the pre-symptomatic phase, which can last several days. The main shedding routes include digestive and respiratory tracts [Citation3–5]. Therefore, to develop effective control measures, it is essential to properly understand the mechanisms underlying HPAIVs infection and dissemination in ducks. In commercial ducks, infection with clade 2.3.4.4b H5 HPAIVs leads to neurological disease and mortality. Typical pathological findings include acute encephalitis, myocarditis, and pancreatitis. However, since these viruses replicate systemically, they can be detected in many other tissues [Citation3,Citation6].
A key feature of viral infection is tropism, which refers to the ability of a virus to infect and replicate in specific cells or tissues. Viral tropism plays a critical role in viral ecology since the range of infected tissues and organs determines clinical signs, mean death time and viral excretion routes [Citation6]. Growing feather follicles have been identified as a site of H5 and H7 HPAIVs replication in ducks [Citation7–9] and other gallinaceous species [Citation10–12]. Feathers are abundant external cutaneous appendages implicated in a variety of functions, including flight, insulation, and communication [Citation13]. Immature feathers are growing tubular structures budding from the skin, covered by a protective external sheath and nourished by a vascularized dermal pulp (supplementary data 1). The feather epithelium grows and differentiates into barbs and barbules. Upon maturation, the external sheath opens and the epithelium comes into contact with the air [Citation14]. Experimental data show that infected feathers pulp is a site of productive viral replication, and feathers obtained from infected duck carcasses contain infectious material for up to 160 and 15 days post-mortem at 4 and 20°C, respectively [Citation12,Citation15]. Furthermore, the stability of avian influenza viruses has been reported to increase with preening oil, which is secreted by the uropygial gland and used for preening behaviour [Citation16].
Dust found in poultry houses is composed of diverse particles in terms of size, density, and shape, originating from feed, faeces, skin, feather and litter debris, microorganisms, and insect parts [Citation17–19]. Feathers are reported to account for 10% of dust found in commercial layer chicken houses [Citation17,Citation18]. Not only is dust known to contribute to viral dispersal within farms, but also inhalable particles (<100 µm) can lead to bird-to-bird airborne transmission [Citation19–23]. While dust is a recognized source of pathogens [Citation19], the specific contribution of feathers in infectious viral shedding, as well as viral environmental spread and ecology, remains unclear.
This study aims to investigate the role of feather epitheliotropism in the environmental dissemination of clade 2.3.4.4b H5 HPAIVs.
Materials and methods
Field cases
Eight flocks of commercial ducks (two flocks of Muscovy ducks [Cairina moschata] and six flocks of mule ducks [Cairina moschata x Anas platyhrynchos]) positive for H5 HPAIVs by official testing during the 2016–2022 HPAIVs outbreaks in France were included in the study. Pathological investigations were conducted on a total of 36 dead animals, naturally infected with H5N8 2016–2017 (n = 8), H5N8 2020–2021 (n = 10), and H5N1 2021–2022 (n = 18) HPAIVs. All samples were obtained under the supervision of the French Official Veterinary Services and in compliance with the French legislation on veterinary practices and notifiable diseases. At necropsy, feathered skin (including wing feathers, caudal tract, and capital tract) was collected and fixed in 10% neutral buffered formalin for histopathological examination and viral in situ detection. Titration of viral infectivity in feather samples was conducted on two flocks of ducks naturally infected with H5N1 2021–2022 HPAIV (5 ducks per flock). Additionally, feathered skin from dead chickens (4 flocks, n = 3–5), turkeys (1 flock, n = 4), quails (1 flock, n = 5), geese (1 flock, n = 6) and wild swans (3 birds) naturally infected with H5 HPAIV were collected in France between 2016 and 2022. Tissue samples were treated and analyzed similarly to ducks. Results are provided in supplementary data.
Experimental infection
Fifty 5-week-old domestic male mule ducks were obtained from a commercial producer (GALLSA, Tarragona, Spain). Ducks were challenged by intrachoanal inoculation with 5 log10 50% egg infectious doses (EID50) of either A/mulard duck/France/171201 g/2017(H5N8) (H5N8/2017) reverse genetics-engineered virus (accession numbers MK859904 to MK859911) [Citation24] or A/Mule duck/France/20320/2020(H5N8) (H5N8/2020) (accession numbers MZ166297 to MZ166304), and monitored for 14 days post-inoculation (dpi). Groups included sham birds (n = 3), inoculated birds (n = 16) and contact birds placed 24 h post-inoculation (n = 6), for each viral strain. Feather pulp samples were collected on 10 inoculated and 6 contact birds at 0, 2, 4, 7, 10 and 14 dpi for viral RNA detection. Complete necropsies were performed at 3 and 5 dpi for each virus on 3 inoculated birds and on 3 sham birds. At necropsy, sections of feathered skin, including capital and caudal tracts [Citation9], as well as detached immature wing feathers were collected for histopathology and stored in 10% neutral buffered formalin. All procedures involving birds were reviewed and approved by the IRTA (#258-2021) and the Catalan Government (#11467) Ethics and Animal Experimentation Committees, subject to national and European regulations. All procedures involving virus were performed in biosafety level-3 (BSL-3) laboratory and animal facilities at IRTA-CReSA, in accordance with procedures approved by IRTA Biosafety Committee (#59-2021).
