ABSTRACT
While sociodemographic predictors of cervical cancer (CC) are well understood, predictors of high-risk (HR) human papillomavirus (HPV) infection have not been fully elucidated. This study explored the HR-HPV infection positivity in relation to sociodemographic, sexual behavior characteristics and knowledge about HPV and CC prevention among women who visited the Arkhangelsk clinical maternity hospital named after Samoylova, Russia. This cross-sectional study was conducted in the city of Arkhangelsk, Northwest Russia. Women who consulted a gynecologist for any reason between 1 January 2015 and 30 April 2015 were residents of Arkhangelsk, 25–65 years of age were included. The Mann–Whitney and Pearson’s χ2 tests were used. To determine the HR-HPV status, we used the Amplisens HPV-DNA test. We used a questionnaire to collect the information on sociodemographic factors. Logistic regression was applied. The prevalence of HR-HPV infection was 16.7% (n = 50). HR-HPV infection was more prevalent in younger women, cohabiting, nulliparae, smokers, having had over three sexual partners and early age of sexual debut. The odds of having a positive HR-HPV status increased by 25% with an annual decrease in the age of sexual debut. Moreover women with one child or more were less likely to have positive HR-HPV status.
Introduction
Human papillomavirus (HPV) is one of the most common sexually transmitted infections worldwide. Most sexually active individuals of both sexes will acquire it at some points in their lives [Citation1–Citation3]. HPV genotypes are classified as high-risk (HR) and low-risk based on their ability to cause cervical cancer (CC) [Citation4]. The presence and persistence of high-risk human papillomavirus infection (HR-HPV) infection is a key etiological factor in the development of carcinoma of the cervix and accounts for approximately 4.8% of all cancers [Citation5]. CC accounts for 7.5% of all female cancers [Citation6]. It therefore significantly affects morbidity and mortality risks worldwide [Citation5]. CC has been termed “a disease of disparity”. Within the European Union the highest CC mortality is seen in countries with the lowest screening programmes participation rates [Citation7]. High participation in screening programmes employing the Papanicolaou test (cytology-based) and subsequent treatment of precancerous lesions has effectively reduced the CC mortality [Citation8,Citation9]. Nevertheless, CC is considered a major public health issue in Sweden, This country has the 9th lowest CC mortality rate in the EU, high participation rate and screening programme in place since 1967 [Citation7,Citation10,Citation11]. In addition, HPV is also associated with increased risk of head and neck, vaginal, vulvar, penile and anal cancers [Citation11,Citation12]. However, the prevalence of HPV in Russia remains unknown or is controversial [Citation13].
CC is the fourth most common female malignancy worldwide with estimates of 528 000 of new cases and 266 000 deaths in 2012 [Citation6]. Most of the cases arise from countries with low-income or middle-income economies, where preventive resources are limited [Citation14]. According to the report of International Agency for Research on Cancer, CC is the second most frequent cancer among women aged 15–44 years in Russia [Citation15]. It is estimated that 64.5 million Russian women aged 15 years and older are at risk of developing this cancer, and 15 342 women are diagnosed with CC, and 7 371 women die from the disease every year [Citation15].
Women positive for HR-HPV DNA have an increased risk of developing CC. HPV infection is the most important but not the only risk factor for CC. Many epidemiological studies have so far focused on the role of co-factors associated with the cancer development and progression [Citation1]. While sociodemographic predictors of CC are well understood, those of HR-HPV infection have not been. Epidemiological chain of infection involves both women and men [Citation16,Citation17]. Both men and women at the same time could be carriers without symptoms, transmitters and the ones who experience active infection [Citation18].
Predictors of HPV may differ from those of CC because the development of the latter is influenced by many variables, including co-factors involved in carcinogenesis and adherence to screening guidelines [Citation16,Citation19,Citation20]. Several studies reported an association between HPV positivity and lifetime number of sexual partners [Citation16,Citation19,Citation21]. On the other hand, the influence of age at sexual debut and condom use on the risk of being HPV positive is questionable [Citation20,Citation22,Citation23]. Other potential risk factors for cervical HPV infection include tobacco smoking [Citation24], nulliparity [Citation25,Citation26], and use of oral contraceptives [Citation25]. Geographic and cultural variations in the sexual behaviour of women and their partners have also been reported to be strongly associated with HPV acquisition [Citation19,Citation27].
