ABSTRACT
Recurrent opportunistic infections (OIs) in patients with severely immunosuppressed AIDS remain an unresolved medical challenge despite advancements in antiretroviral therapy (ART). To address this gap, we developed an HLA-mismatched allogeneic adoptive immune therapy (AAIT) specifically targeting this patient population. The safety and efficacy of this novel therapeutic approach were preliminarily confirmed in our phase 1 trial. Subsequently, a multicenter, open-label, controlled, phase 2a trial was conducted to evaluate the efficacy of AAIT in combination with ART compared with the conventional ART-only regimen. No difference in the incidence of adverse events (AEs) was observed between the two groups at the 96-week follow-up. AAIT treatment improved CD4+ T cell recovery at weeks 72 (P = 0.048) and 96 (P = 0.024) compared to the Control Group. Additionally, stratified analysis of patients in the AAIT Group showed that donor/recipient sex mismatch was significantly associated with the likelihood of patients achieving an immunological response (OR = 8.667; 95% CI, 2.010–37.377; P = 0.004). These findings suggest that AAIT serves as a promising adjunct therapy for improving the outcomes of patients with severely immunosuppressed AIDS. Further studies are needed to elucidate the immunological mechanisms underlying AAIT and identify the subpopulations that respond optimally to this therapeutic approach. This trial is registered at www.clinicaltrials.gov (NCT04098770).
Trial registration: ClinicalTrials.gov identifier: NCT04098770.
Trial registration: ClinicalTrials.gov identifier: NCT02651376.
Introduction
Human immunodeficiency virus-1 (HIV-1) replication leads to the progressive loss of CD4+ T cells and immune disorders. The Universal Test and Treat strategy proposed by the World Health Organization in 2015 markedly curtailed viral transmission and enhanced patient well-being. However, a significant subset of patients are diagnosed in the advanced stages of AIDS, often accompanied by complex opportunistic infections (AIDS stages 3/4), with their immune systems critically impaired [Citation1–4]. These symptoms manifest in drastically reduced CD4 counts, which are sometimes <50 cells/μL. Patients with severely compromised immune systems due to AIDS frequently experience complicated opportunistic infections (OIs), incomplete immune reconstitution, and even mortality despite effective antiretroviral therapy (ART) [Citation5–11]. Thus, patients with severely immunosuppressed AIDS require continuous monitoring and additional therapeutic intervention to promote immune recovery and reduce the incidence of HIV and non-AIDS related events. For example, anti-inflammatory drugs such as 5-hydroxytriptolide and metformin are used in the treatment of HIV immunological non-responders to promote immune recovery [Citation12, Citation13].
In the era preceding combination ART, therapeutic endeavours, such as HLA-matched lymphocytes or stem cell transplantation, were experimentally administered to patients with AIDS [Citation14–17]. Nonetheless, these interventions failed to facilitate immunological reconstitution, primarily due to the lack of antiviral therapy capable of curtailing HIV-1 replication. More recently, research efforts have been directed toward exploring the applicability of genetically modified CD4+ T and hematopoietic stem cells (HSCs) derived from either autologous sources or HLA-matched donors in reinstating the immunological response in individuals infected with HIV-1 [Citation18, Citation19]. For eligibility, participants must exhibit a positive response to ART and maintain adequate CD4+ T cell counts (generally >200 cells/μL). However, to date, therapeutic approaches tailored to the requisites of patients with immunosuppressed AIDS, especially those with CD4+ T cell counts <50 cells/μL, are lacking. Instances of successful “HIV cure” have been documented in patients who underwent allogeneic HLA-matched or haploidentical stem cell transplantations for treating HIV alongside leukemia or lymphoma [Citation20–22]. However, allogeneic transplantation in HIV-infected individuals without hematopoietic malignancies remains impractical. To date, there are no effective immune interventions capable of fully restoring overall immunity in patients with advanced AIDS.
Haploidentical stem cell transplantation (haplo-SCT), an alternative donor source, offers additional opportunities for patients who are transplant candidates [Citation23–25]. On this basis, we have established a new transplantation strategy, micro-transplantation, which does not require immunosuppressive pre-conditioning, avoids clinical GVHD, but enables the formation of minimal donor chimerism and rapid restoration of donor immune function in AML patients [Citation26, Citation27]. Further, we applied micro-transplantation to severely immunosuppressed AIDS patients, aiming to improve their ability to fight against opportunistic infections and immune recovery (AAIT, NCT02651376). The phase I study showed that AAIT was safe and beneficial for better immune restoration for severely immunosuppressed AIDS patients [Citation28]. Consequently, we conducted a two-arm, open-label, multicenter, phase 2a trial to further investigate the safety and efficacy of AAIT in conjunction with the ART regimen in patients with severely immunosuppressed AIDS with CD4+ T cell counts <50 cells/μL.
Methods
Study design, participants, and donors
This parallel-group, open-label, phase 2a trial commenced in October 2019 across three centers in China and enrolled patients with severely immunosuppressed AIDS. The objective of this study was to evaluate the safety and efficacy of AAIT in improving immune reconstitution. The inclusion criteria were as follows: (1) aged between 18 and 65 years, (2) patients with advanced AIDS who are experiencing various opportunistic infections, (3) CD4 count <50 cells/μL before entry and at screening, and (4) sign the informed consent form and refrain from participating in any other clinical trials during this period. Patients were excluded for any of the following reasons: (1) severe organic lesions unrelated to AIDS, (2) HIV-2 infection, (3) allergies to blood products, (4) undergoing long-term immunosuppressive therapy, (5) malignant tumours, or (6) poor adherence to antiviral therapy. After screening for inclusion and exclusion criteria, patients will be assigned to either the AAIT Group or the Control Group based on treatment intention and whether a suitable donor has been screened.