Viral molecular detection
Total RNA was extracted from experimental and selected field samples using the magnetic bead-based ID Gene Mag Fast Extraction Kit and IDEAL 32 extraction robot (Innovative Diagnostics, Grabel, France) according to the manufacturer’s instructions. A one-step, real-time reverse transcription quantitative PCR (rRT-qPCR) targeting the H5 subtype was then performed using the ID GENE Influenza H5/H7 Triplex kit (Innovative Diagnostics, Grabel, France).
Histology and viral in situ detection
Fixed tissue samples were paraffin-embedded and cut at 3 µm. Sections were stained with haematoxylin and eosin (H&E) for histopathological analysis and anti-influenza A virus nucleoprotein immunohistochemistry (anti-IAV NP IHC) using anti-NP influenza A HB65 antibodies (NP, HB65, lot 758121J1, Biozol, Eching, Germany). Briefly, the IHC protocol included an antigen retrieval step with 0.05% pronase applied for 10 min at 37°C, a peroxidase blocking step of 5 min at room temperature (S2023; Agilent) followed by saturation of nonspecific binding sites with normal goat serum (X0907; Agilent) applied for 25 min at room temperature, and overnight incubation with anti-IAV NP antibody (1:2000 dilution) at 4°C. Signal amplification and revelation were assessed using the EnVision FLEX system and 3,3'-diaminobenzidine (DAB) revelation according to the manufacturer’s recommendations. Avian Influenza Matrix gene (M gene) RNAscope in situ hybridization (RNAscope ISH) was performed on selected samples, as previously described [Citation25]. Viral antigen detection was determined for epithelial and mesenchymal tissues of growing feathers, resting feather epidermis and dermis [Citation26]. Results were expressed as frequency (number of positive birds/number of total birds *100) and distribution was determined according to Landman (2021) for each flock [Citation27].
Transmission electron microscopy
Ultrastructural analyzes were performed on samples obtained from an experimentally infected duck with H5N8/2017, which was selected based on histopathological lesions and viral antigen detection. Growing feathers from the caudal tract stored in formalin were cut (6 transversal sections), immersed in 2% glutaraldehyde in Sorensen’s phosphate buffer (0.1 mol/L, pH = 7.4) for 1 h, and washed with Sorensen’s phosphate buffer for 12 h. Then samples were incubated with 1% OsO4 in Sorensen’s phosphate buffer (0.05 mol/L, glucose 0.25 mol/L, OsO4 1%) for 1 h, dehydrated in an ascending ethanol series until ethanol 100° and then with propylene oxide, and embedded with epoxy resin (EMBed 812). After 48h of polymerization at 60°C, ultrathin sections (70 nm) were mounted on 100 mesh collodion-coated copper grids and post-stained with 3% uranyl acetate in 50% ethanol and with 8.5% lead citrate before being examined on a HT 7700 Hitachi electron microscope at an accelerating voltage 80 KV.
Dust morphological and immunofluorescent study
Dust samples were collected from four H5N1 2021–2022 HPAIV-positive farms using a dry cyclonic air sampler Coriolis Compact (Bertin Technologies) for 20 min at 50 L/min. Samples were resuspended in 1 mL of phosphate buffered saline (PBS). Dust microscopic examination was conducted following an agar-based method, with a similar approach to the cell block technique used in cytopathology [Citation28]. Briefly, 100 µL were centrifuged for 10 min at 300 g. The supernatant was removed and the pellet, consisting of dust, was resuspended in 50 µL of PBS and then 100 µL of Histogel® heated at 60 +/− 5°C (Thermo Scientific Richard-Allen Scientific, Waltham, MA). Dust blocks were formalin-fixed, paraffin-embedded and serially cut at 3 µm. One section was stained with H&E for histological analysis. On a second section, a double immunofluorescence (IF) was performed using non-commercial polyclonal rabbit primary antibodies targeting corneous beta-proteins (CBP) and mouse monoclonal anti-NP influenza A antibodies [Citation29]. The antigenic retrieval and saturation steps were similar to those used in the anti-IAV NP IHC. Both antibodies were co-incubated at dilutions of 1/2000 (IAV NP) and 1/100 (CBP) overnight at 4°C. Anti-mouse 594 (Invitrogen, A11032) and anti-rabbit 488 (Jackson ImmunoResearch, 711-546-152) secondary antibodies were then used at a dilution of 1/500, incubated 1 h at room temperature. Slides were mounted with Fluoromount G with DAPI (Thermo-Fischer Scientific, 00-4959-52). Images were collected using a Zeiss LSM 710 confocal microscope.
Viral titration
Growing feathers collected from ten ducks belonging to two flocks (five ducks per flock) that tested positive for H5N1 during the 2021 outbreaks in France were used to determine the infectivity of the feather subcompartments through viral titration. Feather tips were sealed with heated paraffin, and the feather outer sheath was decontaminated by immersion in 70% ethanol for 5 min at room temperature, followed by a washing step in PBS with 0.5% bovine serum albumin (BSA). Feather outer sheath was then longitudinally opened to dissect both the distal epithelial fraction (whitish, matt, filamentous material) and the basal pulp (pink-red, soft, translucent and moist material). Epithelial samples were incubated in PBS-0.5% BSA for 30 min at room temperature. Subsequently, the PBS-0.5% BSA solution was replaced and vortexed for 30 s. Feather outer sheath (before and after decontamination), basal pulp, and distal epithelial fractions (with and without mechanical disruption) were subjected to viral titration. Titrations were performed using Madin-Darby canine kidney (MDCK) cells, followed by an immunoperoxidase monolayer assay. Results were expressed as log10 focus forming units per mL (FFU/mL) according to Matrosovitch et al. [Citation30].