Representative information about HR-HPV infection in relation to different sociodemographic and sexual behaviour characteristics have not been available in Russia. Such data may be useful in designing effective preventive public health strategies to reduce CC and related mortality.
Aims
(i) To estimate prevalence of HR-HPV infection in women who visited Arkhangelsk clinical maternity hospital named after Samoylova in Arkhangelsk, Russia; (ii) to identify sociodemographic characteristics and sexual behaviour associated with HR-HPV status; and (iii) to assess a possible association between the HR-HPV status and knowledge about HPV and CC prevention.
Materials and methods
Study design, setting, participants and data collection
This cross-sectional study was conducted between 1 January and 30 April 2015 in the city of Arkhangelsk, which is the administrative centre of Arkhangelsk County in Northwest Russia. In 2015 the population of Arkhangelsk was 351 488, of whom 194 765 were females, while 109 096 were in the 25–65 age group [Citation28].
Women who were residents of Arkhangelsk, aged 25 to 65 years and consulted a gynaecologist for any reason at the Samoylova Clinical Maternity hospital were recruited. Since there are no national guidelines that regulate the cervical screening age in Russia, we adopted that of the UK NHC Cervical Screening Programme (NHSCSP). The latter recommends that routine screening be conducted during the 25–65 age interval [Citation29]. A sample size of 300 with an HPV prevalence of 10% was calculated to satisfy the condition (1 – β) ≥ 0.80 at α = 0.05. The final analytical sample was 300, which constitutes 86% of women recruited.
All participants completed a questionnaire entitled “HPV and CC Related Factors Questionnaire” which sought information on the participants’ knowledge about HPV and CC prevention and as described previously [Citation30] included 14 questions pertaining to: sociodemographic characteristics (age, education, marital status, parity, smoking) and sexual behaviour characteristics (age of sexual debut, history of sexually transmitted infections, contraception, history of the papanicolaou test (the Pap test) and related abnormal findings and their management).
At enrolment, women underwent a pelvic examination with collection of cervical specimens for cervical cytology and for HR-HPV DNA genotyping. The cervical smears were collected with a cervical cytobrush and were transported to the laboratory and stored in a buffer solution. Liquid-based cytology (LBC) (SurePath Technology; TruePath Imaging Inc, Burlington, NC) samples were sent to the central laboratory for cytological diagnosis, HPV detection and genotyping (Central Research Institute of Epidemiology “Rospotrebnadzor”, Moscow, Russia). Pap smears were prepared and reviewed by the study pathologist who was not aware of the HPV results. Abnormalities were reported according to the Bethesda System 2001 [Citation31]. Smears were classified as normal, atypical squamous cells of undetermined significance (ASCUS), low-grade squamous intraepithelial lesions (LSIL), high-grade squamous intraepithelial lesions (HSIL) or carcinoma. Women with ASCUS and negative HPV test were recommended to repeat both tests in 6 months. Women with other abnormal cytological findings and/or positive HPV test were referred to colposcopy and histologic confirmation.
HPV positivity was determined using an HPV DNA test (AmpliSens® HPV HCR screen-titre-FRT PCR kit, InterLabService, Moscow, Russia), which is an in vitro nucleic acid amplification test for qualitative and quantitative detection in biological materials of DNA of HPV of high carcinogenic risk (HCR). This test is able to detect DNA of HR-HPV of the four phylogenetic groups, namely A7 (HPV types: 18, 39, 45, and 59), and A9 (HPV types: 16, 31, 33, 35, 52, and 58), A5 (HPV type 51) and A6 (HPV type 56). The analytical sensitivity was 1 × 103 copies/ml. An endogenous internal control was present in all our HPV kits, which allowed an assessment of the control stages of PCR (DNA isolation and amplification) and an evaluation of sample quality and storage adequacies. When epithelial swab quality was not sufficient (i.e. insufficient number of epithelial cells in the clinical sample), the signal of β-globin gene was significantly lower. This β-globin based Internal Control protocol significantly reduces false negative results caused by a poor clinical sample quality. Samples were considered to be positive with an HPV-DNA threshold of 1pg/ml, which is recommended by the USA Food and Drug Administration (US FDA). Automated results were confirmed with blind manual readings undertaken by experienced laboratory personnel.