Healthy donors were selected from the HLA-mismatched relatives of the enrolled recipients by typing their HLA haplotypes. Before transplantation, donor and recipient HLA-A, -B, -C, -DRB1, and -DQB1 loci were typed using polymerase chain reaction (PCR) with a sequence-specific primer method [Citation27]. As previously described, each donor underwent physical examinations and laboratory tests, which mainly included blood routine, urine routine and stool routine, liver and kidney function. Donors with viral infections (including human immunodeficiency virus [HIV], hepatitis A virus [HAV], hepatitis B virus [HBV], hepatitis C virus [HCV], hepatitis E virus [HEV], Epstein–Barr virus [EBV], cytomegalovirus [CMV], and Treponema pallidum) were excluded.
We collected demographic and clinical data, HIV transmission category, ART history, opportunistic diseases, comorbidities, and serological and immunological data from the participants. The study protocol was approved by the institutional review board of each study center. This study adhered to the Declaration of Helsinki and ICH Good Clinical Practice guidelines. All the participants provided written informed consent.
Procedures
The AAIT protocol have been reported previously [Citation28], including: (1) screening and selection of healthy donors; (2) G-CSF mobilization; (3) G-MNCs leukapheresis; and (4) G-MNCs transfusion (A). Depending on the needs of the recipients and availability of G-MNCs, two to four rounds of transfusion were performed. All transfusions were administered intravenously infusion in arms.
Figure 1. Study design. (A) Flowchart of clinical trial design. After donor screening, G-CSF mobilization and G-MNCs leukapheresis, patients underwent G-MNCs transfusion. Antimicrobial therapy and ART were given as necessary before or after enrolment. (B) Consolidated standards of reporting trials diagram. G-CSF, granulocyte-colony-stimulating factor; G-MNCs, G-CSF mobilized mononuclear cells; ART, antiretroviral therapy.
Patients attended follow-up checkups for 96 weeks from the beginning of the study. All patients continued to receive ART during the treatment and follow-up periods. Patients who did not attend the last follow-up checkpoint (96 weeks) were considered lost to follow-up. Clinical safety was assessed via interim medical history and physical examination by reporting adverse reactions, such as skin disorders, otolaryngology disorders, cardiac disorders, gastrointestinal disorders, and other disorders (such as fever, fatigue, and insomnia), as well as the occurrence of opportunistic infections and tumours. All patients underwent regular examinations for liver and kidney function, routine blood tests, coagulation markers, virological and immunological parameters at baseline, 1, 2, 4, 8, 12, 24, 36, 48, 60, 72, 84, and 96 week follow-up.
The primary outcome was the variation in CD4+ T cell counts, which were continuously monitored to evaluate immune reconstitution. Secondary outcomes included alterations in CD8+ T cell counts, CD4/CD8 ratio, plasma HIV RNA levels, and incidence of treatment-related adverse events.
Safety evaluation
Clinical safety was assessed via physical examination, measurement of vital signs, laboratory analyses, and monitoring of adverse events and concomitant medications during the study, the criteria for monitoring AEs were accorded to the Common Terminology Criteria for Adverse Events (CTCAE) Version 5.0. An independent safety-monitoring committee reviewed the data and recorded all AEs during the study. Participants were monitored for 24 h post-transfusion for immediate reactions. Participants then used diary cards to record local (pain or tenderness, erythema, and swelling or induration) and systemic (pyrexia or fever, fatigue, headache, nausea, myalgia, and chills) adverse events on the day of injection and for the subsequent 7 days.
Statistical analysis
Descriptive analyses of the demographic and baseline characteristics were conducted for all individuals within the per-protocol set (PPS). Continuous variables are presented as median (interquartile range), and differences between groups were evaluated using the Mann–Whitney U test. Categorical data were reported as frequencies (percentages) and analyzed via the χ2 or Fisher’s exact test, as appropriate. To handle missing data, the last evaluable assessment was carried forward to an endpoint analysis (the LOCF method). Generalized estimating equations (GEE) were employed to examine the impact of treatment intervention, with interaction terms (time vs. treatment group) formulated to investigate the significance of changes across the treatment cohorts.
Kaplan–Meier analysis was used to categorize distinct treatment strategies and calculate the cumulative probability of an immunological response. Furthermore, the log-rank test was used to determine the statistical significance of differences in hazard rates across groups.
Binary logistic regression was applied to explore the association between a suite of independent predictors and dichotomous outcome variables. To ensure the stability and reliability of the regression estimates, only predictors exhibiting a variance inflation factor (VIF) <10 and tolerance level >0.1 were incorporated into the analysis. A Forward Stepwise (Likelihood Ratio) approach was adopted for variable selection in the multivariate regression framework.
Statistical tests for significance were bidirectional, with a p-value of <0.05 indicating statistical significance. The analytical procedures were performed using SPSS version 25.0 and GraphPad Prism (version 9.0).
