?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
Hepatitis E virus (HEV) is an important cause of acute hepatitis, however, is highly neglected and largely underreported. This study aimed to describe the detailed epidemiology of hepatitis E (HE) through a 10-year surveillance. A community-based active hepatitis surveillance was conducted between November 2007 and October 2017 in 11 townships of Dongtai City in China, involving 355,673 residents. Serum samples were obtained from patients presenting with hepatitis symptoms for more than 3 days. Serum alanine aminotransferase (ALT) levels greater than 2.5 times the upper limit of normal (ULN) were considered acute hepatitis. Samples were subsequently tested for IgG and IgM anti-HEV antibodies, HEV RNA, and hepatitis B surface antigen (HBsAg). The data indicated the incidence of HE fluctuated downward from 2007 to 2017, with an average annual age-standardized incidence of 17.50 per 100,000, exceeding the 10.26 per 100,000 in the National Notifiable Disease Report System (NNDRS). The incidence was notably higher among males (20.95 per 100,000) and individuals aged 50–69 years (37.47 per 100,000). Genotype 4 (HEV-4) was the predominantly circulating genotype during the study period. Furthermore, the study revealed the incidence of hepatitis with HEV and hepatitis B virus (HBV) co-infection was 4.99 per 100,000. The active surveillance system identified a higher incidence of HE compared to NNDRS, with a decreased prevalence over a 10-year period. While efforts are still needed to prevent HE in high-risk populations, including individuals with hepatitis B and the elderly.
Background
The hepatitis E virus (HEV) represents an important public health concern globally, especially as a leading cause of acute viral hepatitis in developing countries [Citation1]. An estimated 20 million HEV infections occur annually worldwide, resulting in more than 3 million clinical cases [Citation2]. While most patients with hepatitis E (HE) are asymptomatic and have self-limited disease, a minority can develop liver failure, which is associated with high morbidity and mortality [Citation3, Citation4]. Individuals at increased risk for severe outcomes include pregnant women and individuals with chronic liver disease [Citation5, Citation6].
HEV genotype 1 (HEV-1) and genotype 2 (HEV-2) primarily infect humans, and are predominantly circulating in developing countries, particularly in Asia and Africa, often related to large waterborne outbreaks [Citation7, Citation8]. Recently, a study found that HEV-1 can also experimentally infect Mongolian gerbils [Citation9]. While genotype 3 (HEV-3) and genotype 4 (HEV-4) infect mammals but occasionally transmit to humans, and are prevalent in industrialized and high-income countries [Citation10, Citation11].
Hepatitis E was first recognized during an epidemic of hepatitis, which occurred in India in 1978, involving an estimated 52,000 cases of icteric hepatitis and 1700 deaths [Citation12]. Since then, the outbreaks have been identified in numerous developing countries, among which the largest one occurring in Xinjiang, China, in the 1980s. This outbreak, driven by HEV-1, resulted in approximately 119,280 reported cases and 705 deaths [Citation13]. Current understanding of hepatitis E largely originates from data collected during outbreak situations. Despite decades having passed, hepatitis E continues to be largely overlooked in daily clinical practice. There are still limited efforts to comprehensively assess the true burden of the disease and its evolving epidemiology dynamics. Although the epidemiology of hepatitis E has been partially reported through the National Notifiable Disease Report System (NNDRS) in China, it is believed that these figures do not reflect the true extent of the issue due to significant underreporting [Citation14]. Over the past years, substantial improvements in public health conditions and licensure of HEV vaccine in China have likely altered the epidemiological patterns and trends of hepatitis E [Citation15]. Continuous epidemiological studies are essential to understand the dynamic characteristics of hepatitis E and to develop evidence-based preventive strategies for the future.
Method
From 2007 to 2009, a randomized, controlled Phase III clinical trial of the hepatitis E vaccine (Hecolin®) was conducted in 11 rural townships in Dongtai City [Citation16]. To evaluate the efficacy of the vaccine, a symptom-based active hepatitis surveillance system was established from November 2007 to October 2017. The system comprised virtually all village clinic centres, the township hospitals, and public and private clinics, covering the 355,673 residents of the 11 rural townships. This comprehensive surveillance system provided an invaluable opportunity to analyse and understand the epidemiological trends and characteristics of hepatitis E. Approval of the study by the Independent Ethics Committee was obtained from the Ethics Committee of the Jiangsu Provincial Center for Disease Control and Prevention.