Variables
For the analyses, women were grouped based on their age (25–29, 30–39, ≥ 40), marital status (married, cohabiting, or single including divorced and/or widowed), parity (0, 1, or ≥ 2 deliveries), and education (university level or less). Age at sexual debut (in years) was used as a continuous variable, and the number of lifetime sexual partners was recorded as 3 or less and more than 3. History of sexually transmitted infections was categorised into ever-had or never-had. Smoking was designated as yes or no (ever and never smokers). Contraception was categorised into use of condoms (yes and no) and use of combined oral contraceptive pill for over 5 years (yes and no). Participants’ knowledge about HPV and CC prevention was used as both a discrete variable (number of correct answers from 0 to 14) and a binary variable. We considered having at least 50% of the questions answered correctly (7 out of 14) as a sufficient level of knowledge, and less than 50% of the questions answered correctly (6 or less out of the 14 questions) as a poor.
HR-HPV infection was used as a binary variable (being positive or negative for any type of HR-HPV) and were categorised into the four groups HR-HPV infection types mentioned, as well a mixed group that included HR-HPV types from both group A9 and group A7.
Data analyses
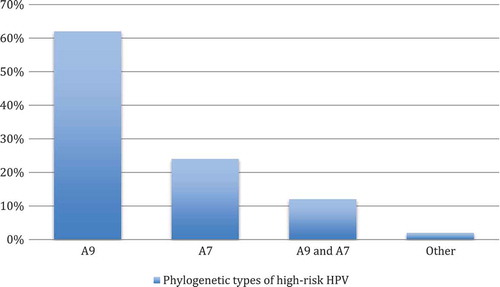
The proportion of HR-HPV types are described and displayed graphically in . Sexual debut and number of correct answers were presented as the median with the first and third quartiles. All the other variables were shown as numbers and percentages. Statistical comparison between women with and without HR-HPV infection was carried out using the Mann–Whitney U test for continuous variables and Pearson’s χ2 test for categorical variables.
Figure 1. Distribution of the phylogenetic types of high-risk HPV in women who visited Arkhangelsk Clinical Maternity Hospital, Russia (n = 50).
Footnote: Phylogenetic types of high-risk HPV (in per cent): A7 (HPV types 18, 39, 45, and 59); A9 (HPV types 16, 31, 33, 35, 52, and 58); and other: A5 (HPV type 51) and A6 (HPV type 56).
We used logistic regression analysis to estimate possible associations between the HR-HPV status as the outcome and age, parity, age at sexual debut and number of sexual partners as predictors. We tested for linear trends by entering ordinal variables as continuous in the regression analyses. As we aimed to explore risk factors that are causally related to the outcome these predictors were chosen based on the previous knowledge taking into account the cross-sectional design of the study and excluding potential mediators. Crude and adjusted odds ratios (ORs) were calculated with 95% confidence intervals (95% CIs). The value of p < 0.05 was considered to be statistically significant. The statistical analyses were carried out using SPSS version 24 (SPSS Inc., Chicago, IL, USA).
Ethical approval was obtained from the Research Ethics Committee of the Northern State Medical University of Arkhangelsk, Northwest Russia (Registered Report Number 08/12–14 from 10.12.2014) and from the Norwegian Regional Committee for Medical and Health Research Ethics, Tromsø, Norway (Registered Report Number 2014/1670). All study participants provided a written informed consent.
Results
HPV prevalence
Of the 300 women recruited and examined in the study, 16.7% (n = 50) were positive for HR-HPV. The most commonly detected HPV types were group A9 and then group A7 (). Multiple infections were detected in 14% (n = 7). Around 97% (n = 292) of the study participants had no pathological findings in the Pap smear, while 2% (n = 6) had L-SIL and 0.7% (n = 2) were assigned as having atypical squamous cell undetermined significance (ASCUS) changes in the smear.
Associations between sociodemographic characteristics, sexual behaviour and knowledge about HPV and cervical cancer prevention and the high-risk HPV infection
Women aged 25–29 years (p = 0.013), those cohabiting (p = 0.011), being nulliparae (p = 0.009), smokers (p = 0.011) and having more than three sexual partners (p = 0.034) were more prevalent to have positive HRH PV status rather than negative (). Moreover, women with a positive HR-HPV status had sexual debut at earlier age than women with a negative HR-HPV status (p = 0.001). The prevalence of positive and negative HR-HPV infections did not differ in women with different educational levels, those with previous abortions, hormonal contraceptive use, condom use and history of sexually transmitted infections ().
Table 1. Associations between sociodemographic and sexual behaviour characteristics and HPV status in women who visited Arkhangelsk Clinical Maternity Hospital named after Samoylova, Russia.