Results
Patient characteristics
From October 2019 to December 2021, 96 patients were screened for eligibility at three centers in China; of which, 71 were recruited for the study. Of the included participants, 48 were assigned to the AAIT combined with ART treatment group (AAIT Group) and 23 were assigned to the conventional ART treatment group (Control Group) (). In the AAIT Group, two (4.17%) patients received G-MNCs from multiple donor sources. Seven (14.58%) participants in the AAIT Group and one (4.35%) in Control Group were not included in the PPS owing to a lack of 96-week follow-up data. Finally, 39 participants (81.25%) in the AAIT Group and 22 participants (95.65%) in the Control Group completed 96 weeks of follow-up.
The baseline characteristics of the 61 participants included in the PPS are summarized (). The clinical variables, including age, sex, weight, BMI, HIV transmission route, viral load, immune parameters (CD4+T cell count, CD8+T cell count, CD4/CD8 ratio), routine blood and blood biochemistry parameters, proportion of clinical events and symptoms, and first-line treatment regimen, were all balanced between the two study arms. Upon enrollment, all patients exhibited 2–6 severe OIs, including Pneumocystis jirovecii pneumonia, cryptosporidiosis, tuberculosis, candidiasis, herpes simplex, herpes zoster infection, Talaromyces marneffei infection, and cryptococcosis. Notably, a subset of the patients displayed detectable levels of CMV (>100 IU/mL) and EBV nucleic acids (>100 IU/mL) in their peripheral blood samples. The prevalence of OIs was statistically equivalent across the study cohorts, and the incidences of concurrent conditions such as syphilis, hepatitis B, hepatitis C, anemia, and sepsis were similarly distributed between the groups.
Table 1. Baseline characteristics of 61 patients with severely immunosuppressed AIDS in the per-protocol analysis set.
Safety assessment
All the 71 participants were included in the safety analysis set and no deaths were observed up to 96 weeks, and a total of 65 patients (91.5%) reported at least one adverse effect after treatment: 45 (93.8%) in the AAIT Group and 20 (87.0%) in the Control Group, with no significant difference between the two groups (P = 0.381). The most frequent immune-related AEs in the participants who received AAIT were fever, rash, nausea, fatigue, vertigo, myalgia, anemia, and diarrhea. Laboratory abnormalities were similar between the two groups, including increased triglyceride, total cholesterol, urinary protein, thrombocytosis, thrombocytopenia, serum glucose, blood urea nitrogen, and transaminase levels. Grade 3 or 4 treatment-related AEs occurred in 10.4% of the participants in the AAIT Group and 8.7% of the participants in the Control Group. This safety profile was consistent with that of our previous study, with no change in the incidence or severity of AEs after long-term follow-up (). Grade 3 or 4 AEs that occurred in participants in both groups were related to fever or anemia, with five participants (10.4%) in the AAIT Group experiencing a fever of ≥40°C for up to 24 h, which could not be dismissed as irrelevant to the infusion of G-MNCs. In contrast, two participants (8.7%) in the Control Group developed a severe anemic condition and received an erythrocyte infusion. In addition, preexisting opportunistic infections were well controlled throughout the follow-up period after the patient’s initial discharge from the hospital, and no additional AIDS-defining tumours were observed. These observations indicate that G-MNC transfusion is clinically and biologically well-tolerated in patients.
Table 2. Selected treatment-emergent adverse events (AEs) and laboratory abnormalities observed in 71 patients of the safety-analysis-set.
Plasma HIV-1 RNA loads were monitored during a follow-up period of up to 96 weeks. Except for two patients in the AAIT Group who were HIV RNA-negative at baseline, the remaining 37 and 22 participants in the AAIT and control groups, respectively, were monitored for post-treatment HIV RNA conversion. The proportion of individuals with plasma HIV-1 RNA concentrations of less than 50 copies/mL at weeks 12, 24, 36, 48, 72, and 96 was found to be statistically indistinguishable between the two groups (supplemental Table 1).
Assessment of clinical manifestation after AAIT
Patients with severely immunosuppressed AIDS are frequently burdened with complex OIs. The use of clinical medications was well documented for all participants, and the use of various anti-infective drugs was based on the latest or recent clinical guidelines at the time of enrolment [Citation29, Citation30]. All participants were treated with sulfamethoxazole and trimethoprim (SMZ-TMP) to prevent OIs. Three participants discontinued the SMZ-TMP because of adverse reactions, two in the AAIT Group and one in the Control Group. All the participants received close monitoring of clinically relevant outcomes, including mortality, emergence of additional symptoms, hospitalization, and frequency and duration of readmission (supplemental Figure 1). In the AAIT Group, a marginal reduction was observed in both the average number of readmission per patient (49.88%, P = 0.222) and the average duration of readmission per capita (33.13%, P = 0.353), although these differences were not statistically significant when compared with the Control Group (). Furthermore, AAIT treatment did not necessitate frequent changes in ART regimens, underscoring its safety profile.
Table 3. Comparative Clinical Outcomes in AAIT and Control Groups for HIV Management.
