Abstract
Four divalent transition chelate polymer compounds (1–4) were synthesised by condensation of ibpmpc (isophthaoyl bis(paramethoxyphenylcarbamide)) with metal acetate in a dimethylformamide medium. The synthesised polymer compounds 1–4 were characterised by elemental analyses, FT-IR, diffuse reflectance, magnetic moment measurements and thermal studies. Each metal ion is coordinated through an oxygen atom of the carboxylate group and a nitrogen atom of the amide group of ligand and two aqua ligands by the coordinated bond which formed a six-member heterocyclic ring and significantly formed octahedral geometry for 1–3, whereas distorted octahedral geometry for 4. The thermal properties were studied by TG/DTG/DTA analyses. It was found that the thermal stabilities of polymer compounds follow the order: 4 > 3 > 1 > 2 Also, their physical properties including solubility, colour and melting point were studied, and the result showed insolubility in common organic solvents. Moreover, SEM and XRD studies were carried out to determine the size and morphological behaviour of chelate polymer compounds. The electrical conductivity of 1–4 compounds were measured in compressed pellet form at 311 K which showed poor semiconducting nature.
1. Introduction
The construction of solid-state chelate polymer compounds has become an area of increasing curiosity in modern years because of their specific properties in useful materials. Transition metal chelate polymer compounds of organometallic material are environmentally benign, and they are lightweight and have outstanding thermal resistivity and insolubility in common organic solvents. Transition organometallic chelate polymer compounds with precisely controlled chain length have rapidly attracted and expanded considerable attention in recent years due to their imperative properties and potential applications, i.e. magnetic materials are used in data storage, electrical conductors, superconductors, electrochromic materials and catalyst including metaloenzymes.[Citation1,Citation2] Moreover, aromatic polyamide compounds of amide groups are –high-performance polymeric materials with outstanding mechanical properties, and excellent thermal and chemical stabilities.[Citation3–Citation7] From the coordination chemistry point of view, the dicarboxylic acid derivative is versatile ligand since it offers strongly basic donor centres in ligand geometry facilitating chelating towards metal ions and constructs excellent supra-molecular architecture with gorgeous artistic and valuable properties.[Citation8–Citation11] The coordination chemistry of organometallic chelate compounds of nitrogen-containing chelating ligands is an active area of research. Recently, valuable interest has been shown in the synthesis and use of functionalised compounds having chelating ability due to their practical convenience, operational flexibility and formation of coordination with high metal to polymer bond energy. In synthetic chemistry, there are many methods employed to synthesise new materials such as macrocyclic compounds or nanoscopic materials via template reactions.
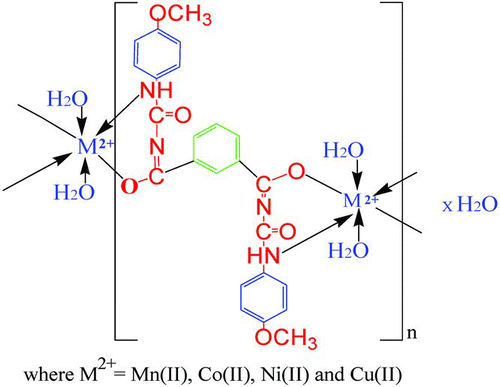
In our previous paper,[Citation12,Citation13] we have reported seven thermally stable chelate polymer compounds of bis(bidentate) ligand and compared their thermal properties by TG/DTG/DTA techniques at a heating rate of 10°C min−1 under nitrogen atmosphere. Herein, we have recently focused attention on synthesis of a series of transition metal chelate polymer compounds of aromatic amide bis(bidentate) ligand and discussed morphological and thermal properties. The main aim of our present work is to describe the thermal degradation studies of chelate polymer compounds (Figure ).
2. Experimental details
2.1. Materials and measurements
The chemicals used for the synthesis of bis(bidentate) ligand and chelate polymer compounds were of analytical grade. The infrared (IR) spectra of the bis(bidentate) chelating ligand and chelate polymer were recorded on Shimandzu FT-IR-8101A instruments. Thermogramometric analyses were carried out in the range of room temperature to 1220°C. Magnetic susceptibilities were determined by the Gouy's method at room temperature using Hg2+ [Co(SCN)4] as a calibrate. Solid reflectance spectral and SEM analyses were recorded at STIC Cochin University, Kerala, India.
2.2. Synthesis of ligand and chelate polymer compounds
Isophthaoyl bis(paramethoxyphenylcarbamide) (ibpmpc) ligand was synthesised in a two-step process and published.[Citation14] The metal acetate solution (0.01 mmol) was prepared into a 100 ml beaker; then it was mixed drop-wise under a hot condition with bis(bidentate) ligand (0.01 mmol) in 20 ml hot, dry dimethylformamide and refluxed [Citation15,Citation16] in an oil bath for 15–24 h at 150°C.