Regardless of their HR-HPV status, the study participants answered correctly more frequently to the following questions/statements: “The chance of getting HPV increases with number of sexual partners”; “What is the main hazard of HPV for female”; and “HPV vaccine is most effective if given to individuals who have never had sex” (). The statement “Most HPV types can clear up on their own if left untreated” was the most poorly answered. We found no difference in the numbers of correct answers between women with positive and negative HPV status (). The prevalence of poor knowledge was not significantly different for participants with positive and negative HPV status (28.0% versus 18.4% p = 0.121, respectively).
Table 2. Associations between knowledge about HPV and cervical cancer prevention and HPV status in women who visited Arkhangelsk Clinical Maternity Hospital named after Samoylova, Russia (n = 300).
Factors associated with positive HPV status
In the crude analyses, risk of being positive for HR-HPV infection increased gradually with being younger and with lower parity: p-values for linear were 0.012 and 0.007, respectively (). Odds of having positive HR-HPV status increased with an increased lifetime number of sexual partners and with a younger age at sexual debut. After adjustment for all variables presented in , associations with age and the number of sexual partners were no longer significant.
Table 3. Risk factors for positive high-risk HPV status among women who visited Arkhangelsk Clinical Maternity Hospital named after Samoylova, Russia (n = 300).
Discussion
To the best of our knowledge, this study is the first in Russia to explore potential risk factors and knowledge about HPV and CC prevention in relation to HR-HPV infection. In addition to young age, a lifetime number of more than 3 sexual partners, nulliparity, cohabiting, smoking and having earlier age at sexual debut were associated with HR-HPV status. The independent risk factors for positive cervical HR-HPV infection among women aged 25–65 years consulting gynaecologists in the Samoylova Clinical Maternity hospital were early age at sexual debut and nulliparity.
The observed prevalence of HR-HPV infection in our study was 16.7%, which is higher than the worldwide prevalence of HPV infection in women without cervical abnormalities of 11–12% [Citation5,Citation32]. Higher prevalence rates have been reported in the Caribbean (35.4%), sub-Saharan African (24%) and Eastern European (21%), while those reported for North America (4.7%) and Western Asia (1.7%) were lower compared to our estimate [Citation5]. The estimates of HR-HPV prevalence vary between the regions, and this could in part be due to different ages of the study populations, use of different methods of HPV detection, different screening programme implemented or variation in study designs and exposure to HPV risk factors [Citation32].
The prevalence of HPV among women in Russia has been poorly documented and is restricted primarily to studies conducted in Saint-Petersburg, where HR-HPV positivity of 13% (n = 107) has been reported [Citation33]. A cohort study of women at risks for HPV infection in three independent states of the former Soviet Union suggested an overall HPV prevalence of 33.4% [Citation34]. Differences in the prevalence estimates within Russia may be partly explained by the disparity in the study populations. Age is one of the major determinants of the HPV infection prevalence. In our study group, younger aged women (age groups 25–29 and 30–39) tended to have an HPV positive status more often than those aged 40 years or older. These findings are in accordance with other studies that show that HPV infection steeply decreases with age [Citation20,Citation22]. In general, the younger a population is, the higher the prevalence of HPV infection. It has been suggested that most HR-HPV infections in young women are transient and often involve sexual contacts with new partners, and that persistent infections occur in a small proportion of women [Citation20].
One possible explanation for our observation that cohabiting is associated with HPV infection could be that the time since last exposure to HPV is longer in married women than in singles. Also sexual activity in single people tends to be sporadic [Citation35]. Nevertheless, there is a growing prevalence of sex outside marriage, and thus marriage status does not reliably safeguard sexual-health status [Citation35–Citation37].
We found an association between HR-HPV infection and reproductive factors. There was a negative linear trend observed for parity. That is consistent with a study of Munoz et al. [Citation38] where parity was a protective factor for positive HPV status. The latter could be explained by prenatal hormonal changes in the transformation zone that contribute to HPV resistance during sexual intercourse [Citation39]. Study from Colombia showed that high parity increases the risk of squamous-cell carcinoma of the cervix among HPV-positive women [Citation40].