In the symptom-driven surveillance, serum samples were obtained from patients presenting hepatitis symptoms such as fatigue and/or loss of appetite for more than 3 days, and tested for alanine aminotransferase (ALT) levels. For patients with ALT levels higher than 2.5 times the upper limit of the normal range (ULN), the serum samples were subsequently tested for IgG and IgM anti-HEV antibodies and hepatitis B surface antigen (HBsAg).
All serologic marker assays were obtained from Wantai Biological Pharmacy Enterprise Co. (Beijing, China). Samples tested positive for IgG anti-HEV were further calibrated with WHO reference serum (WHO 95/584) to determine the IgG anti-HEV concentration. The detection limit of assay was 0.077Wu/ml. Patients with detectable IgM anti-HEV in any sample and/or with ≥2-fold increase of IgG anti-HEV levels in paired samples were selected and tested for HEV RNA using reverse-transcription PCR as previously described [Citation17]. An RT–PCR cycle threshold (Ct) value of ≤38 was considered positive. Serum samples tested positive for HEV RNA were further analysed to identify the HEV genotype via nested RT–PCR. After sequencing, the sequences were aligned with corresponding regions of available reference sequences in the GenBank database. Phylogenetic analysis of the partial ORF1 gene (YK) and ORF2 gene (YE) of HEV was conducted constructed using the neighbour-joining method using MEGA 11 [Citation18].
A positive finding for HBsAg indicated a diagnosis of HBV infection. Acute hepatitis E was diagnosed if any two of following three criteria were met: (1) positive testing for IgM anti-HEV; (2) ≥4-fold increase in IgG anti-HEV levels in serial serum; (3) the presence of HEV RNA [Citation16, Citation19].
Statistical analyses
Participants immunized with HE vaccine during the phase III clinical trial were excluded from the analysis. Hepatitis E cases reported to the NNDRS from 11 rural townships in Dongtai City were collected between November 2007 and October 2017. The age-standardized incidence was calculated based on Dongtai population.
Normally distributed quantitative variables were compared by Student’s t test and expressed as the mean ± standard deviation (SD). Variables that did not follow a normal distribution were described using the median along with the first and third quartiles (Q1, Q3). Differences in characteristics were compared using either the chi-square test for categorical variables or the Mann–Whitney U test for non-normal variables. Cochran–Armitage Trend Test was used to test the association between age group and incidence of hepatitis E. Odds ratios (OR) and 95% confidence intervals (CIs) were calculated. All analyses were conducted using SAS software (version 9.3). All reported P values will be two-sided, and P values < .05 were considered significant.
Result
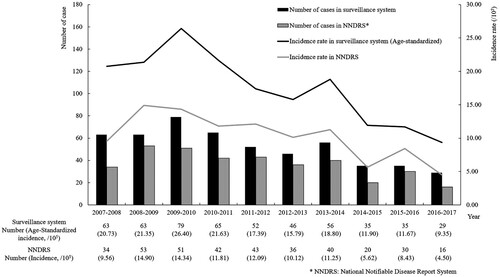
During the study period from November 2007 to October 2017, a total of 11,432 cases presented with hepatitis symptoms and abnormal alanine aminotransferase (ALT) levels. Of these, 523 cases were diagnosed as hepatitis E, resulting in an average annual age-standardized incidence of 17.50 per 100,000 across the 11 rural townships (). Based on surveillance data, the incidence of hepatitis E exhibited an upward trend from 2007 to 2010, followed by a fluctuating downward trend from 2010 to 2017, and similar trend was also observed in the NNDRS data. In 2009-2010, the surveillance system reported the peak incidence, with 79 cases and an age-standardized incidence of 26.40 per 100,000. The lowest incidence occurred in 2016–2017, with an age-standardized incidence of 9.35 per 100,000. Data from NNDRS presented an average annual age-standardized incidence of 10.26 per 100,000.