2.2.1. {[Mn (II) (ibpmpc)(H2O)2] 2H2O}n (1)
The yield of (1): 63.4%; colour: faint brown. Elemental anal: calcd for C24H28N4O10Mn (FW 586.93): C, 49.0%; H, 4.7%; N, 9.5%; Mn, 9.9%. Found: C, 48.9%; H, 4.8%; N, 9.4%; Mn, 9.8%. FT-IR (KBr, cm–1): 3220.23, 754, 3255, 1662, 1538, 2953, 1601, 1412, 475, 554; TG/DTG data (T DTG /°C): 69, 115, 200, 298, 989, 520; conductivity (Ω cm): 4.8 × 10−5; reflectance (λmax/cm−1): 16,025, 18,668, 23,992; magnetic moment, μeff: 5.94.
2.2.2. {[Co (II)(ibpmpc)(H2O)2] 2H2O}n (2)
The yield of (2): 61.1%; colour: purple; elemental anal: calcd for C24H28N4O10Co (FW 590.93): C, 48.7%; H, 4.7%; N, 9.5%; Co, 9.9%. Found: C, 48.3%; H, 4.5%; N, 9.6%; Co, 9.8%. FT-IR (KBr, cm−1): 3315, 749, 3253, 1660, 1556, 2953, 1602, 1431, 567, 667; TG/DTG data (T DTG /°C): 62, 111, 243, 384, 512, 796, 918, 612; conductivity (Ω cm): 5.7 × 10−5; reflectance (λmax/cm−1): 23,870, 19,120; magnetic moment, μeff: 4.82.
2.2.3. {[Ni (II)(ibpmpc)(H2O)2] 3H2O}n (3)
The yield of (3): 69.4%; colour: cement; elemental anal: calcd for C24H30N4O11Ni (FW 608.69): C, 47.3%; H, 4.9%; N, 9.2%; Ni, 9.6%. Found: C, 47.4%; H, 4.8%; N, 9.0%; Ni, 9.5%. FT-IR (KBr, cm−1): 3414, 732, 3391, 1632, 1515, 2953, 1600, 1412, 532, 618; TG/DTG data (T DTG /°C): 65, 209, 351, 1037, 535; conductivity (Ω cm): 6.9 ×10−5; reflectance (λmax /cm−1): 23,250, 26,315; magnetic moment, μeff: 3.10.
2.2.4. {[Cu (II)(ibpmpc)(H2O)2] 2H2O}n (4)
The yield of (4): 59.2%; colour: black; elemental anal: calcd for C24H28N4O10Cu (FW 595.54): C, 48.4%; H, 4.7%; N, 9.4%; Cu, 10.6%. Found: C, 48.3%; H, 4.5%; N, 9.3%; Cu, 10.5%. FT-IR (KBr, cm−1): 3432, 831, 3432, 1635, 1513, 2980, 1601, 1462, 1417, 487, 626; TG/DTG data (T DTG/°C): 86, 222, 334, 526, 1062, 1110, 965; conductivity (Ω cm): 7.2 × 10−5; reflectance (λmax/cm−1): 17421; magnetic moment, μ eff : 1.87.
3. Results and discussion
3.1. Spectral studies of chelate polymer compounds
The structure (Figure ) of resulting divalent transition metal chelate polymer compounds has been proposed on the basis of spectral, magnetic moment and thermal techniques. The IR spectra of 1–4 are compared with ligand to determine the changes that might have taken place during polymerisation. The IR spectrum of free ligand shows a strong band at 3353.99 cm−1 due to the N–H group frequency.[Citation17,Citation18] However, this band was shifted towards the higher or lower frequency in 1–4 [Citation19] due to collaboration of hydroxy group frequency, i.e. 3445–3510 cm−1 may be due to lattice water molecule.[Citation20,Citation21] The N–H group stretching frequency for polymer compound 1 was shifted at 3220.23, 3253.03 (2), 3391.13−1 (3) and 3432.96 cm−1 (4). A perceptible sharp long band was found around 1688.44 cm−1 due to the C=O bond in parent chelating ligand, whereas in polymer compounds 1–4 it is shifted towards the lower frequency [Citation22–Citation25] in the range of 1635.54–1668.00 cm−1, indicating the formation of a metal–oxygen bond,[Citation26,Citation27] which ultimately strengthened C=N bond as a result of six-member heterocyclic chelate ring formation (polymerisation). It may happened due to the tautomerisation of C=O to C=N mode and has been displayed in Scheme .