Statistics
Statistical analyzes were performed using R Studio software (https://www.R-project.org) to compare viral titres among tissue compartments (feather outer sheath before and after decontamination, distal feather epithelium before and after vortexing, and basal pulp). Data for each sample were analyzed with a linear model that used the bird as a random parameter, and the tissue compartment and flock as fixed parameters.
Results
H5 HPAIVs lead to severe damage of growing feather epithelium
Growing feathers of domestic ducks naturally infected with H5 HPAIVs between 2016 and 2022 exhibited segmental and multifocal to diffuse epithelial necrosis (A). Lesions were identified in several layers exhibiting different stages of differentiation, including the epidermal collar and early and late barb ridges, and were associated with epithelial disruption and loss of the original architecture (Supplementary data 2). Epithelial necrosis and signs of dermal pulpitis, such as hyperaemia, exudation, leukocytic infiltration comprised of lymphocytes, plasma cells and occasional heterophils, were also present. In experimentally infected ducks, lesions were similar with those observed in naturally infected birds.
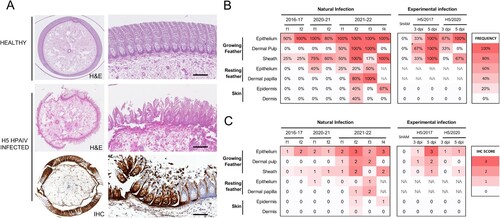
Figure 1. Feather lesions and in situ viral detection in commercial ducks naturally and experimentally infected with clade 2.3.4.4b H5 HPAIVs. (A) Growing feather from a healthy duck showing normal dermal pulp surrounded by intact feather epithelium (H&E, top figure). In contrast, severe epithelial necrosis and disruption associated with inflammation of feather pulp (H&E, middle figure) and viral antigen detection (IHC, bottom figure) is observed in a growing feather from a duck naturally and experimentally infected with H5 HPAIVs, respectively. Bars represent 100 µm. Frequency (B) and average score (C) of viral antigen detection by IHC according to tissue compartment in naturally and experimentally infected ducks. Flocks are labelled as f and are numbered for each period. Flocks included 4–6 birds per flock.
H5 HPAIVs have marked and persistent feather epitheliotropism
Viral antigen was detected within lesions in both cytoplasm and nuclei of cells (A). Overall, in domestic ducks the most frequently and severely affected tegumental compartment was the growing feather epithelium, followed by the feather sheath (B,C). In growing feather epithelium, viral antigen was frequently and extensively detected in barbules cells, barb cells (ramus), rachidial cells, and supportive cells from marginal and axial plates. In contrast, viral antigen detection was less frequent and widely spread in feather dermal pulp and resting feathers, except in ducks naturally infected with H5N1 from 2021 to 2022. In the dermal pulp, viral NP was mainly detected in mesenchymal stromal cells and leukocytes. In resting feathers, viral antigen was inconstantly detected in dermal papilla and lining epithelium. Feathered skin dermis and epidermis were mostly negative, except in some ducks naturally infected with H5N1 from 2021 to 2022. Viral antigen detection in other species including chicken, turkey, quail, geese, and wild swan is presented in Supplementary data 3.
These results indicate that H5 HPAIVs have a marked tropism for feather epithelium in domestic ducks.
H5 HPAIVs are detected in growing feathers early in the course of infection
In experimentally infected ducks, viral RNA was detected in growing feathers as early as 2 dpi, with peak of detection at 4 dpi and positive detection up to 14 dpi in the remaining ducks (A). At 3 dpi, when the majority of the ducks were in the pre-symptomatic phase, viral antigens were multifocally detected within the dermal pulp and basal layer of growing epithelium, extending through marginal plates, and then in the lower portion of barb and barbule cells (B). At 5 dpi, when mortality peaked, the detection of severe and diffuse viral antigens extended to barbs, barbules, and inter-barbular spaces (B). Viral antigen distribution was similar in H5N8/2017 and H5N8/2020 infected ducks within feather tissue. However, immunoreactive areas tended to be more widespread in H5N8/2017 infected ducks.
Figure 2. Viral antigen dynamics in commercial ducks experimentally infected with H5 HPAIV from 2017 and 2020. (A) Viral RNA detections in growing feathers from ducks experimentally infected with H5N8/2017 (red) or H5N8/2020 (blue), represented as 40-Ct values. Means calculated on living birds at the time of sampling are represented as points, and standard deviations as hooks for both inoculated (I) and contact (C) birds. N+, N−, ND dots represent number of positive, negative and dead birds, respectively. H5N8/2017_I: H5N8/2017 inoculated birds, H5N8/2017_C: H5N8/2017 contact birds, H5N8/2020_I: H5N8/2020 inoculated birds, H5N8_C: H5N8/2020 contact birds. (B) Viral antigen detection with anti-IAV NP IHC in growing feathers of experimentally infected ducks with H5N8 2017 at 3 and 5 dpi.
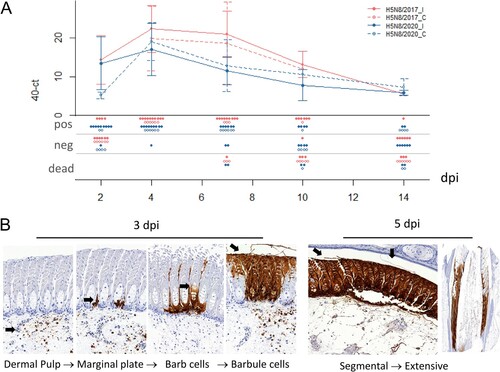
These results indicate that H5 HPAIVs rapidly infect and diffuse into the feather epithelium over the course of infection.