From 2007 to 2017, of all hepatitis E cases, 353 were males, and 170 were females (). A higher risk of incidence was observed in males (OR = 1.61, 95% CI 1.36∼1.91), with an average annual age-standardized incidence of 20.95 per 100,000 compared to 13.02 per 100,000 for females. Overall, the incidence increased with age and tended to decline after 70 years, a trend observed in both males and females (Ptrend < .0001). Among males, the highest prevalence was in the 50–59 age group (46.88 per 100,000), while among females, it was in the 60∼69 age group (32.21 per 100,000). Of 235 samples sequenced, 75 were negative for hepatitis E by sequencing and 160 were positive. All viral isolates were HEV genotypes 1 and 4. A total of 46.88% (75/160) were subtype 4a, 31.88% (51/160) were subtype 4d and 3.13% (5/160) were genotype 1. Notably, HEV-1 has not been detected since November 2012. Phylogenetic analyses of the YE fragments from 38 patients and YK fragments from 58 patients are presented in .
Figure 2. Phylogenetic tree based on YE (A) and YK (B) fragments from hepatitis E cases. Thirty-eight ORF2 gene fragments (YE) and 58 ORF1 gene fragments (YK) obtained from 96 hepatitis E cases and reference sequences are shown. The phylogenetic tree was constructed with the neighbor-joining method.
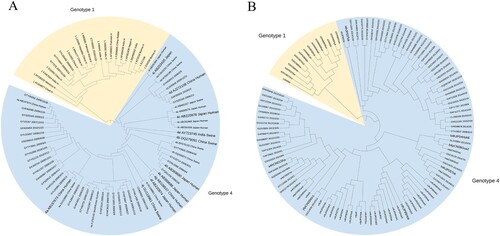
Table 1. Average-annual Incidence rate of hepatitis E from 2007–2017 by age and gender in the surveillance.
Among patients with symptomatic hepatitis, 2,617 were diagnosed with hepatitis B, including 97 (3.71%) who were co-infected with hepatitis E (). From 2007 to 2017, the average annual age-standardized incidence of hepatitis with HBV-HEV co-infection was 4.99 per 100,000. Patients with HBV-HEV co-infection were relatively younger than those with solely hepatitis E, with an average ages of 53.82 and 58.88 respectively (P = .004). Additionally, patients with hepatitis E and those with HBV-HEV co-infection presented significantly higher ALT levels [22.53 (5.94, 42.61) ULN, and 11.57 (3.83, 30.53) ULN, respectively] compared to those with HBV infection alone [4.38 (3.11, 8.13) ULN], with P < .001.
Table 2. Features of hepatitis in the surveillance.
Discussion
Data derived from a community-based surveillance in Dongtai City revealed the average annual age-standardized incidence of symptomatic hepatitis E from 2007 to 2017 was 17.50 per 100,000, which was higher than that reported in the NNDRS (10.26 per 100,000). The incidence exhibited a short-term upward trend followed by a fluctuating downward trend. Considerable higher prevalence occurred in the 50–69 age group, with a notable male preponderance. During the study period, the predominant strain was genotype 4. In addition, the average annual age-standardized incidence of symptomatic HBV-HEV co-infection was 4.99 per 100,000, with 3.71% of symptomatic hepatitis B cases presenting co-infection with HEV.
Fluctuations in the sensitivity of surveillance system are always a concern in long-term studies. The community-based surveillance conducted in this study was designed for the phase III clinical trial of a hepatitis E vaccine. This system encompassed 11 rural townships, covering the majority of residents in Dongtai [Citation16]. The incidence of abnormal ALT increases remained stable throughout the study period, which mirrored the steady status of the surveillance [Citation20]. Consequently, fluctuations in outcome measurement did not significantly bias the analysis. To better reflect the epidemiological characteristics of HEV infection in the natural environment, individuals immunized with the HE vaccine during the clinical trial were excluded from the analysis.