Scheme 1 Keto-enol tautomerism and covalent mode of ibpmpc in polymer compounds during polymerisation
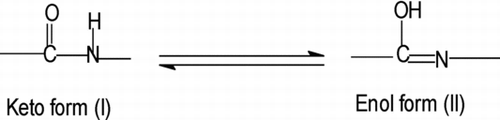
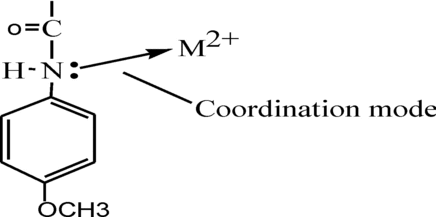
This suggests a covalent mode of ibpmpc to central metal ion by deprotonation, while the coordination mode by nitrogen atom. The coordination mode of ibpmpc in polymers is shown in Scheme .
The modes of coordination further supported by the noteworthy weak bands are in the range 335–557 cm−1 and 554–669 cm−1 in 1–4 due to the metal–oxygen (M–O) and metal–nitrogen (M–N),[Citation28,Citation29] respectively.
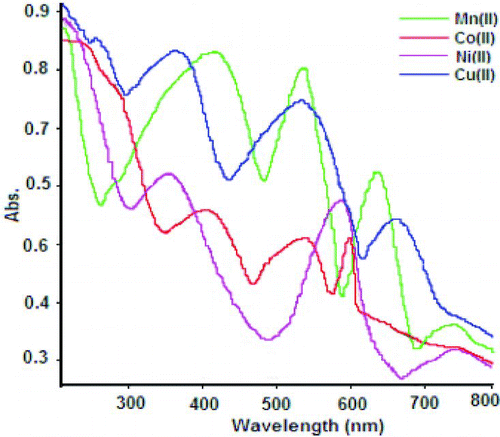
Furthermore, the diffuse reflectance spectral studies were carried out, which were genuinely helpful for determining the geometry of polymer compounds 1–4 The diffuse reflectance spectra of polymer compounds are shown in Figure . The reflectance spectra of 1 show sharp strong bands at 23,866 cm−1, 18,348 cm−1 and 16,025 cm−1, which may be assigned to the transitions 6A1g → 4T1g(G), 6A1g → 4T2g(G) and 6A1g → 4T1g(G), respectively, and the calculated magnetic moment value μ B = 5.74 BM. This indicates spins free, corresponding to highly paramagnetic nature and sp3d2 hybridisation; therefore, it is considered for outer octahedral stereochemistry.[Citation30,Citation22] The diffuse reflectance spectra of 2 and 3 showed three absorption bands, respectively, at 16,891, 18,832, 23,870 cm−1 and 26,881, 23,250, 14,810 cm−1 corresponding to the 4T1g → 4T1g(P), 4T1g → 4T2g, 4T1g → 2T2g(P) and 3A2g → 3T1g(P), 3A2g(F) → 3T1g(P), 3A2g → 3T1g(F) transitions [Citation31,Citation22] and calculated μ B : 4.82 BM and 3.1 BM corresponding to the sp3d2 hybridisation which indicates high-spin complexes; hence, favoured octahedral geometry for 2–3. The spectrum of 4 consists of a high-intensity broad band that is expected to show three permitted transitions 15,998, 17,421 and 20,286 cm−1 corresponding to 2T2g → 2Eg, 2B1g → 2Eg and 2B1g → 2B1g transitions,[Citation22] respectively. Hence, this band suggests a distorted octahedral geometry for Cu (II) ion; also supported by μ B : 1.87 BM.[Citation32,Citation20]
3.2. Physicochemical properties
The physiochemical properties of ligand and 1–4 have been discussed and interpreted. The ligand was recrystallised from a dimethylformamide-alcohol medium and obtained orange colour amorphous powder with yield 72%. Polymers 1–4 were obtained at different time intervals (15–24 h). Polymer 1 found faint brown with yield 63.4%, 2 purple with yield 61.1%, 3 cement with yield 69.4% and 4 black colour with yield 59.2%.
The polymeric products 1–3 were amorphous in nature, whereas 4 obtained in semi-crystalline form. The solubility of polymers were investigated with 0.01 g in 2 ml of solvent and tabulated in Table . All the chelate polymers were found to be insoluble in common organic solvents; however, 4 could be partially dissolved in organic polar solvents under a hot condition such as dimethyl sulfoxide and N,N-dimethyl acetamide. It was also noticed that the solubility of ligand increases suddenly when the temperature increases and partially soluble at 25°C; however, it gets dissolved at higher temperature. The solubility of ligand decreases as bulkiness of ligand increases. The melting point of ligand was determined by standard method and was found to be 355°C, whereas the melting point of 1–4 were determined by thermal analysis (TGA,DTG/DTA) due to their high thermal stabilities.