Virion-shaped particles are observed in infected feather epithelium
Ultrastructural analysis was performed on a total of six different sections of growing feathers obtained from the caudal tract of a duck experimentally infected with H5N8/2017. Regions of interest were selected based on semi-thin sections and observation of epithelial necrosis. Filamentous particles, ranging 60-80 nm in diameter and up to 500 nm in length, were seen budding from the cytoplasmic membrane of the feather epithelium and accumulating between barbules, extracellularly ().
Figure 3. Ultrastructural viral detection in growing feather epithelium of commercial ducks experimentally infected with H5 HPAIVs. (A) Ultrastructural images of feather epithelium of a duck experimentally infected with H5N8/2017 HPAIV at 6 dpi was assessed after selecting regions of interest (ROI) on histological sections. Bar, 200 µm. (B) ROI within feather growing epithelium included barbules, inter-barbular spaces, and necrotic areas. Bar, 50 µm. (C–G) Detection of budding and free, filamentous virion-shaped particles (arrowheads) within intercellular spaces of differentiating barbules. Transmission electron microscopy. Bar, 500 nm.
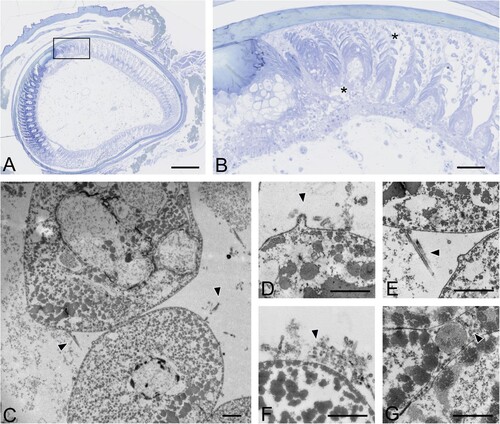
These results indicate that infection of the feather epithelium generates production of virions released extracellularly between barbules.
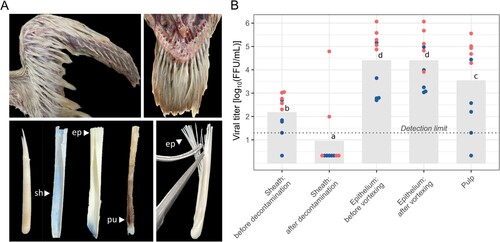
High titres of infectious material are associated with immature feather epithelium
Both the detection of viral RNA and proteins in the feather epithelium and the observation of particles evocative of filamentous influenza virions, prompted us to determine whether the infected feather epithelium produced infectious particles. To do so, immature duck feathers from naturally infected flocks were carefully dissected after sheath disinfection (A). The association of infectious material with the various feather subcompartments was then determined by viral titration in MDCK cells (B). Although moderate levels (mean concentration of 2.16 log10 FFU/mL) of infectious material were present on feather sheaths, ethanol incubation led to a drastic reduction of infectivity, below the assay detection limit, in 8/10 samples, ruling out subsequent contamination of internal feather elements by external infectious material (B). High concentrations of infectious material (mean concentration around 4.39 log10 FFU/mL) were detected after simple incubation, without agitation of epithelium samples. Similar concentrations (mean concentration of 4.39 log10 FFU/mL) were detected after replacement of the diluent and vortexing. Finally, mean concentrations of 3.53 log10 FFU/mL were detected in feather pulp sampled in the area closest to the skin. Overall, viral titres were statistically higher in the epithelial compartment compared to the pulp and sheath compartments (p < 0.05). These results support the hypothesis that infectious viral particles are produced by the infected immature feather epithelium.
Figure 4. Viral infectivity of growing feathers in commercial ducks naturally infected with clade 2.3.4.4b H5N1 HPAIV. (A) Wing (top left) and caudal tract (top right) growing feathers of a 40-day-old male mule duck. Detached growing feathers are comprised of an external outer sheath (sh) and a growing and differentiating epithelium (ep) supported by a central core of dermal pulp (pu) (bottom left). For viral titration, distal epithelium was collected (bottom right). (B) Viral titres in MDCK cells, expressed as log10 FFU/mL, determined on feather outer sheath before and after decontamination, feather epithelium with and without mechanical disruption, and feather pulp. Histograms and the dotted line represent the mean viral titres and the limit of detection, respectively. Each dot represents an individual subject, with blue and red colours indicating the two flocks included in the analysis. Different letters indicate significant statistical difference at p < 0.05 determined by ANOVA analysis
Feather fragments and viral antigens colocalize in dust from influenza outbreak farms
Histologically, dust collected from four H5 HPAIV-positive farms revealed the presence of frequent light to dense, eosinophilic and elongated structures admixed with bacteria, fungi and granular material of unknown origin (). Prior to IF, Corneous beta-protein (CBP) antigen detection was first determined by IHC on normal feathered skin: CBP was detected in cornifying cells, including barbules and the feather outer sheath (Supplementary data 4). Subsequently, double IF, targeting both viral IAV NP and CBP, was conducted on sections of feathered skin of a duck experimentally infected with H5N8/2017 to assess positive detection of both targets, and then on dust blocks obtained from H5 HPAIV-positive farms. In feathered skin, both CBP and IAV NP antigens colocalized in the growing feather epithelium, particularly in the barbule cells (). IAV NP antigen was also observed between barbules. In dust blocks, CBP antigen was detected within the elongated structures. AIV NP antigen colocalized with the CBP-positive elongated structures and the granular material (, Supplementary data 5 and 6).
