ABSTRACT
Objective: Foetuses and neonates of women who use tobacco are exposed to nicotine and tobacco-derived carcinogens. We determined the relationship between urine biomarkers of tobacco toxicant exposure postpartum and in the neonates of Alaska Native (AN) women, comparing smokers and smokeless tobacco (ST) users, including iqmik, a homemade ST product.
Methods: AN women, including 36 smokers, 9 commercial ST and 16 iqmik users their neonates participated. Urine from the woman at the time of delivery and her neonate’s first urine were analysed for cotinine, the major metabolite of nicotine, and 4-(methylnitrosamino)-1-(3) pyridyl-1-butanol (NNAL), a tobacco-specific carcinogen biomarker.
Results: Maternal urine cotinine and neonatal urine cotinine were strongly correlated in all tobacco use groups (r from 0.83 to 0.9, p < 0.002). Correlations between maternal cotinine and neonatal NNAL were moderately strong for cigarettes and commercial smokeless but weaker for iqmik users (r 0.73, 0.6 and 0.36, respectively).
Conclusion: Correlations between maternal and neonatal biomarkers of tobacco toxicant exposure vary, dependent on tobacco product use.
Significance: This study provides novel data on biomarkers of tobacco exposure among postpartum AN women and their neonates. The results could be useful to guide future epidemiological studies of health risks associated with use of various tobacco products during pregnancy.
Introduction
Cigarette smoking is the major preventable risk factor for premature morbidity and mortality in developed countries, and poses a serious threat to pregnancy and the health of the foetus and neonate [Citation1,Citation2]. Cigarette smokers are exposed to numerous toxicants, including 73 established animal carcinogens, of which 16 are known human carcinogens [Citation3]. Two classes of chemicals are specific or nearly specific to tobacco exposure: nicotine and related alkaloids and the tobacco-specific nitrosamines. Cotinine, the proximate metabolite of nicotine, is a measure of tobacco smoke toxicant exposure in general, but neither nicotine nor cotinine are known to cause cancer. The major tobacco-specific nitrosamine in tobacco, 4-(methylnitrosamino)-1-(3)pyridyl-1-butanone (NNK), is a potent carcinogen and is thought to cause lung and pancreatic cancer, and possibly liver and nasopharyngeal cancer [Citation4]. The major metabolite of NNK, 4-(methylnitrosamino)-1-(3) pyridyl-1-butanol (NNAL), is found in the urine of tobacco-exposed individuals, and is in itself carcinogenic. Cotinine and NNAL are widely used as biomarkers of tobacco exposure [Citation4,Citation5].
The prevalence of smoking during pregnancy in the U.S. is 11%, and highest among American Indian/Alaska Native (AI/AN) women (26%) compared with other racial/ethnic groups (14% Whites, 9% Blacks, 3% Hispanics and 2% among Asian women [Citation6]. In Alaska, compared with Non-Native women, the prevalence of prenatal smoking was higher among Alaska Native (AN) women in 2013 (14% vs 31%, respectively), and has not significantly changed in nearly two decades [Citation7]. Similarly, smokeless tobacco (ST) use rates reported for 2013 were greater among pregnant AN women (18%) compared to non-Native Alaskan women (1%) and have remained about the same in the past 10 years. In Southwest Alaska where ST use tends to be the highest, the primary type of ST used among prenatal AN women is iqmik. Iqmik is a homemade chewing tobacco made from dried or cured tobacco leaves and ash, the latter derived from burning willow or other tree bark or from a fungus that grows on birch trees [Citation8]. Iqmik is thought by many women in that region to be less harmful than other forms of ST [Citation9].