Despite the relatively low smoking rate (13%) among our study subjects, we found that positive HPV status was associated with cigarette smoking. A recent study from the Murmansk region of North-West Russia reports that the prevalence of smoking among women was 25.2% during 2006–2011 [Citation41]. Differences in the time frame, age and education level between the study populations might have contributed to the discrepancy. Although smoking is associated with an increased risk of CC [Citation42], its effect on HPV infection is mixed. Poppe et al. [Citation43] found that smoking potentially increased the risk of HPV infection through a local impairment of cell-mediated immunity, although the magnitude of the effect was small. While some studies report an increased risk of HPV infection among smokers [Citation22], others found a reduced risk [Citation44].
Our observation that age at sexual debut and number of lifetime sexual partners are associated with positive HPV status is consistent with previous studies [Citation19–Citation22]. Age at sexual debut appears to have declined over time [Citation45]. Most young women and men become sexually active during their teenage years and generally do so unsafely [Citation35]. Little knowledge and lack of access to contraceptive related services explain the low use and high rates of ineffective use. Greenberg et al. [Citation46] have demonstrated that early age at first sexual intercourse in a female population was associated with risky behaviours, such as multiple sexual partners, intravenous drug-use, higher likelihood of having sexually transmitted diseases. Others have demonstrated that risky behaviours including multiple sexual partners and frequency of vaginal intercourse are associated with HPV infection, particularly by the high oncogenic risk types. Of these, a positive association with the number of sexual partners has most consistently been reported [Citation20–Citation22]. Multiple lifetime sexual partners has been shown to increase the risk of being HPV-DNA positive [Citation19,Citation40]. However, women tend to underreport the number of lifetime sexual partners [Citation22,Citation35,Citation36].
Our findings indicate that condom use was not associated with HPV status. Munoz et al. [Citation22] showed that condoms most likely prevent HPV infection, although the evidence of their effectiveness against other sexually transmitted diseases is mixed. Manhart et al. [Citation23] in their meta-analyses showed that there was no consistent evidence that condom use reduces the risk of becoming HPV-DNA positive. Authors suggest that while condoms may not prevent HPV infection, they may protect against genital warts and CC [Citation23]. Underestimation of the potential protective effect of condom use is possible, as very few women used it consistently [Citation47,Citation48].
Long-term use of oral contraceptives could be a cofactor that increases the risk of cervical carcinoma [Citation25]. Previous studies indicate that compared to never-users, patients who used oral contraceptives for less than 5 years did not have an increased risk of CC [Citation49,Citation50]. However its use for over 5 years resulted in higher risks of CC [Citation25,Citation26]. Almost 30 years ago it was hypothesised that oestrogen and other hormones are capable of reactivating HPV or increase its viral expression [Citation51,Citation52]. However this relation is not likely to be causal. Contraceptive users may generally differ from nonusers in other aspects than sexual behaviour.
Despite the sufficient level of knowledge about HPV and CC prevention among our study participants, it yielded no apparent protection against positive HPV status. Neither was there a difference with education level. Women with sufficient level of knowledge about HPV and CC prevention had the same positive HPV status as those with a poor level. One possible explanation could be that the mode of communication between women and their healthcare providers was that scientific information was shared in a matter-of-fact manner, with no or little attention to a problem’s complexity or the context of a patient’s understanding of a health issue. It may well be necessary to re-conceptualise the education of healthcare workers to include a better understanding of STDs and HPV infection.
There remains little doubt that women who were positive for HPV can be distinguished from those who were HPV negative by a number of characteristics in their reproductive health, their sexual behaviour and preferences. However, many of the sociodemographic characteristic as well as variables used to measure sexual behaviour are closely interrelated and could be potential confounders and/or mediators in multivariable analysis. Moreover, total exposure to some of the potential risk factors, such as for example marital status and smoking, could not be measured properly due to cross-sectional design of the study. In relation to this, it was difficult to build a regression model that would be the best to explore potential independent risk factors. We found that age at sexual debut and parity, but not age or the lifetime number of sexual partners were independent predictors for cervical HR-HPV infection.
Limitations
Our study has several potential limitations related to cross-sectional design. Our study used non-probabilistic sampling and this is why it cannot be generalised. Despite the fact that we excluded the younger age group (< 25 years old), it has been shown that initiation of screening at an early age can lead to an overestimation of CC risk [Citation53]. All survey data based on self-reports are susceptible to bias. Sexual behaviour might suffer more than other variables because of social desirability bias — the tendency for participants to respond according to social expectations of what is acceptable. Our study’s cross-sectional design has the risk of reporting bias, particularly in the context of variables that address aspects of sexual behaviour, such as age at sexual debut and lifetime number of sexual partners. Many surveys find that women tend to under-report the number of sexual partners [Citation35–Citation37]. Thus selection bias might have occurred in our study. It is possible that study participants were more health conscious in terms of HPV and CC than women in the general population.