Table 1 Solubility of ibpmpc chelate polymer compounds in various solvents
3.3. Morphology and electrical conductivity
The morphological structures of 1–4 have been studied by using X-ray diffraction and SEM analysis. The diffractographs of 1–3 showed broad peak features, which indicate the amorphous nature and did not exhibit any anisotropic behaviour. Though an amorphous structure was shown by these polymers, yet they were insoluble in common organic solvents due to the chelate ring in all compounds. The XRD curve of 4 exhibits some long fine peak and data show the hallow pattern in the region 2ø = 10°–90°; which indicates the weak crystallinity with a pseudo-orthorhombic structure.
The scanning electron microscopy technique is remarkably helpful to classify the distinct morphology of ligand and 1–4 and was recorded at an energy of 20 kV with magnification ×10,000 and has been shown in Figure . The micrograph of 1 (Figure (a)) was found to be of flat bead shape, and it also appears to be in the shape of various small globule droplets or beads [Citation33] due to polymerisation of compounds. The bigger single bead has length 1.63 nm and diameter 851.53 nm. Figure (b) shows a flat rod with broken ends.
Figure 3 SEM images of chelate polymer compounds (a) Mn (II), (b) Co (II), (c) Ni (II) and (d) Cu (II)
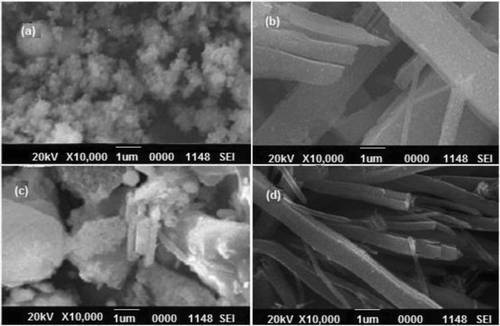
The SEM of 3 (Figure (c)) showed a small irregular shape particle entrapped on a bigger size particle like agglomerisation of thick flake,[Citation33] which indicates the accumulation of a number of small particles and formation of a polymer chain. The micrograph of 4 (Figure (d)) shows a couple of tentacle structures or it is likely to be appeared in a small fine sharp needle shape; which showed that the compounds are in polymeric form. The electrical conductivities of all 1–4 were measured in compressed pellet form at 311 K by the two-probe method. The data were obtained at an average range of 7.2–1.9 × 10−5 Ω cm−1, which indicates the moderate semiconducting nature. The low conductivities are shown by 1 and 2; therefore, they behave as poor semiconducting materials. On the other hand, the medium order of electrical conductivity was shown by the 3 and 4 polymers; hence they might act as moderate semiconducting materials.
3.4. Thermal properties
The thermal degradation TG/DTG curves of chelate polymer compounds has been shown in Figures and . The initial, final decomposition temperature and total mass losses for each step in the thermal decomposition of polymer have been investigated by using thermal analysis at a heating rate 10°C min−1 under nitrogen atmosphere. The obtained data have been reported in Tables and , together with the temperature of greatest rate of decomposition-evolved moiety and theoretical percentages of mass losses. Peak temperatures and initial and final decomposition temperatures of 1–4 were identified at a distinct step by DTG analysis, whereas the DTA method was used to evaluate the endothermic or exothermic weight loss.