AAIT facilitates immune restoration
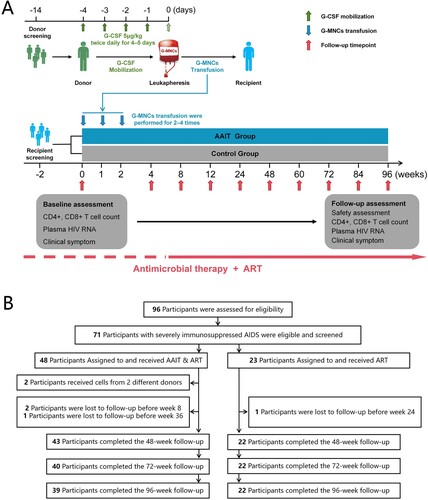
The CD4+ T cell count, CD8+ T cell count, and CD4/CD8 ratio were closely monitored in both groups during the follow-up period of up to 96 weeks (A–C). In the AAIT Group, patients demonstrated a significantly enhanced recovery of CD4+ T cells at weeks 72 [201.00 (145.00, 273.00) versus 135.50 (112.75, 217.75), P = 0.048] and 96 [239.00 (144.00, 307.00) versus 156.00 (123.00, 227.75), P = 0.024], relative to the Control Group. No significant differences were found between the two groups in terms of dynamic changes in CD8+ T cell counts and CD4/CD8 ratios. Moreover, the study compared the differences in ΔCD4+ T cell count, ΔCD8+ T cell count, and ΔCD4/CD8 ratio from baseline to various time points between the two groups (D–F). The patterns of change in ΔCD4+ T cell count and ΔCD4/CD8 ratio were similar to those observed for CD4+ T cell count and CD4/CD8 ratio. Notably, the AAIT Group exhibited a more pronounced increase in ΔCD8+ T cell count compared to the Control Group at weeks 4, 8, and 96.
Figure 2. Differences in CD4+ T cell counts, CD8+ T cell counts, and CD4/CD8 ratio between the two groups at each follow-up point. (A) CD4+ T cell counts; (B) CD8+ T cell counts; (C) CD4/CD8 ratios; (D) ΔCD4+ T cell counts; (E) ΔCD8+ T cell counts; (F) ΔCD4/CD8 ratios. Continuous variables are presented as median (interquartile range), and differences between groups were evaluated using the Mann–Whitney U test.
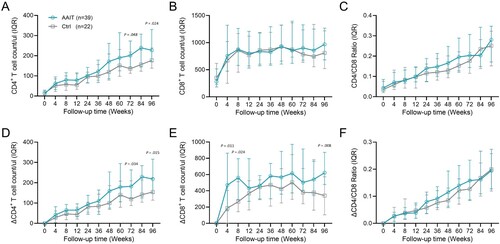
Next, generalized estimation equation (GEE) was used to investigate whether AAIT treatment prompted an increase in the CD4+ T cell count in participants. The result showed no interaction between group and time (χ2 group * time = 13.853, P = 0.128) (A). Therefore, a separate time-effect analysis was performed. The results showed statistically significant (P < 0.05) increases in CD4+ T cell counts at all follow-up time points compared with week 4 within the two groups, except for week 12 follow-up points in the Control Group (P = 0.151) (supplemental Table 2). A separate analysis of the between-group effect showed that the difference in incremental CD4+ T cell counts between the two groups became apparent at week 60 (supplemental Table 3). The AAIT Group’s CD4+ T cell count showed a mean increase of 42.63 cells/μL (P = 0.087), 48.01 cells/μL (P = 0.034), 43.11 cells/μL (P = 0.125), and 67.18 cells/μL (P = 0.011) at weeks 60, 72, 84, and 96, respectively, compared to that of the Control Group. This progression suggests an improvement in CD4+ T cell counts over time in the AAIT Group compared with the Control Group.
Figure 3. Dynamic changes of immune recovery in AAIT and Control groups during 96-week follow-up. (A) Time-group interaction analysis of CD4+ T cell count differences between AAIT and Control Groups. (B) Kaplan–Meier Survival Analysis of Cumulative Immunological Response Probability on patients in AAIT Group compared to those in the Control.
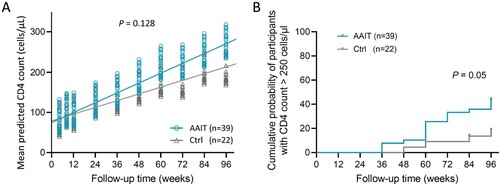
Kaplan–Meier curve and Log-rank test were used to compare the cumulative probability of immunological response (two consecutive CD4 counts >250 cells/μL and plasma HIV RNA <50 copies/mL) within 96 weeks between the two groups. The distribution of censoring values was similar between the groups. The Log-rank test results showed a statistically significant difference in the immunological response of patients in different groups (χ2 = 3.837, P = 0.05) (B).
Beneficial factors for achieving immunological response
To reveal the characteristics of donors and recipients who benefited from AAIT treatment, we analyzed the donor-recipient-related information of 39 patients in the AAIT Group, including donor age, degree of donor-recipient HLA locus matching, donor-recipient blood type and matching, and donor-recipient sex matching, the specific information as listed (supplemental Table 4).
Factors associated with the immunological response (IR) at week 96 were analyzed using binary logistic regression. Univariate regression analysis showed that donor sex, recipient BMI, and donor-recipient sex matching were independent factors for achieving an immunological response after AAIT treatment (). Multivariate logistic regression analysis was performed and the results showed that among the above mentioned variables, donor–recipient sex mismatch alone was significantly associated with the attainment probability of IR (OR = 8.667; 95% CI, 2.010–37.377; P = 0.004). The recipient’s BMI and donor’s sex were not included in the model, indicating that they were not significantly associated with the attainment probability of IR.