Through the symptom-driven and community-based surveillance, this study revealed the epidemiological trend of HE from 2007 to 2017 in eastern China. Each patient presenting with suspected hepatitis symptoms underwent well-established testing and strict diagnostic criteria, ensuring high sensitivity and specificity in diagnosis. The average annual age-standardized incidence of hepatitis E in the surveillance system was higher than that reported in the NNDRS (17.50 per 100,000 vs 10.26 per 100,000), suggesting a significant proportion of HE cases were underdiagnosed or underreported in primary health institutions. In addition, the HEV detection indicators and diagnostic criteria for hepatitis E have varied and remained inconsistent across clinics over a long-time span. This inconsistency could potentially introduce bias in case reporting within the NNDRS, contributing to the disparity when compared with the surveillance system. Age- and sex–specific incidence were further reported in the present study, revealing a higher average annual age-standardized incidence among males (20.95 per 100,000) and individuals aged 50–69 years (37.47 per 100,000). In general, the elderly are more susceptible to hepatitis E due to their decreased immunity, while adult males are engaged in more social activities, may have more exposure opportunities [Citation21]. Overall, a decrease in the prevalence of hepatitis E was observed. However, the potential disease burden and the elevated risk for men and individuals aged 50–69 years remain concerning.
Although HEV-1 infection caused waterborne outbreaks in China decades ago, HEV-4 has been the predominant genotype in Dongtai during the surveillance period, and has been the only circulating genotype since November 2012. Improvements in sanitation and water supply are considered to be one of the reasons for the change in genotypes [Citation22]. Nevertheless, there is uncertainty in the genotype prevalence trends in this study because some cases could not be sequenced due to low viral loads. Although HEV-4 appears to be less virulent than HEV-1 in humans, recent studies indicate that HEV-4 is emerging as a significant disease burden among immunocompromised individuals, patients with chronic liver disease, and the elderly [Citation23].
This study provided valuable data on the average annual age-standardized incidence of hepatitis with HBV-HEV co-infection (4.99 per 100,000). Patients with HBV-HEV co-infection exhibited higher peak ALT levels compared to those with only HBV infection, suggesting that HEV co-infection may accelerate disease progression in HBV patients. Considering the significantly higher risks of liver failure (OR = 5.5, 95% CI: 1.5–20.1) and mortality (OR = 5.0, 95% CI: 1.9–13.3) in hepatitis B patients with HEV superinfection compared to those without HEV superinfection [Citation24], the hepatitis E vaccine should be highly recommended for the population to provide long-term protection [Citation25, Citation26].
By this robust community-based hepatitis surveillance system, the detailed epidemiological trend and characteristics of hepatitis E were revealed in a large-scale rural population in eastern China. However, several limitations should be considered. First, the study was conducted in a single region in eastern China, which limits the generalizability of the conclusion. Second, the study focused on the symptomatic cases, which limited our ability to capture the full spectrum of the HE, including asymptomatic cases. Third, we were unable to follow the long-term clinical outcomes of all patients with HE in this community-based surveillance.
Acknowledgements
The authors extend their gratitude to the participants of the study and acknowledge the valuable contributions of the community-based surveillance members across all 11 rural townships.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Zeng D-Y, Li J-M, Lin S, et al. Global burden of acute viral hepatitis and its association with socioeconomic development status, 1990–2019. J Hepatol 2021;75(3):547–556. doi:10.1016/j.jhep.2021.04.035
- Hepatitis E [Internet]. World Health Organization. (cited 2024 Jan 20). Available from: https://www.who.int/news-room/fact-sheets/detail/hepatitis-e.
- Yang H, Wen J, Zhang Q, et al. Clinical characteristics of 1279 patients with hepatitis E in Tianjin. Epidemiol Infect. 2023;151:e157. doi:10.1017/S0950268823001516
- Bagulo H, Majekodunmi AO, Welburn SC. Hepatitis E in Sub Saharan Africa – a significant emerging disease. One Health. 2020;11:100186. doi:10.1016/j.onehlt.2020.100186
- Labrique AB, Sikder SS, Krain LJ, et al. Hepatitis E, a vaccine-preventable cause of maternal deaths. Emerg Infect Dis. 2012;18(9):1401–1404. doi:10.3201/eid1809.120241
- Kumar Acharya S, Kumar Sharma P, Singh R, et al. Hepatitis E virus (HEV) infection in patients with cirrhosis is associated with rapid decompensation and death. J Hepatol. 2007;46(3):387–394. doi:10.1016/j.jhep.2006.09.016
- Rein DB, Stevens GA, Theaker J, et al. The global burden of hepatitis E virus genotypes 1 and 2 in 2005. Hepatology. 2012;55(4):988–997. doi:10.1002/hep.25505
- Corwin AL, Khiem HB, Clayson ET, et al. A waterborne outbreak of hepatitis E virus transmission in southwestern Vietnam. Am J Trop Med Hyg. 1996;54(6):559–562. doi:10.4269/ajtmh.1996.54.559
- Liu T, He Q., Yang X, et al. An immunocompetent mongolian gerbil model for hepatitis E virus genotype 1 infection. Gastroenterol. 2024. doi:10.1053/j.gastro.2024.03.038
- Dalton HR, Izopet J. Transmission and Epidemiology of Hepatitis E Virus Genotype 3 and 4 Infections. Cold Spring Harb Perspect Med. 2018;8(11):a032144. doi:10.1101/cshperspect.a032144
- Mansuy JM, Abravanel F, Miedouge M, et al. Acute hepatitis E in south-west France over a 5-year period. J Clin Virol. 2009;44(1):74–77. doi:10.1016/j.jcv.2008.09.010
- Khuroo MS. Discovery of hepatitis E: The epidemic non-A, non-B hepatitis 30 years down the memory lane. Virus Res 2011;161(1):3–14. doi:10.1016/j.virusres.2011.02.007
- Cao XY, Ma XZ, Liu YZ. The epidemiological study of enterically transmitted non-A, non-B hepatitis in the south area of Xinjiang. Chinese Journal of Public Health; 1989;(04):193–199.