Table 2 Thermal behaviour data of ibpmpc chelate polymer compounds
Table 3 TG/DTG/DTA data and assignments of ibpmpc chelate polymer compounds
Figure 4 TG/DTG thermograph of chelate polymer compounds (a) Mn (II) and (b) Co (II) at a heating rate of 10ºC min−1 in nitrogen atmosphere
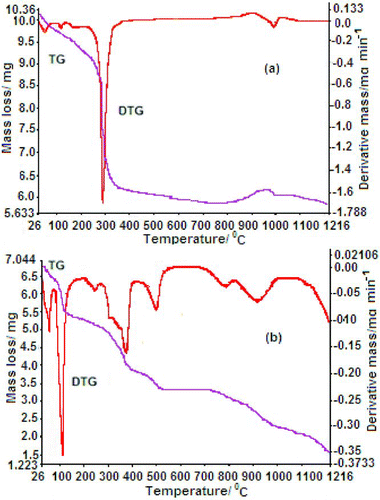
Figure 5 TG/DTG thermograph of chelate polymer compounds (a) Ni (II) and (b) Cu (II) at a heating rate of 10ºC min−1 in nitrogen atmosphere
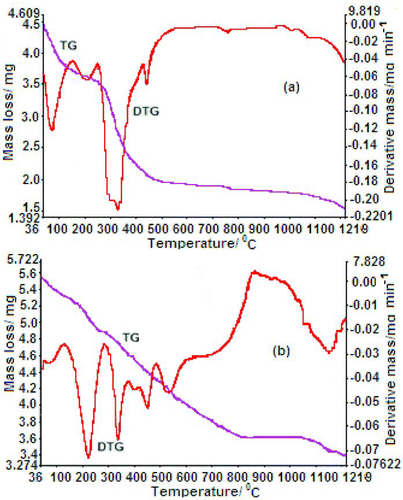
The thermal stability properties of divalent transition chelate polymer compounds were evaluated by using the TG/DTG and DTA methods, and the results revealed good thermal stability for 1–4. Polymers compounds 1–4 start to lose weight at around 40°C–145°C, which may be due to the removal of the uncoordinated water, and start to lose weight at 145°C–270°C due to the loss of coordinated water which was supported by elemental analyses and IR data. From DTG curves (Figures and ), the T DTG peak temperature (i.e. the temperature at which maximum weight loss was observed by DTG) for 1, 2, 3 and 4 was observed to be 69°C, 62°C, 65°C and 86°C, respectively, which may correspond to the loss of lattice water. Hence, it is revealed that all polymer compounds must have initial decomposition at lower temperature due to the presence of lattice water and it was also supported by endothermic and exothermic T DTA and T DTG peaks that are given in Table . However, T DTG peaks were observed at 200°C, 243°C, 209°C and 222°C for polymers 1–4, respectively, which correspond to the removal of coordinated water. The endothermic and exothermic peaks were evaluated by DTA studies, and data have been tabulated in Table . After that, the gradual weight loss was observed in the range of 265°C–1200°C for 1–4, which corresponds to decomposition of organic moieties attached to the central metal ion and later on it forms stable species (metal oxide) since no weight loss was observed after 1220°C.
The last step of the degradation process in polymer compounds was organic moieties. The backbone degradation in 1 (Figure (a)) passed through two steps, i.e. third and fourth steps. The mass loss in the range 272°C–700°C (60%) for the third step with a T DTG peak (298°C) and two T DTA weak peaks was observed at 297°C and 326°C which may correspond to mass loss (obs. 47.8%, calcd 47.2%). In the fourth step (700°C–1216°C), the mass loss was found to be 26.6% out of remaining ligand and formed metal oxide with T DTG peak at 989°C and broad weak endothermic T DTA peak at 887°C found due to the released organic ligand. In the third step (270°C–600°C) for 2 (Figure (b)) two strong T DTG peaks were found at 384°C and 512°C corresponding to the release of 40% of ligand, and 60% mass loss of ligand in the fourth step (600°C–1216°C) with T DTG peak at 796°C and 918°C. According to DTA curve, in the third step three T DTA weak peaks were observed at 387°C, 489°C and 507°C and in the fourth step two endothermic T DTA peaks were observed at 855°C and 891°C, corresponding to the release of ligand and the formation of metal oxide. The mass loss (100%) in the third step (260°C–1219°C) were observed two long T DTG peaks at 336°C and 1037°C and showed two broad endothermic peaks T DTA at 451°C and 661°C corresponding to the mass loss of 75.6% (calcd 75.9%) is assigned to the release of organic moiety in 3 (Figure (a)) with the formation of metal oxide. The mass losses of 45% (35.3%) for 4 (Figure (b)) at third step (276°C–800°C) with two strong T DTG peaks at 334°C and 516°C and was supported by two T DTA peaks at 700°C and 354°C; hence, it indicates the mass loss of ligand. The slow rate mass loss at fourth step (800°C–1219°C) were observed with two broad T DTG peaks at 1062°C and 1110°C, and according to the DTA curve, two T DTA peaks at 961°C and 1065°C which correspond to the loss of 55% of ligand (obs. 37.4%, calcd 42.6%) and at last, formed metal oxide.[Citation34]
Moreover, the thermal stabilities of polymer compounds were studied based on 5%, 10% and 20% weight loss (T 5, T 10 and T 20) and provided in Table . Furthermore, the thermal stabilities of transition (II) chelate polymer compounds are measured on the basis of char yield that is also summarised in Table .
