Abstract
In this study, after doping Tb4O7 to α-Bi2O3 in the range of 1% ≤ n ≤ 50% in a series of different mol ratios, heat treatment was carried out by applying cascade temperature rise in the range of 600°C–950°C for 72 hours, and new phases were obtained in Bi2O3–Tb4O7 system. After 72 hours of heat treatment at 800°C, 1% and 2% mol of Tb4O7-containing mixtures formed tetragonal phase; 6%, 13%–20% and 30% mol of Tb4O7-containing mixtures formed face-centered cubic phase; 7%–10%, 15% and 18% mol of Tb4O7-containing mixtures were put in quenching process at 750°C and face-centered cubic phases were obtained. Similarly, 1% and 3%–5% mol of Tb4O7-containing mixtures formed a tetragonal phase. With the help of x-ray diffraction, crystal systems and lattice parameters of solid solutions were obtained and their characterization was carried out. Their surface and particle properties were detected and microprobe analysis was performed by scanning electron microscope.
1. Introduction
Until now, six polymorphs of bismuth trioxide (Bi2O3) have been reported by researchers. These are monoclinic (α-Bi2O3), body-centered cubic (bcc) (γ-Bi2O3), face-centered cubic (fcc) (δ-Bi2O3), tetragonal (β-Bi2O3), triclinic (ω-Bi2O3) and orthorhombic (ɛ-Bi2O3) phases.[Citation1–Citation7] The α-phase is stable at room temperature while the other five forms are unstable crystal modifications that are formed at high temperatures. If pure α-Bi2O3, whose melting temperature is 824°C, is heated until around 729°C, it transforms to δ-Bi2O3 phase which is stable at high temperatures, even at the melting point. When it is cooled again, it transforms to β-Bi2O3 phase at ∼650°C and γ-Bi2O3 phase at ∼639°C. If β- and γ-phases are cooled to lower temperatures, they transform to α-Bi2O3 phase again at around ∼500°C. Orthorhombic crystal (ɛ-Bi2O3) and triclinic phases (ω-Bi2O3), upon which there is scarce information, can be obtained with notable special-synthesis reactions and hydrothermal heat-treatment processes at 240°C and 800°C, respectively.[Citation3,Citation8]
Bi2O3 polymorphs have important scientific and industrial uses. For example, they are used in the construction of ceramic solid-fuel electrodes and membranes, in the catalysis of some heterogeneous reactions, in the partial oxidation of hydrocarbons, in the removal of harmful effects of exhaust gases as catalytic converters, etc. The other most important area of use is production of electrochemical energy.[Citation1,Citation10–Citation13] Conductive polymorphs are used in photoconductivity applications, modern solid-state technology and electronic and ceramic industry.[Citation9–Citation15] β- and δ-Bi2O3 phases display oxygen ionic-conductivity properties and can be stabilized by doping with small amounts of other oxides. The ionic conductivity in β- and δ-Bi2O3 phases, doped with Tb4O7 system, was measured in the composition range between 1 and 30 mol % Tb4O7 at different temperatures. According to the differential thermal analysis/thermogravimetry results, this tetragonal-type solid solution is stable up to about ∼740°C, and the solubility limit is found at ∼5 mol % Tb4O7 in the β-phase; this fcc type solid solution is stable up to about ∼740°C, and the solubility limit is found at ∼30 mol % Tb4O7 in the δ-phase. All phases show predominant oxide-ionic conduction.[Citation16]
In this study, doping Tb4O7 in a series of different mol ratios, with pure α-Bi2O3, unstable β- and δ-phases at room temperature were transformed into a stable structure by performing solid-state reactions at high temperature. After detecting the crystal systems of stable solid solutions, their surface and particle properties were detected, and microprobe analyses were conducted.
2. Experiment
2.1. Experimental setup
The Tb4O7 was added to α-Bi2O3 in different mol ratios in the range of 1% ≤ n ≤ 50% mol. The combined substances were grinded with agate mortar to achieve a solid state reaction, after 72 hours of heat treatment in a porcelain crucible. Mixtures were heat treated from 600°C to 950°C, with a rise of 50°C per pace. After each reaction, products were cooled gradually until they reached room temperature.