Figure 5. Histological analysis of aerosols (dust) collected from H5 HPAIV-positive farms. (A) Histogel-based Coriolis Compact (Bertin Technologies) sampled dust blocks (arrows). (B–F) Dust blocks from H5 HPAIV-positive farms stained with H&E revealed the presence of frequent eosinophilic elongated structures (arrowheads), admixed with bacteria (asterisks), fungi and granular material of unknown origin. Bar, 50 µm (A) and 20 µm (B).
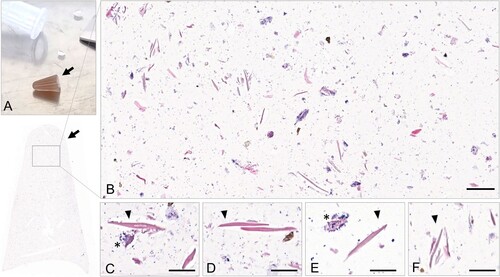
Figure 6. Immunofluorescent detection of avian CBP and influenza A NP antigen in growing feather and aerosols (dust). Immunofluorescent detection of avian CBP and influenza A NP antigen in a growing feather of an H5N8/2017 experimentally infected mule duck, and in histogel-based dust block sampled by Coriolis Compact (Bertin Technologies) from four HPAIV-positive farms. 4’,6-diamino-2-phenilidole (DAPI, blue), Corneous beta-protein (CBP, green), Nucleoprotein influenza A (NP, red).
These results indicate that fragments detached from the infected feather epithelium are aerosolized in infected poultry houses and remain infected.
Discussion
The contagiousness of avian influenza viruses and, in particular, of HPAIVs derived from the Gs/GD lineage, is now universally recognized, especially in ducks. Experimental studies showed that both oropharyngeal and cloacal viral shedding occur as early as a few hours post-infection and can last up to 14 days [Citation5,Citation31–33]. In comparison, the duration of viral excretion in infected chickens is much shorter (<2 days) due to their short MDTs, contributing to the higher excretion potential of ducks compared to gallinaceous birds [Citation4,Citation5,Citation24].
Feather tropism of HPAIVs is now well documented, particularly for viruses of the Gs/GD lineage [Citation7–9,Citation32,Citation34]. However, virological investigation of feather tropism have mainly focused on the feather pulp, regarded both as a marker of systemic infection and a target for diagnostic purposes, but not on the feather epithelium, as a potential excretion route of infectious viruses. In these studies, detached feather follicles were homogenized following extraction and processed as a whole biological matrix. As a consequence, the precise tissue location was lost [Citation8,Citation12,Citation31,Citation35]. In our study, particular attention was given to the dissection of growing feathers, allowing to differentiate viral titres obtained from the feather epithelium, pulp, and sheath ().
Our data show a marked tropism of clade 2.3.4.4b H5 viruses for the epithelium of growing feathers in ducks. A similar marked epitheliotropism was identified in other waterfowl species, including commercial geese and wild mute swans naturally infected with H5 HPAIVs (supplementary data 3). On the contrary, in gallinaceous birds naturally infected with H5 HPAIVs, including commercial chickens, quail, and turkeys, viral antigen detection was more frequent and widely spread in the feather pulp and skin dermis compared to the feather epithelium (supplementary data 3). These results are consistent with experimental infections [Citation9] and suggest rapid death of infected galliformes is associated with limited viral extension from pulp to the feather epithelium.
The total number of feathers present in a single bird is poorly known although, on average, it could account for 3-6% of the total adult body weight [Citation36]. In 1937, George Andrew Ammann allegedly counted 25,216 feathers in a swan. Phoebe Knappen reported a total of 11,903 feathers in a female adult duck [Citation37]. In young birds, feather growth is an extremely fast and relatively synchronous process that can reach up to 4 cm over 10 days in 40-day-old ducks [Citation38]. For this reason, the feather epithelium compartment represents an extremely large surface area and volume in young individuals (A). In contrast, adult birds have a higher number of mature feathers that are associated with lower viral detection [Citation32]. However, moulting can result in increased proportion of growing feathers in adults. Different periodic and spontaneous moulting processes can be observed in wild birds, which can be influenced by photoperiod, nutritional deficiency, migration timing constraints, and global warming [Citation39,Citation40]. Therefore, attention should not only be given to the study of host species but also to host age, breeding practices, plumage moult, and their potential impact on viral dissemination.
Normal and pathological behaviours may also lead to increased viral transmission from birds with infected feathers to other individuals. Ducks are well known for their preening behaviour, which is very specific of waterfowl and to which they devote several hours a day. This behaviour can involve only one bird (self-preening) or several congeners (allo-preening) [Citation33]. Additionally, feather pecking is a pathological injurious behaviour resulting in removal and eating of feather’s congeners [Citation41]. Consequently, feathers represent an external organ strongly involved in normal and pathological ethograms of wild and domestic birds. This suggests possible direct transmission and ingestion of viral particles from feathers.