- Dalton HR, Bendall R, Ijaz S, et al. Hepatitis E: an emerging infection in developed countries. Lancet Infect Dis. 2008;8(11):698–709. doi:10.1016/S1473-3099(08)70255-X
- Park SB. Hepatitis E vaccine debuts. Nature. 2012;491(7422):21–22. doi:10.1038/491021a
- Zhu F-C, Zhang J, Zhang X-F, et al. Efficacy and safety of a recombinant hepatitis E vaccine in healthy adults: a large-scale, randomised, double-blind placebo-controlled, phase 3 trial. Lancet. 2010;376(9744):895–902. doi:10.1016/S0140-6736(10)61030-6
- Huang S, Zhang X, Jiang H, et al. Profile of acute infectious markers in sporadic hepatitis E. PLoS One. 2010;5(10):e13560. doi:10.1371/journal.pone.0013560
- Tang ZM, Wen GP, Ying D, et al. Profile of clinical characteristics and serologic markers of sporadic hepatitis E in a community cohort study. Emerg Microbes Infect. 2023;12(1):2140613. doi:10.1080/22221751.2022.2140613
- Huang S, Zhang X, Su Y, et al. Long-term efficacy of a recombinant hepatitis E vaccine in adults: 10-year results from a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2024;403(10429):813–823. doi:10.1016/S0140-6736(23)02234-1
- Chinese Society of Hepatology, Chinese Medical Association. Consensus on prevention and treatment of hepatitis E. Zhonghua Gan Zang Bing Za Zhi. 2022;30(8):820–831. doi:10.3760/cma.j.cn501113-20220729-00401
- Shi Y, Shen W, Liu W, et al. Analysis of the spatial-temporal distribution characteristics of hepatitis E in Jiangsu province from 2005 to 2020. Front Public Health. 2023;11:1225261. doi:10.3389/fpubh.2023.1225261
- Ren X, Wu P, Wang L, et al. Changing epidemiology of hepatitis A and hepatitis E viruses in China, 1990-2014. Emerg Infect Dis. 2017;23(2):276–279. doi:10.3201/eid2302.161095
- Thakur V, Ratho RK, Kumar S, et al. Viral hepatitis E and chronicity: a growing public health concern. Front Microbiol. 2020;11:577339. doi:10.3389/fmicb.2020.577339
- Qiu LX, Huang Y, Quan JL, et al. Prognosis of hepatitis E infection in patients with chronic liver disease: a meta-analysis. J Viral Hepat. 2023;30(2):101–107. doi:10.1111/jvh.13754
- Zhang J, Zhang XF, Huang SJ, et al. Long-term efficacy of a hepatitis E vaccine. N Engl J Med 2015;372(10):914–922. doi:10.1056/NEJMoa1406011
- Wu T, Huang SJ, Zhu FC, et al. Immunogenicity and safety of hepatitis E vaccine in healthy hepatitis B surface antigen positive adults. Hum Vaccin Immunother. 2013;9(11):2474–2479. doi:10.4161/hv.25814