Higher the value of residue, higher will be thermal stability.[Citation35] The char yield may be applied as a decisive factor for estimating the limited oxygen index of chelate polymers using Van Krevelen and Hoftyzer's equation [Citation36] and calculated data presented in Table . Char residue left after decomposition was in the range 85%–87%. Char residue data show that more residue value is obtained for 4 at 1218°C and the order of char residue was the same as obtained by the T i (TG), T DTG and T f (T DTG) values of decomposing ligand. In conclusion, the thermal parameters of polymer compounds were significantly inferred in the presence of hydration water and high thermal stability. In summary, the complete backbone (ligand) degradation were taken place in two steps (283°C–1216°C) for 1, two steps (281°C–1216°C) for 2, one step (285°C–1219°C) for 3 and two steps (310°C–1219°C) for 4.
Therefore, the inflation of DTG curves of chelate polymer compounds at temperature below 1220°C indicates the decomposition of carbamide moiety, leaving metal oxide. On the basis of organic moiety degradation T i (TG) was found for 1 at 283°C, for 2 at 281°C, for 3 at 285°C and for 4 at 310°C, T f (T DTG) was found for 1 at 993°C, for 2 at 942°C, for 3 at 1037°C and for 4 at 1110°C, whereas T h (T DTG) was observed for 1 at 520°C, 2 at 512°C, 3 at 535°C and 4 at 965°C. Hence, in order to check out the thermal stability of polymer on the basis of char residue, T i (TG), T DTG, T h (T DTG) and T f (T DTG) follow the order 4 > 3 > 1 > 2.
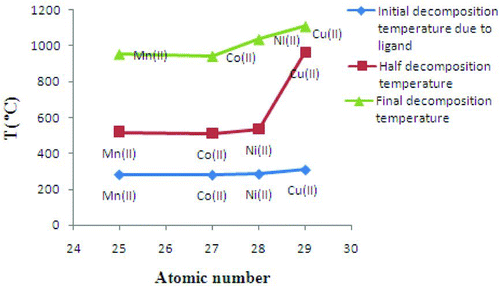
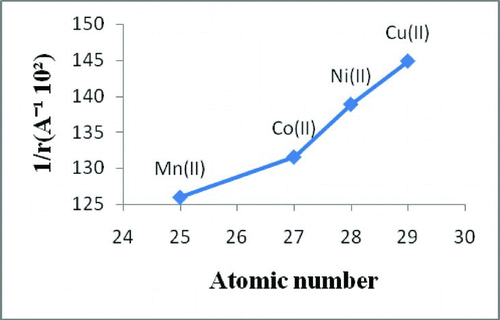
Besides, in order to check out the more thermal stability of divalent chelate polymer compounds, the initial decomposition temperature due to ligand, half decomposition temperature and final decomposition temperature are compared. A graph of decomposition temperature versus atomic number has been plotted and shown in Figure . It reveals greater thermal stability for copper (II) ion, while cobalt (II) shows less stability. Additionally, a graph of reciprocal of ionic radii of divalent transition metal ions against the atomic number has been plotted and shown in Figure . It unveils the same order of thermal stability for chelate polymer compounds. Herein, the study shows that there is a relationship between the ionic radius of the divalent transition metal ions and the thermal stability of the chelate polymer compounds. The reciprocal of ionic radii of divalent transition metal ions of polymer compounds increases from manganese to copper with increasing initial decomposition temperature due to ligand, and the thermal stability increases with the exception of manganese ion. Consequently, the thermal stability of chelate polymer compounds increases as the ionic radii of divalent transition metals decrease.
4. Conclusions
Divalent transition metal chelate polymer compounds with chelating ligand were synthesised and characterised with spectral, magnetic moment, thermal, SEM, XRD and physicochemical studies including solubility. The results inferred the insolubility in common organic solvents. The presence of coordinated or uncoordinated water for all polymers was also found out. The size and morphological behaviour of chelate polymers compounds were studied by SEM. In this research article, the thermal degradation behaviour of all chelate polymer compounds has been investigated by TG/DTG and DTA techniques at a heating rate of 10°C min−1 under nitrogen atmosphere.
Acknowledgements
The research was supported from the University Grants Commission New Delhi, under Rajiv Gandhi National Fellowship.
Notes
+, soluble at room temperature; +h, soluble on heating; −, insoluble; ± h, partially soluble on heating.
a, the initial degradation temperature for ligand in chelate polymer compounds; b, peak temperature; c, final step peak temperature; d, half decomposing temperature; e–g, temperature corresponding to 5%, 10%, 20% weight losses; and h, decomposed material left after TG analysis at 1200ºC; i, LOI – limiting oxygen index.
References
- Hussein , M A and Asiri , A M . 2012 . Organometallic ferrocene and phosphorus-containing polymers: synthesis and characterization . Des Monomers Polym. , 15 : 207 – 251 .