The quantitative importance of the feather excretion route, compared to the respiratory and digestive shedding routes, still needs to be assessed [Citation33]. Nevertheless, although a definitive conclusion is still premature, the intensity of the viral signal detected in the feather fraction identified in dust samples is remarkable. Current data available in chicken suggest that feather particles make up as much as 10% of the total mass of the dust present in poultry houses [Citation17], underlying the quantitative importance of animal exposure to this type of substrate. A clear parallel can be drawn between AIVs and the aetiological agent of Marek's disease in chickens; Gallid alphaherpesvirus 2 replicates to high titres in the epithelium of the feather follicles, and mature virions are released in association with debris originating from the epithelial layer. In this form, herpesvirus can survive for several months in the poultry house, which is much longer than expected for a herpesvirus [Citation42]. A similar excretion route was found for the chicken infectious anaemia virus [Citation43].
Staining experiments on dust samples collected from the environment of infected duck farms confirmed that viral antigens are mostly co-detected with avian CBPs. In feathered skin, CBP antigens could be detected in barbs, barbules, feather sheath, and the outermost layer of epidermis, but viral antigen detection was mostly positive in feather epithelium. Determining the infectious viral titre of organic material released into the environment is currently a challenge. Optimizations, including pre-treatments, are underway in our laboratory to overcome methodological limitations related to the study of environmental matrices. In order to overcome this issue, we tested the infectivity of the epithelial layer in a controlled setting, as this sub-tissular compartment is in direct contact with the environment, compared to the feather pulp. This experiment showed that the infectious viral load was higher in the epithelial layer compared to the pulp and feather sheath, further supported by the observation of filamentous virion-shaped particles by electron microscopy in infected feather epithelium. Filamentous influenza viral particles are indeed preferentially observed after infection of epithelial cells and their genesis appears to depend on both host and virus-derived factors [Citation44]. The relative frequency of filamentous versus spherical viral particles produced by infected feather epithelium is currently under investigation. Altogether, these data support the notion that infected duck feather debris are infectious. Persistence of infectivity over time and dispersion of such infectious debris in the environment remains to be assessed, in particular for long-distance contamination and between-farm dissemination.
Active viral shedding occurs along the time course of infection from pre-clinical to clinical phases [Citation4]. However, attention should also be given to the passive post-mortem viral shedding from infected carcasses littering the ground. Stability in detached infected feathers has been demonstrated for both H5 and H7 HPAIVs in experimental conditions [Citation12,Citation15]. In birds, feather loss and disruption take place throughout the decomposition process [Citation45], underlying the importance of rapid disposal of infected carcasses and complete environmental disinfection, considering also the risk of exposing cadaveric fauna (insects, arthropods etc.), scavenger birds, and mammals to infectious tegument. Dispersion of feather debris may also occur during transportation of ducks from the farm to the slaughterhouse, during processing, or in live poultry markets, which play a critical role in the epidemiology and evolution dynamics of HPAIVs in many low- and middle-income countries [Citation46].
By identifying another route of viral shedding for ducks, these results open the way to a paradigm shift in the epidemiology of avian influenza. Transmissibility is influenced by viral shedding and environmental stability [Citation6]. Infectious virions shed by the feather epithelium may be protected from physical factors or even disinfectants, resulting in an unexpected persistence of infectivity in the environment. However, whether any particular resistance properties are associated with this route of excretion, thanks to the protection provided by cornified cells, endogenous or uropygial lipids, still needs to be clarified [Citation16,Citation47,Citation48].
Combining natural and experimental infections, our findings support the environmental shedding and dissemination of H5 HPAIVs through the infected plumage of domestic ducks, which may constitute an underestimated route of transmission. Further investigations are needed to establish the importance of this alternative route compared to the fecal-oral and the respiratory-aerosol routes, and to define its impact in the implementation of biosecurity measures.
Supplemental Material
Download ()Acknowledgments
The authors would like to thank Manel Vinyes (GALLSA) for providing the ducks for the experimental infections, and Miquel Nofrarías, Maria José Valdez, and Rosa Valle for their technical assistance. We thank Thomas Figueroa, Pierre Bessière and Romain Volmer for providing viral stock of reverse genetics-engineered virus. We would like to express our sincere gratitude to Professor Lorenzo Alibardi from the University of Bologna for providing primary antibody aliquots for the detection of corneous beta proteins. We gratefully acknowledge Cell Imaging Facility of INFINITy for access and assistance to the confocal microscope. The authors are thankful to Ms Aurore Mathon and Ms Grace Delobel for the design of the graphical Abstract and the English proofreading, respectively.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
The data supporting the findings of this study are available within the article and its supplementary materials.
Additional information
Funding
References
- World Organization for Animal Health (WOAH). Avian Influenza [Internet]. Available from: https://www.woah.org/en/disease/avian-influenza/.
- Shi J, Zeng X, Cui P, et al. Alarming situation of emerging H5 and H7 avian influenza and effective control strategies. Emerg Microbes Infect. 2023;12:2155072.
- Pantin-Jackwood MJ, Costa-Hurtado M, Bertran K, et al. Infectivity, transmission and pathogenicity of H5 highly pathogenic avian influenza clade 2.3.4.4 (H5N8 and H5N2) United States index viruses in Pekin ducks and Chinese geese. Vet Res. 2017;48(1):33. doi:10.1186/s13567-017-0435-4
- Germeraad EA, Sanders P, Hagenaars TJ, et al. Virus shedding of avian influenza in poultry: A systematic review and meta-analysis [Internet]. Viruses. 2019;11(9):812. doi:10.3390/v11090812. Available from: https://www.mdpi.com/1999-4915/11/9/812.
