ABSTRACT
Bone mineral density (BMD) and fracture risk are elevated in adults with impaired fasting glucose (IFG) or type 2 diabetes mellitus (T2D). This study aimed to compare bone health among Inuit women with IFG, T2D and normoglycemia. The study included Inuit women (≥40 y) with IFG (n = 57), T2D (n = 72) or normoglycemia (n = 340) from the International Polar Year Inuit Health Survey 2007–2008 in Canada. Distal one-third forearm BMD (FaBMD) was measured using a peripheral instantaneous x-ray imager. Anthropometry, fasting plasma glucose (FPG), serum adiponectin, leptin and 25-hydroxyvitamin D (25(OH)D) were measured. Traditional food intakes were surveyed. Data were analysed using mixed model ANOVA and regression models. The median age was 53 (IFG: IQR 48, 67) y and 56 (T2D: IQR 49, 63) y. Compared to normoglycemic women, FaBMD and T-scores were significantly lower in women with T2D, but not with IFG. Frequency of marine mammal intakes (ß = 0.145; 95%CI: 0.018, 0.053, p = 0.0001) positively related to FaBMD. The odds ratio of having a T-score consistent with osteoporosis was lower among women with T2D and higher BMI, while aging increased the risk. Although T2D associates with lower BMD among Inuit women, risk of osteoporosis is tempered, possibly by maintenance of a traditional lifestyle.
Introduction
Type 2 diabetes mellitus (T2D) is paradoxically related to increased risk of fractures despite normal to elevated areal bone mineral density (BMD) relative to normoglycemic controls [Citation1]. In a meta-analysis, T2D was associated with positive Z-scores for BMD of the lumbar spine (0.41 ± 0.01) and hip (0.27 ± 0.01) [Citation2], which could be primarily ascribed to elevated body mass index (BMI). This is an important consideration since in a study of postmenopausal women, those with T2D and an overweight BMI had greater BMD of the spine and hip compared to controls, whereas those with T2D and a healthy BMI had lower BMD [Citation3]. Furthermore, poorly controlled T2D on the basis of a haemoglobin A1c (HbA1c) ≥ 7.5% associates with greater BMD of lumbar spine and hip and greater fracture risk relative to controls, but this is not observed when T2D is adequately controlled [Citation4]. Similarly, lumbar spine BMD of postmenopausal women with T2D on average in poor control (fasting plasma glucose (FPG) of 9.1 mmol/L), is higher than controls, but still in the osteoporotic range (T-score −2.84 ± 0.42) [Citation5]. There is also evidence that T2D associates with greater loss of BMD at the femoral neck, which may predispose to fracture [Citation6].
In addition to T2D, ethnic variation in BMD and fracture risk may relate to genetic, anthropometric, and lifestyle factors [Citation7]. Being of First Nations (Aboriginal Canadian) ethnicity was among the predictors of fracture in an age, sex and region matched case control study in Canada [Citation8]. In that study, having T2D increased the risk of any fracture (rate ratio: 1.26; 95% CI: 1.19, 1.34). Similarly, the incidence of fracture was higher for Inuit women over 64 y in Alaska relative to white US women [Citation9]. Neither of these studies was able to report upon BMD and its association with BMI and T2D. In the International Polar Year Inuit Health Survey (IPY-IHS), BMI and age were the most important correlates of BMD among Inuit women [Citation10]. In Greenland, age was the most important predictor of fragility fracture independent of family history, lifestyle and medication use [Citation11]. The prevalence of T2D, once considered rare among Inuit, is 12.2% (95% CI: 8.7%, 15.7%) among adults ≥ 50 y [Citation12]. To our knowledge, no studies have examined the association between BMD and impaired fasting glucose (IFG) or T2D among Inuit, both of which increase with adiposity and age.
Other factors that accompany the rise in IFG and T2D among Inuit include the nutrition transition, characterised by reduced traditional food consumption, n-3 fatty acid intakes [Citation13] and vitamin D status [Citation10]. Traditional food patterns including an abundance of fish and seafood associate with lower HbA1c [Citation14] and both vitamin D and n-3 fatty acids are, in part, considered protective against fracture risk [Citation15,Citation16]. In addition, elevated leptin and reduced adiponectin are observed in First Nations adults with T2D relative to normoglycemic adults [Citation17]. Higher circulating leptin associates with increased BMD and lower fracture risk, whereas adiponectin inversely associates with BMD [Citation18]. Data on BMD and its association with IFG or T2D in Inuit women are scarce. Hence, the goals of this study were to (1) compare forearm BMD (FaBMD), and FaBMD T-scores between Inuit women with IFG or T2D to normoglycemic women; (2) assess whether biomarkers and dietary factors that accompany IFG and T2D associate with FaBMD; and (3) explore whether the risk of osteoporosis is elevated by IFG and T2D. We hypothesised that FaBMD would not be significantly higher in T2D and IFG cases compared to normoglycemic women, and that IFG and T2D risk factors including low traditional food intakes would associate with BMD.