Previously published studies have documented the presence of cotinine and NNAL in foetal amniotic fluid and in the urine of neonates of mothers who were cigarette smokers [Citation10–Citation14]. Less is known about biomarkers of tobacco exposure for neonates born to mothers using ST or among AI/AN populations. Recently, we published data on the relationship between maternal urine cotinine and neonatal urine NNAL concentrations from AN participants in the Biomarker Feedback to Motivate Tobacco Cessation in Pregnant Alaska Native Women (MAW) study [Citation15]. We described the use of the findings in developing a biomarker feedback intervention to motivate smoking cessation during pregnancy [Citation16]. The intent was to use maternal cotinine results as an educational tool about carcinogen exposure in their babies, which would then motivate them to quit smoking. The present analysis provides a more in-depth examination of biomarker concentrations in mothers and neonates, focusing on differences in exposures and correlations among biomarkers in mothers who used different tobacco products – cigarettes, commercial ST and iqmik.
Methods
Ethics statement
The study was approved by Institutional Review Boards at the Mayo Clinic, the Alaska Area Indian Health Service, and the University of California San Francisco. Tribal approval was granted by the Alaska Native Tribal Health Consortium and Southcentral Foundation.
Study participants and procedures
Details of study participants and procedures have been published previously [Citation15]. In brief, the study was conducted in Anchorage, Alaska on the Alaska Native Health campus, which provides prenatal care to AN women statewide. Recruitment for the study occurred when women presented for prenatal care at the primary care centre. Women were approached in an exam room before or after seeing their provider at a prenatal visit. Potential participants were screened for eligibility criteria, which included Alaska Native heritage, 18 years of age or older, currently pregnant with singleton and intention to deliver at the tertiary care hospital. Women were excluded if they were using nicotine replacement therapy or medication for tobacco cessation, or had participated in a tobacco cessation programme within the past 30 days. Those who were eligible and agreed to participate provided written informed consent and enrolled into the study. At enrolment, a questionnaire inquiring about socio-demographic factors, pregnancy history and personal and family smoking history was administered. A urine sample was collected to biochemically verify self-reported tobacco use status.
A brief interview was administered during the postpartum period to determine maternal tobacco use status and type of tobacco product use since enrolment. We attempted to collect the first void after delivery from postpartum women and their neonates. Nursing staff in Labor and Delivery collected the maternal urine. Research and nursing staff worked with mothers to collect neonatal urine. Urine was collected from neonates by placing pieces of cotton pad in the diaper. Soaked pieces of cotton pad were subsequently collected and placed in a urine cup, sealed and refrigerated. Within 24 h, the urine was expressed from pads and frozen. In some instances the first void could not be collected – if the neonate voided before a diaper was placed, if the diaper was discarded accidentally, or if there was too much meconium in the diaper. The time interval between delivery and the neonatal urine collection was recorded in 53 participants. The mean value was 7.2 h with a median of 7.1 h.
Urine samples were sent to the Clinical Pharmacology Tobacco Research Laboratory at the San Francisco General Hospital/University of California San Francisco (UCSF). Cotinine concentrations in maternal urine were measured by gas liquid chromatography and in neonatal urine by liquid chromatography – tandem mass spectrometry, with limits of quantitation of 10 ng/ml and 0.05 ng/ml, respectively [Citation17,Citation18]. NNAL concentrations were assayed by liquid chromatography – tandem mass spectrometry, with a limit of quantitation of 1 pg/ml [Citation19].
Data analysis
Given the non-normal distribution of urine analyte concentrations, descriptive data were expressed as geometric means and 95% confidence intervals. Spearman correlation coefficients were computed comparing urine concentrations as follows: (1) maternal cotinine vs maternal NNAL; (2) neonatal cotinine vs neonatal NNAL; (3) maternal cotinine vs neonatal cotinine and (4) maternal cotinine vs neonatal NNAL analyses were performed with and without normalisation of maternal cotinine and NNAL concentrations by urine creatinine. Creatinine corrected neonatal urine concentrations are presented for reference purposes, but were not used for statistical analysis because concentrations of creatinine in neonatal urine are determined by the maternal creatinine concentration at the time of delivery, not the neonate’s kidney function [Citation20]. The Kruskal-Willis test was used to compare biomarkers across tobacco types. Data analyses were performed using SAS and R software.