- Beerens N, Germeraad EA, Venema S, et al. Comparative pathogenicity and environmental transmission of recent highly pathogenic avian influenza H5 viruses. Emerg Microbes Infect. 2021;10(1):97–108. doi:10.1080/22221751.2020.1868274
- Pantin-Jackwood MJ, Swayne DE. Pathogenesis and pathobiology of avian influenza virus infection in birds. OIE Rev Sci Tech. 2009;28(1):113–136. doi:10.20506/rst.28.1.1869
- Yamamoto Y, Nakamura K, Kitagawa K, et al. Pathogenesis in call ducks inoculated intranasally with H5N1 highly pathogenic avian influenza virus and transmission by oral inoculation of infective feathers from an infected call duck. Avian Dis. 2007;51(3):744–749. doi:10.1637/0005-2086(2007)51[744:PICDII]2.0.CO;2
- Yamamoto Y, Nakamura K, Okamatsu M, et al. Detecting avian influenza virus (H5N1) in domestic duck feathers. Emerg Infect Dis. 2008;14(10):1671–1672. doi:10.3201/eid1410.080415
- Nuradji H, Bingham J, Payne J, et al. Highly pathogenic avian influenza (H5N1) virus in feathers. Vet Pathol. 2017;54(2):226–233. doi:10.1177/0300985816666608
- Bertran K, Dolz R, Busquets N, et al. Pathobiology and transmission of highly and low pathogenic avian influenza viruses in European quail (Coturnix c. coturnix). Vet Res. 2013;44(1):1–11. doi:10.1186/1297-9716-44-23
- Bertran K, Pérez-Ramírez E, Busquets N, et al. Pathogenesis and transmissibility of highly (H7N1) and low (H7N9) pathogenic avian influenza virus infection in red-legged partridge (Alectoris rufa). Vet Res. 2011;42(1):1–9. doi:10.1186/1297-9716-42-24
- Busquets N, Abad FX, Alba A, et al. Persistence of highly pathogenic avian influenza virus (H7N1) in infected chickens: feather as a suitable sample for diagnosis. J Gen Virol. 2010;91(9):2307–2313. doi:10.1099/vir.0.021592-0
- Terrill RS, Shultz AJ. Feather function and the evolution of birds. Biol Rev. 2023;98(2):540–566. doi:10.1111/brv.12918
- Alibardi L. Review: cornification, morphogenesis and evolution of feathers. Protoplasma. 2017;254(3):1259–1281. doi:10.1007/s00709-016-1019-2
- Yamamoto Y, Nakamura K, Yamada M, et al. Persistence of avian influenza virus (H5N1) in feathers detached from bodies of infected domestic ducks. Appl Environ Microbiol. 2010;76(16):5496–5499. doi:10.1128/AEM.00563-10
- Karunakaran AC, Murugkar HV, Kumar M, et al. Survivability of highly pathogenic avian influenza virus (H5N1) in naturally preened duck feathers at different temperatures. Transbound Emerg Dis. 2019;66(3):1306–1313. doi:10.1111/tbed.13148
- Ahaduzzaman M, Milan L, Morton CL, et al. Characterization of poultry house dust using chemometrics and scanning electron microscopy imaging. Poult Sci. 2021;100(7):101188. doi:10.1016/j.psj.2021.101188
- Cambra-López M, Hermosilla T, Lai HTL, et al. Particulate matter emitted from poultry and Pig houses: source identification and quantification. Trans ASABE. 2011;54(2):629–642. doi:10.13031/2013.36466
- Zhao Y, Aarnink AJA, De Jong MCM, et al. Airborne microorganisms from livestock production systems and their relation to dust. Crit Rev Environ Sci Technol. 2014;44(10):1071–1128. doi:10.1080/10643389.2012.746064
- Filaire F, Lebre L, Foret-Lucas C, et al. Highly pathogenic avian influenza A(H5N8) clade 2.3.4.4b virus in dust samples from poultry farms, France, 2021. Emerg Infect Dis. 2022;28(7):1446–1450. doi:10.3201/eid2807.212247
- James J, Warren CJ, De Silva D, et al. The role of airborne particles in the epidemiology of clade 2.3.4.4b H5N1 high pathogenicity avian influenza virus in commercial poultry production units. Viruses. 2023;15(4):1002. doi:10.3390/v15041002
- Spekreijse D, Bouma A, Koch G, et al. Quantification of dust-borne transmission of highly pathogenic avian influenza virus between chickens. Influenza Other Respi Viruses. 2013;7(2):132–138. doi:10.1111/j.1750-2659.2012.00362.x
- Wang CC, Prather KA, Sznitman J, et al. Airborne transmission of respiratory viruses. Science. 2021;80:373.
- Bessière P, Figueroa T, Coggon A, et al. Opposite outcomes of the within-host competition between high- and Low-pathogenic H5N8 avian influenza viruses in chickens compared to ducks. Subbarao K, editor. J Virol. 2022;96(1):1–16. Available from: https://journals.asm.org/doi/10.1128/JVI.01366-21.
- Gaide N, Crispo M, Jbenyeni A, et al. Validation of an RNAscope assay for the detection of avian influenza A virus. J Vet Diagnostic Investig. 2023: 1–7.