Methods
Study population and ethical approval
Data were obtained from the cross-sectional IPY-IHS of adults residing in 36 Arctic communities in the late summer and early fall of 2007 (August–September) and 2008 (August-–October). Details of the IPY-IHS survey are published in full elsewhere [Citation19]. The survey included all communities in three jurisdictions: Inuvialuit Settlement Region (ISR), Nunavut and Nunatsiavut. The communities are located between 54°10ʹ and 74°43ʹ north. Stratified random sampling was used to select households where communities were strata and where homes were randomly selected using either a computer random generation of numbers or a random digit table. The household participation rate was 68% and 2595 male and non-pregnant female adults were included in the main survey.
Assessment of FaBMD was performed only in women ≥40 y of age (n = 570, 22.0% of the survey participants). From the total sample, women with IFG or T2D with data available for FaBMD were compared to normoglycemic women (n = 340; FPG values < 5.6). IFG (n = 57) was defined by FPG values ≥ 5.6 to 6.9 mmol/L [Citation20], whereas T2D (n = 72) was ascertained if a participant had a prior diagnosis of T2D (n = 53); or if the survey results for FPG exceeded 7 mmol/L (n = 15) or if 2-h plasma glucose exceeded 11.1 mmol/L (n = 4) following an oral glucose tolerance test [Citation21]. Among those with a prior diagnosis of T2D, n = 21 of 53 reported details of medical therapy for T2D (n = 12 were using metformin, n = 3 were using glyburide and n = 1 was using insulin and metformin, in addition to 5 with non-specified type of medications).
The study was reviewed and approved by the McGill University, Faculty of Medicine Research Ethics Board, the Nunavut Research Institute and the Aurora Research Institute. Signed informed consent was obtained from each participant.
Dietary assessment
Data on dietary habits were collected using a food frequency questionnaire (FFQ) administered by trained bilingual (English and Inuit dialects) interviewers [Citation19]. The FFQ was customised to capture information within the past year about Inuit traditional food items that are abundant in the regions of the study. Inuit adults were asked about how often in the last year they consumed (in season or off season) traditional food items from a list of 47 items. Data were combined into total traditional foods, total marine mammals and total fish and expressed as a frequency of intake per day.
Clinical assessment
A portable stadiometer (Road Rod 214 Portable stadiometer, Seca, Maryland, USA) was used to measure height to the nearest 0.1 cm. Body weight was recorded to the nearest 0.1 kg and 0.4 kg was subtracted to account for clothes; body fat % was measured using the same scale (Tanita TBF-300GS, Tanita Corporation of America Inc. Arlington Height, Illinois). Participants who had pacemakers had their weight measured by a Seca Scale (Medical Scale Model 214, Seca Corp., Toronto, Ontario), BMI was then calculated.
Areal BMD of the distal one-third forearm (FaBMD) was evaluated by a registered x-ray technologist using a peripheral instantaneous x-ray imager (PIXI; GE/Lunar, Fort Myers, FL). Before performance of any assessment, the equipment underwent quality control using the forearm phantom every day; long-term precision error was 0.5%. In vivo variability was calculated as percent coefficient of variation (CV) by doing three repeated measures on randomly selected participants per day; one was measured in the beginning of the day and the other at the end of the day yielding an average CV of 1.7%. The proprietary reference data were collected in the United States using the same PIXI model. T-scores were calculated using manufacturer’s reference data of ambulatory white premenopausal women who were 20–45 years of age with no history of chronic disease or medications affecting bone and no history of symptomatic, atraumatic fractures. The World Health Organization definition for osteoporosis and osteopenia was used [Citation22]: a FaBMD T-score of ≤−2.5 was considered as osteoporosis, whereas a FaBMD T-score of −1 to −2.4 was considered to be osteopenia (low bone mass) and values >−1 considered normal.
Smoking habits were surveyed. Medication use in the previous year (2006 or 2007) was ascertained from answers to standardised questionnaires. Participants were instructed to bring their medications and supplements to their survey appointment for accurate documentation by nurses. For the purposes of this analysis, antihypertensive/cardiac, lipid lowering and proton pump inhibitor medications were explored due to the possible relationship to BMD and fracture risk [Citation23,Citation24].