Results
The 148 women completed enrolment included 54 smokers, 10 commercial ST users and 20 iqmik users. Of these, paired samples from 36 smokers, 9 commercial ST users and 16 iqmik users were obtained from postpartum women and their neonates and are included in the present analysis. The women averaged 28.3 years in age (range 19–42). The participants smoked an average of 5.4 cigarettes per day (range 1–15) in the week prior to delivery. Three of the women smoked roll your own cigarettes. Commercial ST users chewed an average of 1.3 (SD 1.6) cans per week, and iqmik users 0.52 (SD 0.31) cans per week.
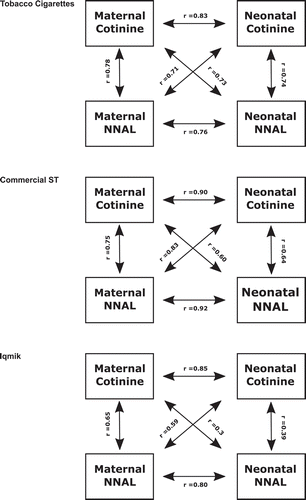
Geometric mean values for urine cotinine and NNAL are presented in . Average maternal cotinine concentrations were highest for iqmik users and similar for cigarette smokers and commercial ST users, although the differences were not statistically significant. However, iqmik users had significantly lower maternal NNAL levels (p < 0.01). Neonatal cotinine concentrations were similar for all tobacco product groups. Urine NNAL was similar for neonates of smokers and commercial ST users, but lower for iqmik users (p < 0.01). Ratios of NNAL to cotinine in maternal and neonatal urine were significantly lower for iqmik users compared to other groups (p < 0.01). Maternal cotinine and NNAL concentrations were higher than those in their neonates (); ratios did not differ by product ().
Table 1. Maternal and neonatal cotinine and NNAL concentrations by tobacco product.
Table 2. Maternal to neonatal ratios of urine cotinine and NNAL by tobacco product.
Spearman correlations among various biomarkers are shown in and . In general, correlations were higher for creatinine-normalised maternal concentrations, therefore correlations with creatinine-normalised maternal analyte concentrations are presented below. For smokers and commercial ST users, the strongest maternal to neonate correlations were between maternal cotinine and neonatal cotinine (r = 0.83 and 0.9, p < 0.001) and between maternal NNAL and neonatal NNAL (r = 0.76 and 0.92, p < 0.001). For smokers and commercial ST users, the correlations between maternal urine cotinine and neonatal NNAL were 0.73 (p < 0.001) and 0.6 (NS), respectively. For mothers who smoked cigarettes urine NNAL and cotinine concentration were strongly correlated both within mother and within neonates. (p < 0.001). For mothers who used commercial ST, urine cotinine and NNAL concentrations were strongly correlated within mother (p < 0.001) but not significantly within neonates. For mothers who were iqmik users, urine cotinine and NNAL concentrations were more weakly but still significantly correlated within mothers (p < 0.05) but not significantly correlated within neonates.
Table 3. Spearman’s correlations of urinary biomarkers by tobacco product.
Discussion
In a prior study, we presented data on the relationship between maternal urine cotinine and neonatal urine NNAL, the aim of which was to provide data that could be used to assist in smoking cessation counselling during pregnancy among AN women [Citation15]. In the present study, we examine relationships between urine cotinine and NNAL concentrations within and between mothers and neonates, and we compare ratios for mothers using different tobacco products. We find that, irrespective of tobacco product, within pairs, mother to neonate cotinine concentrations are highly correlated; and the same is observed for urine NNAL. Maternal concentrations were on an average 2.5-fold and 6.5 fold higher than neonatal concentrations for cotinine and NNAL, respectively.