- Holliday , B J and Swager , T M . 2005 . Conducting metallopolymers: the role of molecular architecture and redox matching . Chem Commun. , 1 : 23 – 36 .
- Koohmareh , G A and Souri , Z . 2011 . Synthesis and characterization of some new thermally stable crystalline polyamides and co-polyamides bearing bipyridine side-chain groups . Des Monomers Polym. , 14 : 475 – 485 .
- Ravikumar , L , Saravanan , R , Saravanamani , K and Karunakaran , M . 2009 . Synthesis and characterization of new polyamides with substitutions in the pendent benzylidene rings . Des Monomers Polym. , 12 : 291 – 303 .
- Isfahani , H N , Faghihi , K , Hajibeygi , M and Bokaei , M . 2010 . Synthesis and properties of new optically active polyamides containing 1,3 dioxoisoindolin-2-yl units as pendent groups and aromatic diamines . Des Monomers Polym. , 13 : 237 – 247 .
- Cassidy , P . 1980 . Thermally stable polymers , New York : Marcel Dekker .
- Sheng-Hue , I H , Guey-Sheng , L , Yi-Chun , K and Tzu-Jung , H . 2010 . Synthesis and properties of new aromatic polyamides with redox active 2,4-dimethoxytriphenylamine moieties . J Polym Sci Part A: Polym Chem. , 48 : 3392 – 3401 .
- Xie , Y , Bai , F Y , Xing , Y H , Wang , Z , Pu , Z F and Ge , M F . 2010 . Lanthanide (III) coordination polymers with longer-spanning flexible ligand: Synthesis, crystal structure and luminescence property . J Inorg Organomet Polym. , 20 : 258 – 263 .
- Yang , L , Yu , W , Zhang , T-L , Zhang , J-G , Liu , Z-H and Wu , R . 2009 . Crystal structure of dimeric units Cd2(ncpo)2(phen)2(H2O)2 constructed by flexible dicarboxylic acid with fluorescent emission . Chinese J Struct Chem. , 4 : 405 – 408 .
- Qin , L , Liu , L W , Xu , D and Cui , G . 2013 . Two dinuclear copper (II) complexes based on 5,6-dimethylbenzimidazole ligands: synthesis, crystal structures and catalytic properties . Transition Met Chem. , 38 : 85 – 91 .
- Xia , H Y , Liu , G X , Nishihara , S and Wang , Y . 2010 . Syntheses and characterizations of three Cu(II) complexes with 2,20-bipyridine-3,30-dicarboxylate and n-donor ancillary ligands . J Inorg Organomet Polym. , 20 : 110 – 117 .
- Chaudhary , R G , Juneja , H D and Gharpure , M P . 2012 . Thermal degradation behaviour of some metal chelate polymer compounds with bis (bidentate) ligand by TG/DTG/DTA . J Therm Anal Calorim. , 112 : 637 – 647 .
- Chaudhary , R G , Juneja , H D and Gharphure , M P . 2012 . “ Thermal decomposition studies of some metal chelate polymers ” . In Eighteenth International Symposium and Workshop on Thermal analysis. Thermans-2012. Proceeding (Paper presented) , 252 – 254 . Mumbai , , India : BARC . Jan 31–Feb 4
- Chaudhary , R G , Gharpure , M P and Juneja , H D . 2012 . Facile synthesis of chelating bis ligands: spectroscopic, physicochemical and biological studies . Int J Appl Biol Pharm Technol. , 3 : 88 – 98 .
- Bonde , A D , Juneja , H D , Ukey , V V , Ghubde , R S and Husain , R . 2006 . Synthetic, spectromagnetic and thermal studies of chelate polymers of azelaoyl-bis-p-chlorophenyl urea . J Mate Sci Eng: B , 132 : 16 – 19 .
- Chaudhary , R G , Juneja , H D , Gandhare , N V and Gharpure , M P . 2013 . Synthesis, characterization and morphology behaviour of Mn (II), Co (II), Ni (II) and Cu (II) chelate polymer compounds based on chelating ligand . Res J Pharm Biol Chem Sci. , 4 : 1625 – 1636 .
- Asundariya , S T , Patel , P R and Patel , K C . 2009 . New copolyesters based on s-triazine derivatives . Int J Polym Mater. , 58 : 692 – 705 .
- Shockravi , A , Javadi , A and Abouzari-lotf , E . 2011 . Fluorinated ortho-linked polyamides derived from non-coplanar 1,1 thiobis (2-naphthol): synthesis and characterization . Polym J. , 43 : 816 – 825 .