The cross-sectional study design is limited in revealing a causal relationship between HR-HPV positivity and selected sociodemographic and sexual characteristics. It measures prevalence and does not reflect any temporal influence. Moreover, changes in social attitudes may have affected the results of the survey since what is being captured could in part reflect a change in behaviour itself. Furthermore, our study results cannot be generalised. We can extrapolate our data only to women who have visited gynaecologists for any reason in the Arkhangelsk Clinical Maternity Hospital named after Samoylova.
Conclusion
The observed prevalence of HR-HPV is comparable to estimates mentioned in previous studies. We have established that women in the HR-HPV positive group differed from the HR-HPV negative group by age, marital status, parity, age at sexual debut, number of sexual partners and cigarette smoking. Women with early age at sexual debut and those nulliparous have had a higher risk of having a positive HR-HPV status. Our results reinforce the importance of sexual behaviour educational programmes for women and healthcare providers in the prevention of HR-HPV infections. There is no universal approach or a single intervention that would work everywhere, as regional and local social contexts can contribute to risky sexual practices. Surveys of cervical HPV prevalence and related risk factors can help to increase general understanding and provide a basis for the design and establishment of educational programmes and their assessment.
Competing interests
The authors declare that they have no competing interests.
Availability of supporting data
According to the Russian Federal Law №152 from 27th of July 2006 “Personal data” “cross-border data transfer may be prohibited or restricted in order to protect constitutional rights of the Russian citizens”.
However, it allows attaching the dataset from corresponding author on the basis of properly submitted requests.
Consent for publication
Not applicable.
Disclosure statement
ER designed the study, collected the data, created the database, carried out the statistical analyses, participated in the sequence alignment and drafted the manuscript. ES assisted in the statistical analyses and critically revised the manuscript. OK assisted in the statistical analyses. EN and VP participated in the drafting/editing of the manuscript. JOO conceived and coordinated the study, participated in its design and in the writing of the manuscript. All authors read and approved the final manuscript.
References
- Baseman JG, Koutsky LA. The epidemiology of human papillomavirus infections. J Clin Virol. 2005;32(Suppl 1):S16–10.
- Human Papillomavirus (HPV) Statistics. Available from: https://www.cdc.gov/std/hpv/stats.htm
- 6 Reasons to Get HPV Vaccine for Your Child. Available from: https://www.cdc.gov/hpv/infographics/vacc-six-reasons.html
- Munoz N, Bosch FX, de Sanjose S, et al. International agency for research on cancer multicenter cervical cancer study G: epidemiologic classification of human papillomavirus types associated with cervical cancer. N Engl J Med. 2003;348(6):518–527.
- Forman D, de Martel C, Lacey CJ, et al. Global burden of human papillomavirus and related diseases. Vaccine. 2012;30(Suppl 5):F12–23.
- Globocan. Cervical Cancer Estimated Incidence, Mortality and Prevalence Worldwide in; 2012. Available from: http://globocan.iarc.fr/old/FactSheets/cancers/cervix-new.asp
- Altobelli E, Lattanzi A. Cervical carcinoma in the European Union: an update on disease burden, screening program state of activation, and coverage as of March 2014. Int J Gynecol Cancer. 2015;25(3):474–483.
- Smith RA, Andrews KS, Brooks D, et al. Cancer screening in the USA, 2017: A review of current American Cancer Society guidelines and current issues in cancer screening. CA Cancer J Clin. 2017;67(2):100–121.
- Davis M, Feldman S. Making Sense of Cervical Cancer Screening Guidelines and Recommendations. Curr Treat Options Oncol. 2015;16(12):55.
- Elfstrom KM, Arnheim-Dahlstrom L, von Karsa L, et al. Cervical cancer screening in Europe: quality assurance and organisation of programmes. Eur J Cancer. 2015;51(8):950–968.
- Ostensson E, Silfverschiold M, Greiff L, et al. The economic burden of human papillomavirus-related precancers and cancers in Sweden. PLoS One. 2017;12(6):e0179520.
- Parkin DM, Bray F. Chapter 2: the burden of HPV-related cancers. Vaccine. 2006;24(Suppl 3):S3/11–25.