However, the relationships between maternal cotinine and neonate NNAL and between maternal NNAL and neonatal cotinine are variable in absolute ratios as well as in strength of association. For pairs in which the mother is a cigarette smoker, the correlations between maternal cotinine and neonatal NNAL are fairly strong (r = 0.73). Compared to pairs in which the mother was a smoker, for commercial ST using mothers, the correlation between maternal cotinine and neonatal NNAL is weaker (r = 0.60) and for iqmik users the correlation between maternal cotinine and neonatal urine NNAL is weaker still (r = 0.36) (both correlations non-significant).
The finding of strong correlations between mother and neonate for the same analyte are expected, given the known penetration and presumed equilibrium of cotinine and NNAL between maternal and neonatal circulations regardless of the type of tobacco product used. The product-related differences in ratios and strength of correlations between maternal cotinine and neonatal NNAL are likely related to the variability and across-product differences in nicotine and NNK content and bioavailability. Commercial ST has on average much higher ratios of NNK to nicotine than has cigarette tobacco [Citation21]. In addition, there is variability in the ratio as well as the pH across smokeless products, and the bioavailability of nicotine depends on the pH of the particular ST product [Citation21,Citation22]. Iqmik is homemade from tobacco and ash and has a very high pH, resulting in very high nicotine bioavailability [Citation8,Citation21]. NNK levels in iqmik are lower than those in other forms of tobacco. This explains the low absolute ratios of NNAL/cotinine in urine of iqmik-using mothers and their neonates. Among iqmik users, the variability in types of tobacco used and amount of ash added most likely explain why the correlations between maternal cotinine and neonatal NNAL are weak.
In addition to our prior study, we are aware of two previously published studies that have measured concentrations of NNAL in neonates. Lackmann studied 31 neonates of women who smoked, and were able to measure NNAL in 71% [Citation13]. They found correlations between neonatal urine cotinine and neonatal NNAL (r = 0.56) which is similar to the findings in our population. Lackmann did not examine maternal urine. Florek et al. studied 62 pregnant smokers in Poland, collecting urine samples from women at the time of admission and urine from the neonates with their first void [Citation11]. They reported similar absolute concentrations of cotinine and NNAL in the maternal urine as we report in this study. While we report a significant correlation between maternal cotinine concentration and neonatal NNAL, Florek et al. did not report a significant correlation (2011). This difference may be due to Florek’s use of creatinine-corrected neonatal concentrations.
Cotinine and NNAL are known to readily pass through the placenta into the foetal circulation [Citation23,Citation24]. This most likely explains the high correlations between maternal and neonatal analyte levels in our population. The weaker correlation between maternal cotinine and neonatal NNAL is not unexpected, as it may reflect differences in nicotine and NNK metabolism and excretory pathways. Thus, a person could be a slow metaboliser of nicotine or cotinine and a fast metaboliser of NNK, or the reverse. This would result in weaker correlations comparing two different analytes as compared to the same analyte in maternal and neonatal urines.
Limitations to our study include the small number of women who were commercial smokeless tobacco or iqmik users, the fact that iqmik is homemade and its chemical constituents could vary from household to household, inaccuracies in self-report of cigarette or smokeless tobacco consumption, the variable time interval between maternal and neonatal urine collections, and possible differences across women in renal clearance of cotinine or NNAL. These potential confounders would be expected to reduce the strength of correlations between maternal and neonatal toxicant concentrations. However, despite these sources of variability, the within-analyte maternal-neonate concentrations were strongly correlated, and provide a sound basis for using maternal biomarker levels to estimate neonatal exposure.
Maternal transmission of NNK to foetal mice has been demonstrated and can cause tumours, including malignant tumours that appear as the mice offspring mature [Citation23,Citation24]. Although there are conflicting reports, some human epidemiology studies suggest that exposure to tobacco smoke during pregnancy or during infancy is associated with an increased risk of cancer later in life [Citation25,Citation26]. Urine concentrations of cotinine and NNAL in mother and neonate provide a quantitative marker of tobacco constituent and carcinogen exposure during pregnancy, and could serve as a predictor of tobacco-related cancer as well as other health risks later in life. We provide data on exposure of neonates to nicotine and NNAL and relationships between maternal and neonatal exposures, which might be useful in guiding future epidemiologic studies on health effects of using various tobacco products during pregnancy.