- Basavaraju , B , Dolaz , M and Tumer , M . 2007 . Transition metal complexes of quinolino [3, 2, b] benzodiazepine and quinolino [3, 2, b] benzoxazepine: synthesis, characterization and antimicrobial studies . Bioinorg Chem Applicat. , 29 : 516
- Mohammad , A and Inamuddin A , A . 2012 . Surfactant assisted preparation and characterization of carboxymethyl cellulose Sn (IV) phosphate composite nano-rod like cations exchanger . J Therm Anal Calorim. , 107 : 127 – 134 .
- Habibi , M H and Askari , E . 2012 . Synthesis of nanocrystalline zinc manganese oxide by thermal decomposition of new dinuclear manganese (III) precursors . J Therm Anal Calorim. , 111 : 1345 – 1349 .
- Patel , H S and Patel , D J . 2010 . Coordination polymer based on bis-ligand containing indole and 8-hydroxyquiniloine moieties . Int J Polym Mater. , 39 : 307 – 317 .
- Sharma , K , Singh , R , Fahmi , N and Singh , R V . 2010 . Microwave assisted synthesis, characterization and biological evaluation of palladium and platinum complexes with azomethines . Spectrochim Acta Part A. , 75 : 422 – 427 .
- Messimeri , A , Nastopoulos , V , Terzis , A , Raptopoulou , C P , Papadimitriou , C and Perlepes , S P . 2005 . The succinamate (–1) ligand in 4f-metal chemistry: First example of amide coordination in the double-chain 1D coordination polymer catena-aquatris (succinamato (–1)) erbium (III) . Inorg Chem Comm. , 8 : 578 – 584 .
- Xie , Y , Bai , F Y , Xing , Y H , Wang , Z , Pu , Z F and Ge , M F . 2010 . Lanthanide (III) coordination polymers with longer-spanning flexible ligand: synthesis, crystal structure and luminescence property . J Inorg Organomet Polym. , 20 : 258 – 263 .
- Syamal , A , Singh , M M and Kumar , D . 1999 . Synthesis and characterization of chelating resin containing ONNO donor quadridentate Schiff base and its coordination complexes with copper (II), nickel (II), cobalt (II), iron (III), zinc (II), cadmium (II), molybdenum (IV) and uranium (VI) . React Funct Polym. , 39 : 27 – 35 .
- He , L B , Mao , H P , Chao , D M and Zhang , W J . 2007 . Electroactive azo polyamide based oligoaniline: synthesis and characterization . Polym J. , 39 : 1172 – 1176 .
- Yina , M , Wua , C , Yanga , X , Wangab , Y and Fana , Y . 2010 . Synthesis, crystal structure and characterization new zinc complex flexible ligand (4-amino-1,2,4-triazole-3,5-diyldithio) diacetic acid and 4,4′-bipyridine. . Synth React Inorg Met Org Chem , 40 : 798 – 804 .
- Foziah , A , Saif , A L and Refat , M S . 2012 . Synthesis spectroscopic and thermal investigation of transition and non transition complexes of metformin as potential insulin-mimetic agents . J Therm Anal Calorim. , 111 : 2079 – 2096 .
- Dixit , B C , Dixit , R B and Desai , D J . 2009 . Synthesis, characterization and material application of novel polyimide . Int J Polym Mater. , 58 : 229 – 242 .
- Refat , M S . 2010 . Synthesis, spectroscopic characterization, thermal, and photostability studies of 2-(2-hydroxy-5-phenyl)-5-aminobenzotriazole complexes . J Therm Anal Calorim. , 102 : 1095 – 1103 .
- Mohamed , G G , Omar , M M and Hindy , A M . 2006 . Metal complexes of Schiff bases: preparation, characterization and biological activity . Turk J Chem. , 30 : 361 – 382 .
- Sadek , S A , Solyman , S M , Abdel-Samd , H S and Hasan , S A . 2010 . Catalytic behaviour of cobalt (II) pthalocyanine immobilized on bentonite clay in bulk polymerization of methyl methacrylate . Int J Polym Mater. , 59 : 353 – 559 .
- Aslanidis , P , Gaki , V , Chrissafis , K and Kantouri , M L . 2011 . Luminescence and thermal behavior by simultaneous TG/DTG–DTA coupled with MS of neutral copper (I) complexes with heterocyclic thiones . J Therm Anal Calorim. , 103 : 525 – 531 .
- Arora , S , Aneja , D K , Kumar , M , Sharma , C and Prakash , O . 2012 . Thermal studies of some biological active oxadiazoles . J Therm Anal Calorim. , 111 : 17 – 25 .
- Van Krevelen , D W and Hoftyzer , P J . 1976 . Properties of polymers , New York : Elsevier .