- Aad G, Abajyan T, Abbott B, et al. Measurement of top quark polarization in top-antitop events from proton-proton collisions at radicals=7 TeV using the ATLAS detector. Phys Rev Lett. 2013;111(23):232002.
- Jones SB. Cancer in the developing world: a call to action. BMJ. 1999;319(7208):505–508.
- Bruni LB-RL, Albero G, Serrano B et al.: Human papillomavirus and related diseases in russian federation. Summary Report; 2017.
- Kjaer SK, Chackerian B, van den Brule AJ, et al. High-risk human papillomavirus is sexually transmitted: evidence from a follow-up study of virgins starting sexual activity (intercourse). Cancer Epidemiol Biomarkers Prev. 2001;10(2):101–106.
- Castellsague X, Bosch FX, Munoz N, et al. Male circumcision, penile human papillomavirus infection, and cervical cancer in female partners. N Engl J Med. 2002;346(15):1105–1112.
- Castellsague X, Bosch FX, Munoz N. The male role in cervical cancer. Salud Publica Mex. 2003;45(Suppl 3):S345–353.
- Vaccarella S, Franceschi S, Herrero R, et al. Sexual behavior, condom use, and human papillomavirus: pooled analysis of the IARC human papillomavirus prevalence surveys. Cancer Epidemiol Biomarkers Prev. 2006;15(2):326–333.
- Chan PK, Chang AR, Cheung JL, et al. Determinants of cervical human papillomavirus infection: differences between high- and low-oncogenic risk types. J Infect Dis. 2002;185(1):28–35.
- Peyton CL, Gravitt PE, Hunt WC, et al. Determinants of genital human papillomavirus detection in a US population. J Infect Dis. 2001;183(11):1554–1564.
- Kjaer SK, van den Brule AJ, Bock JE, et al. Determinants for genital human papillomavirus (HPV) infection in 1000 randomly chosen young Danish women with normal Pap smear: are there different risk profiles for oncogenic and nononcogenic HPV types? Cancer Epidemiol Biomarkers Prev. 1997;6(10):799–805.
- Manhart LE, Koutsky LA. Do condoms prevent genital HPV infection, external genital warts, or cervical neoplasia? A meta-analysis. Sex Transm Dis. 2002;29(11):725–735.
- Vaccarella S, Herrero R, Snijders PJ, et al. Smoking and human papillomavirus infection: pooled analysis of the International Agency for Research on Cancer HPV Prevalence Surveys. Int J Epidemiol. 2008;37(3):536–546.
- Vaccarella S, Herrero R, Dai M, et al. Reproductive factors, oral contraceptive use, and human papillomavirus infection: pooled analysis of the IARC HPV prevalence surveys. Cancer Epidemiol Biomarkers Prev. 2006;15(11):2148–2153.
- Munoz N, Franceschi S, Bosetti C, et al. International agency for research on cancer. multicentric cervical cancer study G: role of parity and human papillomavirus in cervical cancer: the IARC multicentric case-control study. Lancet. 2002;359(9312):1093–1101.
- Bosch FX, Munoz N, de Sanjose S, et al. Importance of human papillomavirus endemicity in the incidence of cervical cancer: an extension of the hypothesis on sexual behavior. Cancer Epidemiol Biomarkers Prev. 1994;3(5):375–379.
- Territorial office of the federal state statistics service of the Arkhangelsk region. Available from: http://arhangelskstat.gks.ru/wps/wcm/connect/rosstat_ts/arhangelskstat/ru/statistics/population/
- The UK NHC cervical screening program. Available from: https://www.bsccp.org.uk/assets/file/uploads/resources/NHSCSP_20_Colposcopy_and_Programme_Management_(3rd_Edition)_(2).pdf
- Roik EE, Sharashova EE, Nieboer E, et al. Knowledge about human papillomavirus and prevention of cervical cancer among women of Arkhangelsk, Northwest Russia. PLoS One. 2017;12(12):e0189534.
- Solomon D, Davey D, Kurman R, et al. The 2001 Bethesda System: terminology for reporting results of cervical cytology. JAMA. 2002;287(16):2114–2119.
- Bruni L, Diaz M, Castellsagué X, et al. Cervical human papillomavirus prevalence in 5 continents: meta-analysis of 1 million women with normal cytological findings. J Infect Dis. 2010;202(12):1789–1799.