- Yue Z, Jiang T-X, Widelitz RB, et al. Mapping stem cell activities in the feather follicle. Nature [Internet]. 2005;438(7070):1026–1029. doi:10.1038/nature04222. Available from: https://www.nature.com/articles/nature04222.
- Landmann M, Scheibner D, Graaf A, et al. A semiquantitative scoring system for histopathological and immunohistochemical assessment of lesions and tissue tropism in avian influenza. Viruses. 2021;13(5):868. doi:10.3390/v13050868
- Joiner KS, Spangler EA. Evaluation of HistoGelTM-embedded specimens for use in veterinary diagnostic pathology. J Vet Diagn Invest. 2012;24(4):710–715. doi:10.1177/1040638712445763
- Alibardi L. Immunodetection of type I acidic keratins associated to periderm granules during the transition of cornification from embryonic to definitive chick epidermis. Micron. 2014;65:51–61. doi:10.1016/j.micron.2014.04.002
- Matrosovich M, Matrosovich T, Garten W, et al. New low-viscosity overlay medium for viral plaque assays. Virol J. 2006;3(1):1–7. doi:10.1186/1743-422X-3-63
- Spackman E, Pantin-Jackwood MJ, Lee SA, et al. The pathogenesis of a 2022 North American highly pathogenic clade 2.3.4.4b H5N1 avian influenza virus in mallards (Anas platyrhynchos). Avian Pathol. 2023;52(3):219–228. doi:10.1080/03079457.2023.2196258
- Gaide N, Foret-Lucas C, Figueroa T, et al. Viral tropism and detection of clade 2.3.4.4b H5N8 highly pathogenic avian influenza viruses in feathers of ducks and geese. Sci Rep. 2021;11(1):5928. doi:10.1038/s41598-021-85109-5
- Wille M, Bröjer C, Lundkvist Å, et al. Alternate routes of influenza A virus infection in Mallard (Anas platyrhynchos). Vet Res. 2018;49(1):110. doi:10.1186/s13567-018-0604-0
- Perkins LEL, Swayne DE. Pathobiology of A/chicken/Hong Kong/220/97 (H5N1) avian influenza virus in seven gallinaceous species. Vet Pathol. 2001;38(2):149–164. doi:10.1354/vp.38-2-149
- Nuradji H, Bingham J, Lowther S, et al. A comparative evaluation of feathers, oropharyngeal swabs, and cloacal swabs for the detection of H5N1 highly pathogenic avian influenza virus infection in experimentally infected chickens and ducks. J Vet Diagnostic Investig. 2015;27(6):704–715. doi:10.1177/1040638715611443
- Leeson S, Walsh T. Feathering in commercial poultry I. Feather growth and composition. Worlds Poult Sci J. 2004;60(1):42–51. doi:10.1079/WPS20033
- Abrahams M. Bird-feather counting: small result when the feathers fly. Guard [Internet]. 2014; Available from: https://www.theguardian.com/education/2014/dec/16/bird-feather-counting-small-result.
- Baéza E, Chartrin P, Bordeau T, et al. Omega-3 polyunsaturated fatty acids provided during embryonic development improve the growth performance and welfare of Muscovy ducks (Cairina moschata). Poult Sci. 2017;96(9):3176–3187. doi:10.3382/ps/pex147
- Beltran RS, Burns JM, Breed GA. Convergence of biannual moulting strategies across birds and mammals. Proc R Soc B Biol Sci. 2018;285(1878):20180318. doi:10.1098/rspb.2018.0318
- Kiat Y, Vortman Y, Sapir N. Feather moult and bird appearance are correlated with global warming over the last 200 years. Nat Commun. 2019;10(1):2540. doi:10.1038/s41467-019-10452-1
- Dong Y. Injurious pecking behavior of Pekin ducks on commercial farms: characteristics, development and duck welfare. Faculty of Purdue University; 2019.
- Calnek BW, Adldinger HK, Kahn DE. Feather follicle epithelium: a source of enveloped and infectious cell-free herpesvirus from Marek’s disease. Avian Dis. 1970;14(2):219–233. doi:10.2307/1588466
- Davidson I, Artzi N, Shkoda I, et al. The contribution of feathers in the spread of chicken anemia virus. Virus Res. 2008;132(1-2):152–159. doi:10.1016/j.virusres.2007.11.012
- Dadonaite B, Vijayakrishnan S, Fodor E, et al. Filamentous influenza viruses. J Gen Virol. 2016;97(8):1755–1764. doi:10.1099/jgv.0.000535
- Delogu M, De Marco MA, Di Trani L, et al. Can preening contribute to influenza A virus infection in wild waterbirds? Brown J, editor. PLoS One [Internet]. 2010;5(6):e11315. Available from: https://dx.plos.org/10.1371/journal.pone.0011315.
- Brooks JW. Postmortem changes in animal carcasses and estimation of the postmortem interval. Vet Pathol. 2016;53(5):929–940. doi:10.1177/0300985816629720
- Moraleda V, Gómez-Catasús J, Schuster C, et al. Decomposition stages as a clue for estimating the post-mortem interval in carcasses and providing accurate bird collision rates. Sci Rep. 2022;12(1):16188. doi:10.1038/s41598-022-20628-3
- Bertran K, Balzli C, Kwon Y-K, et al. Airborne transmission of highly pathogenic influenza virus during processing of infected poultry. Emerg Infect Dis. 2017;23(11):1806–1814. doi:10.3201/eid2311.170672