Table 4. Factors contributing to the benefit of achieving an immune response in 39 patients of the AAIT group.
Discussion
According to the The Joint United Nations Program on HIV/AIDS, 6,30,000 people died of HIV-related illnesses worldwide in 2022 [Citation31]. Alarmingly, approximately 5% of recently diagnosed HIV-1 cases with baseline CD4+ T cell counts below 50 cells/μL succumbed within the first year. The complexity of OIs indicates that some patients with severely immunosuppressed AIDS are unable to tolerate ART and are at an increased risk of early mortality. Moreover, even with effective ART, only 30% of patients whose baseline CD4+ T cell counts are under 100 cells/μL can achieve complete immune restoration [Citation3]. The profound impact of CD4+ T cell count on the rate of AIDS-related deaths or deterioration, regardless of viral load in HIV-infected individuals who have not yet received ART, underscores the critical importance of recovering CD4+ T cell counts to improve patient outcomes [Citation32]. To date, no successful immune intervention has been established that can be administered alongside ART to counteract the widespread damage to holistic immunity in patients with severely immunosuppressed AIDS at high risk of compromised immune reconstitution and increased mortality.
In this report, we presented the findings of a phase II clinical trial utilizing AAIT administered in conjunction with a standard ART regimen to patients with severely immunosuppressed AIDS. Adoptive transfer of G-MNCs from HLA-mismatched healthy donors was well tolerated. Notably, compared with the Control Group, patients undergoing AAIT experienced a better increase in the CD4+ T cell count after the 96-week follow-up period. Stratified analysis revealed that patients who received G-MNCs from sex-mismatched donors exhibited an even greater increase in CD4+ T-cell counts. These findings highlighted the potential of innovative cell-based immunotherapies. Our study suggests that AAIT could serve as a viable adjunct therapy alongside conventional ART and anti-infection therapy for patients with severely immunosuppressed AIDS, offering novel avenues for enhancing patient outcomes.
The efficacy observed in patients undergoing transplantation with reduced-intensity conditioning aimed at curing HIV challenges the previously held belief that completely eradicating both adaptive and innate immune responses is essential in patients with AIDS [Citation33]. This observation suggests that the unique clinical scenario of patients with severely immunosuppressed AIDS may facilitate the acceptance of allogeneic G-MNCs from donors. Furthermore, reports suggest that the transfer of HLA-mismatched allogeneic leukocytes could trigger a graft-versus-viral reservoir effect in patients with non-ablated AIDS undergoing cART, thereby assisting in the restoration of host HIV-specific immune defenses [Citation34, Citation35].
HLA-mismatched allogeneic cell therapy has emerged as a promising solution to overcome the limitations in the availability of cell therapy donors and mitigate the risk of GVHD reaction [Citation26, Citation36, Citation37]. This is corroborated by our AE monitoring, which documented no instances of chronic or acute GVHD and observed no alteration in the frequency or severity of AEs following extended follow-up periods. Furthermore, comparisons of AEs between the donor-recipient sex-matched and sex-mismatched groups revealed no significant differences in the incidence rates of AEs of any grade or grade 3–4 AEs (supplemental Table 5). This emphasized that the efficacy of the therapy did not compromise its safety.
In the phase I clinical study, we evaluated microchimerism levels in patients’ peripheral blood for up to 50 days after receiving haploidentical donor AAIT treatment, identifying minimal donor-derived cells 1 week after treatment [Citation28]. Given the lack of bone marrow biopsies and the absence of both early and long-term GVHD throughout the 96-week follow-up period, it was inferred that HSC engraftment was either non-existent or suboptimal. This suggests that AAIT likely facilitates long-term immune recovery by initially modulating the overall immune environment of patients with severely immunosuppressed AIDS. Additionally, dynamic changes in routine blood parameters were recorded between the two groups of patients throughout the study. The results indicated that in the early phase of treatment (2–4 weeks), a slight increase in the levels of white blood cells, lymphocytes, and monocytes was observed in the AAIT Group compared with those in the Control Group (supplemental Figure 2). These findings suggest the potential of early immune modulation interventions to promote sustained immune health.
Various immunotherapy methods have been examined to boost CD4+ T cell counts and improve clinical outcomes in advanced AIDS patients. The SILCAAT study, involving 1695 patients, tested IL-2 plus ART versus ART alone over 7–8 years and found no clinical benefit despite a small increase in CD4+ cell count [Citation38]. Meanwhile, Lévy’s team conducted a trial with recombinant human IL-7 (rhIL-7) that showed dose-dependent increases in CD4 T-cell numbers and TCR diversity with good tolerance [Citation39]. However, another trial indicated that IL-7 led to a significant rise in CD4+ T cells with integrated HIV DNA, deeming it unsuitable for HIV eradication efforts due to its potential to expand HIV reservoirs [Citation40]. In contrast, our investigation offers novel insights: first, AAIT is safe and capable of diminishing the viral load independently of ART, as evidenced by our phase I study [Citation28]. Second, although there were no fatalities among participants in both the AAIT and Control Groups, AAIT proved effective in lowering inflammatory markers (data not shown). This finding prompted further exploration of the prolonged impact on non-AIDS related incidents.