- Shipitsyna E, Zolotoverkhaya E, Kuevda D, et al. Prevalence of high-risk human papillomavirus types and cervical squamous intraepithelial lesions in women over 30 years of age in St. Petersburg, Russia. Cancer Epidemiol. 2011;35(2):160–164.
- Syrjanen S, Shabalova I, Petrovichev N, et al. Sexual habits and human papillomavirus infection among females in three New Independent States of the former Soviet Union. Sex Transm Dis. 2003;30(9):680–684.
- Wellings K, Collumbien M, Slaymaker E, et al. Sexual behaviour in context: a global perspective. Lancet. 2006;368(9548):1706–1728.
- Nnko S, Boerma JT, Urassa M, et al. Secretive females or swaggering males? An assessment of the quality of sexual partnership reporting in rural Tanzania. Soc Sci Med. 2004;59(2):299–310.
- Curtis SL, Sutherland EG. Measuring sexual behaviour in the era of HIV/AIDS: the experience of Demographic and Health Surveys and similar enquiries. Sex Transm Infect. 2004;80(Suppl 2):ii22–27.
- Munoz N, Mendez F, Posso H, et al. Instituto Nacional de Cancerologia HPVSG: incidence, duration, and determinants of cervical human papillomavirus infection in a cohort of Colombian women with normal cytological results. J Infect Dis. 2004;190(12):2077–2087.
- Louie KS, de Sanjose S, Diaz M, et al. Early age at first sexual intercourse and early pregnancy are risk factors for cervical cancer in developing countries. Br J Cancer. 2009;100(7):1191–1197.
- Munoz N, Kato I, Bosch FX, et al. Risk factors for HPV DNA detection in middle-aged women. Sex Transm Dis. 1996;23(6):504–510.
- Kharkova OA, Krettek A, Grjibovski AM, et al. Prevalence of smoking before and during pregnancy and changes in this habit during pregnancy in Northwest Russia: a Murmansk county birth registry study. Reprod Health. 2016;13: 18.
- Chichareon S, Herrero R, Munoz N, et al. Risk factors for cervical cancer in Thailand: a case-control study. J Natl Cancer Inst. 1998;90(1):50–57.
- Poppe WA, Ide PS, Drijkoningen MP, et al. Tobacco smoking impairs the local immunosurveillance in the uterine cervix. An immunohistochemical study. Gynecol Obstet Invest. 1995;39(1):34–38.
- Lazcano-Ponce E, Herrero R, Munoz N, et al. Epidemiology of HPV infection among Mexican women with normal cervical cytology. Int J Cancer. 2001;91(3):412–420.
- Burchell AN, Winer RL, de Sanjose S, et al. Chapter 6: epidemiology and transmission dynamics of genital HPV infection. Vaccine. 2006;24(Suppl 3):S3/52–61.
- Greenberg J, Magder L, Aral S. Age at first coitus. A marker for risky sexual behavior in women. Sex Transm Dis. 1992;19(6):331–334.
- Potter LB, Anderson JE. Patterns of condom use and sexual behavior among never-married women. Sex Transm Dis. 1993;20(4):201–208.
- Jama Shai N, Jewkes R, Levin J, et al. Factors associated with consistent condom use among rural young women in South Africa. AIDS Care. 2010;22(11):1379–1385.
- Peng Y, Wang X, Feng H, et al. Is oral contraceptive use associated with an increased risk of cervical cancer? An evidence‐based meta‐analysis. J Obstet Gynaecol Res. 2017;43::913–922.
- Moreno V, Bosch FX, Munoz N, et al. International Agency for Research on Cancer. Multicentric Cervical Cancer Study G: effect of oral contraceptives on risk of cervical cancer in women with human papillomavirus infection: the IARC multicentric case-control study. Lancet. 2002;359(9312):1085–1092.
- Mitrani-Rosenbaum S, Tsvieli R, Tur-Kaspa R. Oestrogen stimulates differential transcription of human papillomavirus type 16 in SiHa cervical carcinoma cells. J Gen Virol. 1989;70(Pt 8):2227–2232.
- Mittal R, Tsutsumi K, Pater A, et al. Human papillomavirus type 16 expression in cervical keratinocytes: role of progesterone and glucocorticoid hormones. Obstet Gynecol. 1993;81(1):5–12.
- Landy R, Birke H, Castanon A, et al. Benefits and harms of cervical screening from age 20 years compared with screening from age 25 years. Br J Cancer. 2014;110(7):1841–1846.