After each solid-state reaction, it was detected whether there was a change in each powder sample's mass. Powder patterns were recorded using the x-ray diffraction (XRD) method and their crystal systems were detected. XRD data were recorded with a Bruker AXS D8 Advance model diffractometer (Bragg–Brentano geometry, graphite monochromator with CuKα radiation, 0.002° pitch angle, 2θ = 10°–90°).
The microstructure properties of the powder samples’ surfaces and microprobe analysis were performed in three different locations of solid solutions with LEO 440 model scanning electron microscope (SEM).
3. Results and discussion
3.1. Results
The minimum temperature needed to obtain a crystal system that is stable in its simple phase under reaction circumstances is 800°C.
Solid solutions were obtained in β-Bi2O3, crystallized in a tetragonal crystal system in (Bi2O3)1–χ (Tb4O7)χ, in the range of 0.01 ≤ χ ≤ 0.05 mol fractions. Stable β-phase was obtained by applying quenching process at 750°C from the powder samples which could not preserve their phase stability while being cooled to room temperature after a 72-hour heat treatment. The powder patterns of 1% mol Tb4O7-doped solid solution are given in Figure as a sample.
Figure 1 XRD patterns of β-Bi2O3 doped with 1% mol Tb4O7 at (a) 700°C, (b) 750°C, (c) 800°C and (d) 750°C, water quench
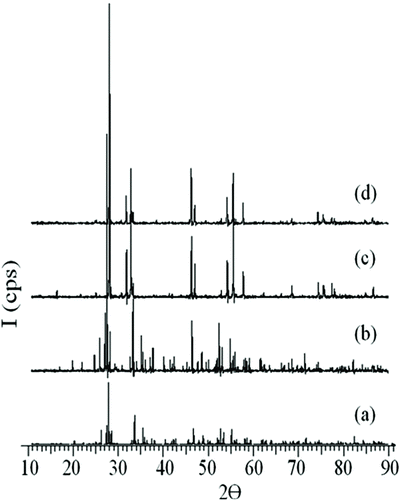
All the designs of samples indexed in the tetragonal crystal system show similarity with the designs in Figure . The unit cell parameters of the β-phase are given in Table .
Table 1 The relationship between the amount of Tb4O7 doping and the lattice parameter of β-Bi2O3
In the (Bi2O3)1−χ (Tb4O7)χ system, when χ is 0.06 ≤ χ ≤ 0.10 and 0.13 ≤ χ ≤ 0.30, solid solutions are obtained in δ-Bi2O3 phase, crystallized in face-centered cubic structure. Stable δ-phase is obtained by applying a quenching process at 750°C from the powder samples which could not preserve their phase stability while being cooled to room temperature after 72 hours of heat treatment. The powder patterns of 18% mol Tb4O7-doped solid solution, obtained at different temperatures, are given in Figure .
Figure 2 XRD patterns of δ-Bi2O3 doped with 18% mol Tb4O7 at (a) 700°C, (b) 750°C, (c) 800°C, (d) 850°C, (e) 900°C and (f) 750°C, water quench
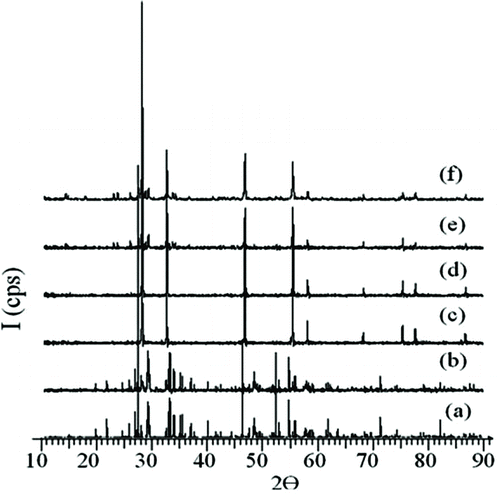
All the patterns of samples crystallized in face-centered cubic crystal systems show similarity with the design in Figure . The change of unit cell parameters in these powder samples, with the amount of Tb4O7 doped, is given in Figure (a) and (b). All the given unit cell parameters and XRD patterns agree with other Bi2O3 systems doped with La2O3 (La: rare earth oxide).[Citation1,Citation15]
Figure 3 The relationship between the amount of Tb4O7 doping and the lattice parameter of δ-Bi2O3: (a) a and (b) V
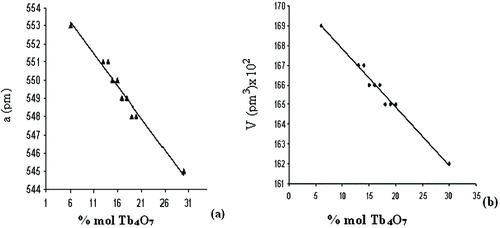
It is seen that as the amount of doped Tb4O7 in Figure (a) and (b) increases, a and V parameters decrease. Virtually this situation is coherent with the effect of ionic radii. Ionic radii are 96 pm for Bi3+, 89 pm for Tb4+, 93 pm for Tb3+. Since the radii of Tb4+ and Tb3+ are smaller than the radii of Bi3+, the change of Tb4+ and Tb3+ ions with Bi3+ ions in crystal lattice causes a decrease in lattice parameters a and V. Since more terbium cation is diffused in the lattice as the amount of dope increases, parameters increase further.