Supplemental Material
Download MS Word (17.5 KB)Acknowledgments
We thank Dr. Delia Dempsey for advice on study design, Dr. Natalie Nardone for assistance in data management and analysis, Gretchen Day for statistical support, the staff at SCF PCC and ANMC for assistance in recruitment and specimen collection. Additionally, we would like to thank Dr. Peyton Jacob III and Lisa Yu for supervision of laboratory activities, Trisha Mao, Polly Cheung, Lawrence Chan and Christopher Havel for performing analytical chemistry, and Newton Addo for editorial assistance
Disclosure statement
No potential conflict of interest was reported by the authors.
Supplementary Material
Supplemental data for this article can be accessed here.
Additional information
Funding
References
- HHS. How tobacco smoke causes disease: the biology and behavioral basis for smoking-attributable disease: a report of the surgeon general. Atlanta, GA: U.S: Department of Health and Human Services, Centers for Disease Control and Prevention, Coordinating Center for Health Promotion, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health; 2010.
- Dechanet C, Anahory T, Mathieu Daude JC, et al. Effects of cigarette smoking on reproduction. Hum Reprod Update. 2011 Jan-Feb;17(1):76–6. PMID: 20685716; eng.
- Hecht SS. Research opportunities related to establishing standards for tobacco products under the family smoking prevention and tobacco control act. Nicotine Tob Res. 2012 Jan;14(1):18–28. . PMID: 21324834PMCID: PMC3242967. eng.
- Hecht SS. Tobacco carcinogens, their biomarkers and tobacco-induced cancer. Nat Rev Cancer. 2003 Oct;3(10):733–744. PMID: 14570033; eng.
- Avila-Tang E, Al-Delaimy WK, Ashley DL, et al. Assessing secondhand smoke using biological markers. Tob Control. 2013 May;22(3):164–171. PMID: 22940677; PMCID: PMC3639350. eng.
- Tong VT, Dietz PM, Morrow B, et al. Trends in smoking before, during, and after pregnancy–pregnancy risk assessment monitoring system, USA, 40 sites, 2000–2010. Morb Mortal Wkly RepSurveillance Summar(Washington, DC: 2002). 2013 Nov 8;62(6):1–19. PMID: 24196750; eng.
- Kim SY, England L, Dietz PM, et al. Patterns of cigarette and smokeless tobacco use before, during, and after pregnancy among Alaska native and white women in Alaska, 2000–2003. Matern Child Health J. 2010 May;14(3):365–372. PMID: 19139981; eng.
- Renner CC, Enoch C, Patten CA, et al. Iqmik: a form of smokeless tobacco used among Alaska natives. Am J Health Behav. 2005 Nov-Dec;29(6):588–594. PMID: 16336113; eng.
- Renner CC, Patten CA, Enoch C, et al. Focus groups of Y-K Delta Alaska natives: attitudes toward tobacco use and tobacco dependence interventions. Prev Med. 2004 Apr;38(4):421–431. PMID: 15020175.
- Dempsey DA, Hajnal BL, Partridge JC, et al. Tone abnormalities are associated with maternal cigarette smoking during pregnancy in in utero cocaine-exposed infants. Pediatrics. 2000 Jul;106(1 Pt 1):79–85. PMID: 10878153
- Florek E, Piekoszewski W, Basior A, et al. Effect of maternal tobacco smoking or exposure to second-hand smoke on the levels of 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanol (NNAL) in urine of mother and the first urine of newborn. J Physiol Pharmacology: Off J Polish Physiol Soc. 2011 Jun;62(3):377–383. PMID: 21893699; eng
- Kirchheiner J, Klein C, Meineke I, et al. Bupropion and 4-OH-bupropion pharmacokinetics in relation to genetic polymorphisms in CYP2B6. Pharmacogenetics. 2003 Oct;13(10):619–626. PMID: 14515060; eng.