Although the recovery of CD4+ T cell counts was not significantly superior in the AAIT Group compared to the Control Group, stratified analysis highlighted a significant improvement in CD4+ T cell counts in patients who received G-MNCs from sex-mismatched donors, suggesting that AAIT therapy may have a long-term benefit independent of HSC transplantation. Although the exact mechanism – related to microchimerism, engraftment, or enhancement of antiviral immunity – remains uncertain, research led by Huang et al. has identified that the age of both donor and recipient, the transfer from female donors to male recipients, and significant ABO mismatches constitute the primary risk factors for transplant-related mortality in patients receiving transplants from HLA haplotype-matched relatives [Citation41]. Considering the influence of sex compatibility on immune recovery and the induction of immunosuppressive cells in donors following G-CSF mobilization, it is hypothesized that the functional differences in cells donated by males and females could play a crucial role. Additionally, insights from H-Y antibody-related studies have shed light on this issue. Miklos and colleagues tested 121 patients undergoing HSCT for IgG antibodies against mHAs encoded by genes on the Y chromosome. The presence of H-Y antibody was found to be associated with chronic GVHD and the maintenance of remission in 75 male patients with hematological malignancies who received female HSC transplantation [Citation42].
This investigation is subject to several limitations, notably the limited sample size, which undermines the robustness of our conclusions. Incomplete long-term follow-up of safety and efficacy introduces uncertainty regarding the enduring effects of the intervention. Additionally, the efficacy of AAIT might be affected by different ART regimens, which was not thoroughly addressed by current data. Furthermore, the specific mechanisms by which sex-mismatched donors confer benefits remain unclear, highlighting a significant gap in our understanding of sex-specific influences.
In summary, the findings of this study underscore the sustained positive effect of AAIT on immune restoration in patients with severe AIDS-induced immunosuppression. Given the complexities associated with immune reconstitution, it is imperative to conduct a more extensive regional clinical trial that includes a larger and ethnically diverse cohort. Additionally, focusing on the immunological characteristics and causes of poor immune reestablishment in patients with advanced AIDS after ART treatment [Citation43–45], it is essential to clarify the underlying mechanism of AAIT for refining and enhancing the therapeutic strategies for this patient population.
Authorship
Contribution: All authors made substantial contributions to this work. F-S.W. and R-N.X. conceived the initial idea and designed the study; H-S.A., R-N.X., Z.X., B.T., C. Zhang and T.Y. collaboratively refined the study protocol; T.Y., R-N.X., F-S.W. and C. Zhang drafted the manuscript, including figures and tables; Z-M.X., Z.X., B.T., H.L., H-H.H., L.H.,L-M.L., L-Y.G, P.M., J.Z. and S-X.Y. were responsible for patient care and follow-up; L.J., R-N.X., T.Y., Y-Y.G., Y.Y., Y-L.C., L.C., C-B.Z., Y-Y.L. and J-H.Y. oversaw the AAIT cell preparation protocol and quality control; T.Y., R-N.X., C. Zheng and M.S. managed data collection and analysis; J-W.S., Y-M.J., T.Y., S-R.M. and C. Zhang conducted immunology assays; and all authors carefully read the final manuscript and consented to their authorship.
Supplemental Material
Download PDF (507.1 KB)Acknowledgments
The authors thank the patients who participated in this trial and their families. This work was supported by grants from National Key Research and Development Program of China (2022YFC2304403), National Science and Technology Major Program (2018ZX10302104) and the National Natural Science Foundation of China (grants 81721002 and grants 82171732).
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Spino C, Kahn JO, Dolin R, et al. Predictors of survival in HIV-infected persons with 50 or fewer CD4 cells/mm3. J Acquir Immune Defic Syndr Hum Retrovirol. 1997 Aug 15;15(5):346–355. doi:10.1097/00042560-199708150-00004
- Palella FJ, Delaney KM, Moorman AC, et al. Declining morbidity and mortality among patients with advanced human immunodeficiency virus infection. HIV Outpatient Study Investigators. N Engl J Med. 1998 Mar 26;338(13):853–860.
- Liu J, Wang L, Hou Y, et al. Immune restoration in HIV-1-infected patients after 12 years of antiretroviral therapy: a real-world observational study. Emerg Microbes Infect. 2020 Dec;9(1):2550–2561. doi:10.1080/22221751.2020.1840928
- AIDS and Hepatitis C Professional Group, Society of Infectious Diseases, Chinese Medical Association; Chinese Center for Disease Control and Prevention. Chinese guidelines for the diagnosis and treatment of HIV/AIDS (2021 edition). Infectious Diseases & Immunity. 2022 Jul 20;02(03):145–167.