When χ = 0.11, 0.12, 0.40 and 0.50, reaction conditions and doping ratio are inadequate to perform crystallization in a stable system.
Moreover, performing a phase in (Bi2O3)1–χ (Tb4O7) χ system requires a long duration (72 h) of heat application. During solid-state reactions that take place at high temperature, terbium ions are diffused gradually into the Bi2O3 lattice. If the doping process is successful, diffused terbium (III, IV) cations prefer to change place with bismuth (III) cations in the lattice. This situation is thought to cause non-stoichiometry and transformation to a defect structure in the lattice as well as O2− ion conductivity.
An empirical formula calculated according to the microprobe analysis data performed at different points of solid solutions confirms the subject mechanism, assertion that a solid solution has non-stoichiometry. Empirical formulas of some solid solutions are given in Table .
Table 2 Empirical formula of Bi2O3 doped with 5, 10, 15 and 20 mol % Tb4O7
Percentage composition values calculated and obtained from SEM microprobe analysis are harmonious (Table ). This harmony can be thought as a proof that a doping process is successful and that a solid solution has non-stoichiometry. Fifteen and 20 mol % Tb4O7 doped solid solutions' SEM images are given in Figures and , respectively. The microstructures of the substances consisted of regular fine grains with an average size of about 0.5–2.5 μm.
Table 3 The microprobe analysis for the solid solution (Bi2O3)1−χ (Tb4O7)χ
4. Conclusions
As a result of this research, β- and δ- phases of Bi2O3, that are unstable at room temperature, were obtained by doping a Tb4O7 substance with α-Bi2O3 substance through solid-state reactions. The effective factors in the synthesis of these polymorphs are high-temperature application, reaction duration and the amount of Tb4O7 doped. It was observed that increasing the Tb4O7 amount influenced phase stability and those solid solutions that had greater doping amount were more resistant to high temperature.
It can be concluded from the change of terbium cations with crystal structured Bi3+ cations that non-stoichiometric phases are synthesized. Since the synthesis process was performed using high temperature application that lasted for a long period of time, we can say that terbium cations diffuse in the crystal structure very gradually. Non-stoichiometry of β-Bi2O3 and δ-Bi2O3 phases is thought to lead to interesting electrical properties.
Acknowledgements
This work was supported by Erciyes University (EUBAP).