- Lackmann GM, Salzberger U, Tollner U, et al. Metabolites of a tobacco-specific carcinogen in urine from newborns. J Natl Cancer Inst. 1999 Mar 3;91(5):459–465. PMID: 10070946; eng.
- Milunsky A, Carmella SG, Ye M, et al. A tobacco-specific carcinogen in the fetus. Prenat Diagn. 2000 Apr;20(4):307–310. PMID: 10740203; eng
- Flanagan CA, Koller KR, Wolfe AW, et al. Fetal exposure to carcinogens with tobacco use in pregnancy: phase 1 MAW study findings. Nicotine Tob Res. 2016 Nov;18(11):2162–2168. PMID: 27190400; PMCID: PMC5055741.
- Koller KR, Flanagan CA, Day GE, et al. Developing a biomarker feedback intervention to motivate smoking cessation during pregnancy: phase II MAW study. Nicotine Tob Res. 2017 Aug 1;19(8):930–936. PMID: 28003506.
- Jacob P 3rd, Wilson M, Benowitz NL. Improved gas chromatographic method for the determination of nicotine and cotinine in biologic fluids. J Chromatogr. 1981 Jan 02;222(1):61–70. PMID: 6783675
- Jacob P 3rd, Yu L, Duan M, et al. Determination of the nicotine metabolites cotinine and trans-3ʹ-hydroxycotinine in biologic fluids of smokers and non-smokers using liquid chromatography-tandem mass spectrometry: biomarkers for tobacco smoke exposure and for phenotyping cytochrome P450 2A6 activity. J Chromatogr B, Anal Technologies Biomed Life Sci. 2011 Feb 01;879(3–4):267–276. PMID: 21208832; PMCID: PMC3050598. eng.
- Jacob P 3rd, Havel C, Lee DH, et al. Subpicogram per milliliter determination of the tobacco-specific carcinogen metabolite 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanol in human urine using liquid chromatography-tandem mass spectrometry. Anal Chem. 2008 Nov 01;80(21):8115–8121. PMID: 18841944; PMCID: PMC3167662.
- Kastl JT. Renal function in the fetus and neonate - the creatinine enigma. Semin Fetal Neonatal Med. 2017 Apr;22(2):83–89. . PMID: 28109705; eng.
- Benowitz NL, Renner CC, Lanier AP, et al. Exposure to nicotine and carcinogens among Southwestern Alaskan Native cigarette smokers and smokeless tobacco users. Cancer epidemiology, biomarkers & prevention: a publication of the American association for cancer research. Cosponsored by Am Soc Prev Oncol. 2012 Jun;21(6):934–942. PMID: 22490317; PMCID: PMC3444141. eng.
- Stanfill SB, Connolly GN, Zhang L, et al. Global surveillance of oral tobacco products: total nicotine, unionised nicotine and tobacco-specific N-nitrosamines. Tob Control. 2011 May;20(3):e2. PMID: 21109685; eng.
- Correa E, Joshi PA, Castonguay A, et al. The tobacco-specific nitrosamine 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone is an active transplacental carcinogen in Syrian golden hamsters. Cancer Res. 1990 Jun 01;50(11):3435–3438. PMID: 2334940; eng
- Schuller HM, Jorquera R, Lu X, et al. Transplacental carcinogenicity of low doses of 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone administered subcutaneously or intratracheally to hamsters. J Cancer Res Clin Oncol. 1994;120(4):200–203. PMID: 8288673; eng.
- John EM, Savitz DA, Sandler DP. Prenatal exposure to parents’ smoking and childhood cancer. Am J Epidemiol. 1991 Jan 15;;133(2):123–132. PMID: 1822074; eng
- Sasco AJ, Vainio H. From in utero and childhood exposure to parental smoking to childhood cancer: a possible link and the need for action. Hum Exp Toxicol. 1999 Apr;18(4):192–201. . PMID: 10333301; eng.