- Deeks SG, Phillips AN. HIV infection, antiretroviral treatment, ageing, and non-AIDS related morbidity. Br Med J. 2009 Jan 26;338:a3172. doi:10.1136/bmj.a3172
- Opportunistic Infections Project Team of the Collaboration of Observational HIV Epidemiological Research in Europe (COHERE) in EuroCoord, Young J, Psichogiou M, et al. CD4 cell count and the risk of AIDS or death in HIV-infected adults on combination antiretroviral therapy with a suppressed viral load: a longitudinal cohort study from COHERE. PLoS Med. 2012;9(3):e1001194. doi:10.1371/journal.pmed.1001194
- Chalmers JD, Rother C, Salih W, et al. Healthcare-associated pneumonia does not accurately identify potentially resistant pathogens: a systematic review and meta-analysis. Clin Infect Dis. 2014 Feb;58(3):330–339. doi:10.1093/cid/cit734
- Serrano-Villar S, Sainz T, Lee SA, et al. HIV-infected individuals with low CD4/CD8 ratio despite effective antiretroviral therapy exhibit altered T cell subsets, heightened CD8+ T cell activation, and increased risk of non-AIDS morbidity and mortality. PLoS Pathog. 2014 May;10(5):e1004078. doi:10.1371/journal.ppat.1004078
- Okulicz JF, Le TD, Agan BK, et al. Influence of the timing of antiretroviral therapy on the potential for normalization of immune status in human immunodeficiency virus 1-infected individuals. JAMA Intern Med. 2015 Jan;175(1):88–99. doi:10.1001/jamainternmed.2014.4010
- Mussini C, Lorenzini P, Cozzi-Lepri A, et al. CD4/CD8 ratio normalisation and non-AIDS-related events in individuals with HIV who achieve viral load suppression with antiretroviral therapy: an observational cohort study. Lancet HIV. 2015 Mar;2(3):e98–106. doi:10.1016/S2352-3018(15)00006-5
- Roul H, Mary-Krause M, Ghosn J, et al. CD4 + cell count recovery after combined antiretroviral therapy in the modern combined antiretroviral therapy era. AIDS. 2018 Nov 13;32(17):2605–2614. doi:10.1097/QAD.0000000000002010
- Cao W, Liu X, Han Y, et al. (5r)−5-hydroxytriptolide for HIV immunological non-responders receiving ART: a randomized, double-blinded, placebo-controlled phase II study. Lancet Reg Health West Pac. 2023;34:100724.
- Zaongo SD, Chen Y. Metformin may be a viable adjunctive therapeutic option to potentially enhance immune reconstitution in HIV-positive immunological non-responders. Chin Med J (Engl). 2023 Sep 20;136(18):2147–2155. doi:10.1097/CM9.0000000000002493
- Hassett JM, Zaroulis CG, Greenberg ML, et al. Bone-marrow transplantation in AIDS. N Engl J Med. 1983 Sep 15;309(11):665.
- Lane HC, Masur H, Longo DL, et al. Partial immune reconstitution in a patient with the acquired immunodeficiency syndrome. N Engl J Med. 1984 Oct 25;311(17):1099–1103. doi:10.1056/NEJM198410253111706
- Vilmer E, Rhodes-Feuillette A, Rabian C, et al. Clinical and immunological restoration in patients with AIDS after marrow transplantation, using lymphocyte transfusions from the marrow donor. Transplantation. 1987 Jul;44(1):25–29. doi:10.1097/00007890-198707000-00007
- Holland HK, Saral R, Rossi JJ, et al. Allogeneic bone marrow transplantation, zidovudine, and human immunodeficiency virus type 1 (HIV-1) infection. Studies in a patient with non-Hodgkin lymphoma. Ann Intern Med. 1989 Dec 15;111(12):973–981. doi:10.7326/0003-4819-111-12-973
- Tebas P, Stein D, Tang WW, et al. Gene editing of CCR5 in autologous CD4 T cells of persons infected with HIV. N Engl J Med. 2014 Mar 6;370(10):901–910. doi:10.1056/NEJMoa1300662
- Xu L, Wang J, Liu Y, et al. CRISPR-edited stem cells in a patient with HIV and acute lymphocytic leukemia. N Engl J Med. 2019 Sep 26;381(13):1240–1247. doi:10.1056/NEJMoa1817426
- Hütter G, Ganepola S, Schneider T, et al. Long-term control of HIV by CCR5 Delta32/Delta32 stem-cell transplantation. N Engl J Med. 2009;360(7):692–698. doi:10.1056/NEJMoa0802905
- Gupta RK, Abdul-Jawad S, McCoy LE, et al. HIV-1 remission following CCR5Δ32/Δ32 haematopoietic stem-cell transplantation. Nature. 2019 Apr;568(7751):244–248. doi:10.1038/s41586-019-1027-4
- Jensen BEO, Knops E, Cords L, et al. In-depth virological and immunological characterization of HIV-1 cure after CCR5Δ32/Δ32 allogeneic hematopoietic stem cell transplantation. Nat Med. 2023 Mar;29(3):583–587. doi:10.1038/s41591-023-02213-x
- Huang XJ. Current status of haploidentical stem cell transplantation for leukemia. J Hematol Oncol. 2008;1:27. doi:10.1186/1756-8722-1-27
- McCurdy SR, Kanakry JA, Showel MM, et al. Risk-stratified outcomes of nonmyeloablative HLA-haploidentical BMT with high-dose posttransplantation cyclophosphamide. Blood. 2015;125(19):3024–3031. doi:10.1182/blood-2015-01-623991
- Kong Y, Wang Y, Zhang YY, et al. Prophylactic oral NAC reduced poor hematopoietic reconstitution by improving endothelial cells after haploidentical transplantation. Blood Adv. 2019;3(8):1303–1317. doi:10.1182/bloodadvances.2018029454
- Guo M, Hu KX, Yu CL, et al. Infusion of HLA-mismatched peripheral blood stem cells improves the outcome of chemotherapy for acute myeloid leukemia in elderly patients. Blood. 2011;117(3):936–941. doi:10.1182/blood-2010-06-288506
- Guo M, Hu KX, Liu GX, et al. HLA-mismatched stem-cell microtransplantation as postremission therapy for acute myeloid leukemia: long-term follow-up. J Clin Oncol. 2012;30(33):4084–4090. doi:10.1200/JCO.2012.42.0281
- Xu R, Zhang JY, Tu B, et al. HLA-mismatched allogeneic adoptive immune therapy in severely immunosuppressed AIDS patients. Sig Transduct Target Ther. 2021 May 7;6(1):174. doi:10.1038/s41392-021-00550-2
- AIDS and Hepatitis C Professional Group. Society of Infectious Diseases, Chinese Medical Association; Chinese Center for Disease Control and Prevention. Zhonghua Nei Ke Za Zhi. 2018;57(12):867–884.