References
- Sammes , N M , Tompsett , G A , Nafe , H and Aldinger , F. 1999 . Bismuth based oxide electrolytes – structure and ionic conductivity . J Eur Ceram Soc , 19 : 1801 – 1826 . doi: 10.1016/S0955-2219(99)00009-6
- Leontie , L , Caraman , M , Delibaş , M and Rusu , G I . 2001 . Optical properties of bismuth trioxide thin films . Mater Res Bull , 36 : 1629 – 1637 . doi: 10.1016/S0025-5408(01)00641-9
- Harwig , H A . 1978 . On the structure of bismuthsesquioxide: the α, β, γ and δ phase . Anorg Allg Chem , 444 : 151 – 166 . doi: 10.1002/zaac.19784440118
- Chehab , S , Conflant , P , Drache , M , Boivin , J-C and McDonald , G. 2003 . Solid-state reaction pathways of sillenite-phase formation studied by high-temperature X-ray diffractometry and differential thermal analysis . Mater Res Bull , 38 : 875 – 897 . doi: 10.1016/S0025-5408(03)00025-4
- Takahashi , T , Esaka , T and Iwahara , H . 1977 . Oxide ion conduction in the sintered oxides of MoO3-doped Bi2O3 . J Appl Electrochem , 7 : 31 – 35 . doi: 10.1007/BF00615527
- Crumpton , T E , Francesconi M , G and Greaves , C . 2003 . The structural chemistry of Bi14MO24 (M = Cr, Mo, W) phases: bismuth oxides containing discrete MO4 tetrahedra . J Solid State Chem , 175 : 197 – 206 . doi: 10.1016/S0022-4596(03)00246-9
- Ekhelikar , S and Bichile , G K . 2004 . Synthesis and structural characterization of (Bi2O3)(1−x) (Y2O3)(x) and (Bi2O3)(1−x) (Gd2O3) x solid solutions . Bull Mater Sci , 27 : 19 – 22 . doi: 10.1007/BF02708478
- Drache , M , Roussel , P and Wignacourt , J-P. 2007 . Structures and oxide mobility in Bi-Ln-O materials: heritage of Bi2O3 . Chem Rev. , 107 : 80 – 96 . doi: 10.1021/cr050977s
- Leontie , L , Caraman , M , Visinoiu , A and Rusu , G I . 2005 . On the optical properties of bismuth oxide thin films prepared by pulsed laser deposition . Thin Solid Films , 473 : 230 – 235 . doi: 10.1016/j.tsf.2004.07.061
- Fruth , V , Popa , M , Berger , D , Ionica , C M and Jitianu , M . 2004 . Phases investigation in the antimony doped Bi2O3 system . J Eur Ceram Soc , 24 : 1295 – 1299 . doi: 10.1016/S0955-2219(03)00506-5
- Kharton , V , Marques , F MB , Tsipis , E V , Viskup , A P , Vyshatko , N P , Patrakeev , M V , Naumovich , E N and Frade , J R. 2004 . İnterfacial effects in electrochemical cells for oxygen ionic conduction measurements: III. Transference numbers vs. grain-boundary resistivity . Solid State Ionics , 168 : 137 – 151 . doi: 10.1016/j.ssi.2004.01.033
- Kobayashi , K and Tsunoda , T . 2004 . Oxygen permeation and electrical transport properties of 60 vol.% Bi1.6Y0.4O3 and 40 vol.% Ag composite prepared by the sol–gel method . Solid State Ionics , 175 : 405 – 408 . doi: 10.1016/j.ssi.2003.11.046
- Löfberg , A , Boujmiai , S , Capoen , E , Steil , M C , Pirovano , C , Vannier , R N , Mairesse , G and Bordes-Richard , E. 2004 . Oxygen permeation versus catalytic properties of bismuth-based oxide ion conductors used for propene oxidation in a catalytic dense membrane reactor . Catal Today , 91 : 79 – 83 . doi: 10.1016/j.cattod.2004.03.013
- Basu , A , Brinkman , A W and Hashemi , T . 2001 . NTC characteristics of bismuth based ceramic at high temperature . Int J Inorg Mater. , 3 : 1219 – 1221 . doi: 10.1016/S1466-6049(01)00132-5
- Kalaycioglu , N O and Çırçır , E . 2011 . Synthesis, characterization and oxide ionic conductivity of binary (Bi2O3)1−x (Lu2O3) x system . J Chin Chem Soc , 58 : 1 – 4 . doi: 10.1002/jccs.201190067
- Ozpozan , N and Çırçır , E . 2012 . Measurement and properties of the oxide ionic conductivity of β- and δ-phases in the binary (Bi2O3)1−x (Tb4O7) x system . Synth React Inorg Met-Org Nano-Met Chem , 42 : 398 – 401 . doi: 10.1080/15533174.2011.611565