- AIDS and Hepatitis C Professional Group. Society of Infectious Diseases, Chinese Medical Association; Chinese Center for Disease Control and Prevention. Zhonghua Nei Ke Za Zhi. 2021;60(12):1106–1128.
- Global HIV & AIDS statistics — Fact sheet [Internet]. [cited 2024 Mar 3]. Available from: https://www.unaids.org/en/resources/fact-sheet.
- Vlahov D, Graham N, Hoover D, et al. Prognostic indicators for AIDS and infectious disease death in HIV-infected injection drug users: plasma viral load and CD4+ cell count. JAMA. 1998 Jan 7;279(1):35–40. doi:10.1001/jama.279.1.35
- Dickter JK, Aribi A, Cardoso AA, et al. HIV-1 Remission after allogeneic hematopoietic-cell transplantation. N Engl J Med. 2024 Feb 15;390(7):669–671. doi:10.1056/NEJMc2312556
- Shearer GM, Clerici M, Graham DR, et al. Curing HIV/AIDS beyond hematopoietic stem cell transplant. AIDS. 2015 Nov;29(17):2364–2366. doi:10.1097/QAD.0000000000000861
- Wang Y, Tao L, Mitchell E, et al. Allo-immunization elicits CD8+ T cell-derived chemokines, HIV suppressor factors and resistance to HIV infection in women. Nat Med. 1999 Sep;5(9):1004–1009. doi:10.1038/12440
- Durand CM, Capoferri AA, Redd AD, et al. Allogeneic bone marrow transplantation with post-transplant cyclophosphamide for patients with HIV and haematological malignancies: a feasibility study. Lancet HIV. 2020 Sep;7(9):e602–e610. doi:10.1016/S2352-3018(20)30073-4
- Melenhorst JJ, Leen AM, Bollard CM, et al. Allogeneic virus-specific T cells with HLA alloreactivity do not produce GVHD in human subjects. Blood. 2010;116(22):4700–4702. doi:10.1182/blood-2010-06-289991
- INSIGHT-ESPRIT Study Group, SILCAAT Scientific Committee, Abrams D, et al. Interleukin-2 therapy in patients with HIV infection. N Engl J Med. 2009 Oct 15;361(16):1548–1559. doi:10.1056/NEJMoa0903175
- Lévy Y, Sereti I, Tambussi G, et al. Effects of recombinant human interleukin 7 on T-cell recovery and thymic output in HIV-infected patients receiving antiretroviral therapy: results of a phase I/IIa randomized, placebo-controlled, multicenter study. Clin Infect Dis. 2012 Jul;55(2):291–300. doi:10.1093/cid/cis383
- Vandergeeten C, Fromentin R, DaFonseca S, et al. Interleukin-7 promotes HIV persistence during antiretroviral therapy. Blood. 2013 May 23;121(21):4321–4329. doi:10.1182/blood-2012-11-465625
- Wang Y, Wu DP, Liu QF, et al. Donor and recipient age, gender and ABO incompatibility regardless of donor source: validated criteria for donor selection for haematopoietic transplants. Leukemia. 2018 Feb;32(2):492–498. doi:10.1038/leu.2017.199
- Miklos DB, Kim HT, Miller KH, et al. Antibody responses to H-Y minor histocompatibility antigens correlate with chronic graft-versus-host disease and disease remission. Blood. 2005 Apr 1;105(7):2973–2978. doi:10.1182/blood-2004-09-3660
- Liu Y, Li Z, Lu X, et al. Dysregulation of memory B cells and circulating T follicular helper cells is a predictor of poor immune recovery in HIV-infected patients on antiretroviral therapy. J Med Virol. 2023;95(2):e28559. doi:10.1002/jmv.28559
- Yang X, Su B, Zhang X, et al. Incomplete immune reconstitution in HIV/AIDS patients on antiretroviral therapy: challenges of immunological non-responders. J Leukoc Biol. 2020;107(4):597–612. doi:10.1002/JLB.4MR1019-189R
- Su B, Kong D, Yang X, et al. Mucosal-associated invariant T cells: a cryptic coordinator in HIV-infected immune reconstitution. J Med Virol. 2022;94(7):3043–3053. doi:10.1002/jmv.27696