Laboratory analyses
Serum 25-hydroxyvitamin D (25(OH)D) reflects both endogenous and exogenous vitamin D sources. Serum total 25(OH)D and intact parathyroid hormone (PTH) 1–84 were measured using chemiluminescent assays (Liaison, Diasorin Inc, Stillwater, MN, USA) [Citation10]. The inter- and intra-assay CV% were 4.5% and 11.1% for the low 25(OH)D control (38.2 nmol/L) and 6.2% and 5.3% for high control (127.2 nmol/L), respectively. The inter-assay CV% for PTH was 19.1 (5.2 pmol/L) for the low control and 8.7 (52.1 pmol/L) for the high PTH control. The accuracy using the mid-range of the manufacturer specifications was 95% for 25(OH)D and 86.7% for PTH. The laboratory that performed the measurement for 25(OH)D was certified by the Vitamin D External Quality Assessment Scheme for the year 2009–2010 when the IPY-IHS samples were measured, which reflects that at least 80% of the results in this report are within 30% of all laboratory trimmed mean.
Serum adiponectin was measured using an Ultra-Sensitive Human Cytokine Assay kit using a Meso Scale Discovery Multi-Array Assay (MSD cat#K151BXC) System and a detection limit of 0.005 µg/mL. Serum leptin was measured using a manual sandwich ELISA assay (Linco cat# EZHL-80SJK, Linco Research, St Charles, Missouri) with a detection limit of 0.5 µg/L. For adiponectin, the intra-assay and inter-assay CV were 5% and 15%, respectively, whereas for leptin, the intra-assay and inter-assay CV were 10%. Samples for FPG and serum insulin were kept at 4°C, until plasma was separated and frozen at −80°C, and then assayed for glucose by Glucose Hexokinase II method. All of these analyses were conducted by Nutrasource Diagnostics, Guelph, ON.
Red blood cell (RBC) membrane fatty acid (FA) profiles, expressed as percent of total FA, were determined using the methodology of Folch et al. [Citation25]. Fatty acid methyl esters were prepared with boron trichloride-methanol to reduce artifact formation and separated on a Varian 3400 gas–liquid chromatograph with a 60-m DB-23 capillary column (0.32 mm diameter). Methods are described in detail elsewhere [Citation26]. The RBC proportion of omega-3 fatty acid (RBC-Omega-3) included all of the n-3 FA from 18:3 n-3 to 22:6 n-3.
Statistical analyses
Differences between IFG and T2D groups and normoglycemic women were evaluated using mixed model ANOVAs; group, region and medication use were tested as fixed effects. Proportions were compared using Chi-square or Fisher’s exact tests.
The association between biomarkers and FFQ variables and FaBMD, and T-scores were tested using multiple linear regression models adjusting for group, age, height, BMI, smoking and region; and medication use which is often different for IFG and T2D. Frequency of alcohol consumption and exercise were not included due to the high frequency of unanswered survey questions. Final regression models were checked for residual distribution and normality, influential points, and collinearity using the variance inflation factor. Risk of having a T-score consistent with osteoporosis was explored using logistic regression. Statistical significance was evaluated using a p-value < 0.05 (2-tailed).
Results
Age, adiposity, and anthropometric measurements were the lower in normoglycemic women compared to IFG and T2D cases (). Serum 25(OH)D and leptin concentrations and prevalence of smoking, antihypertensive and lipid lowering medications, and vitamin and mineral supplement use were higher in T2D cases than normoglycemic women. Arctic region of residence did not vary by glycemic status.
Table 1. Health and lifestyle characteristics of Inuit women with impaired fasting glucose (IFG) or diabetes mellitus type 2 (T2D) and a normoglycemic group
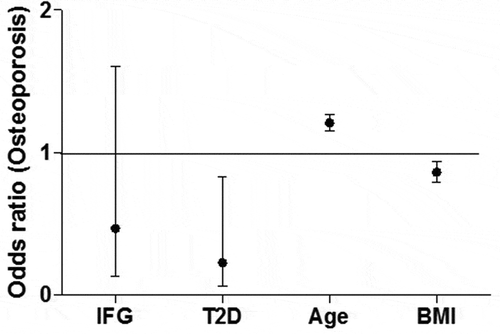
No differences were observed in FaBMD, T-scores or proportions with osteopenia and osteoporosis among IFG cases and normoglycemic women (). However, FaBMD and T-scores were lower in T2D cases compared to normoglycemic women in models adjusting for age and region. The parameters in the regression models that explained FaBMD included age, height, BMI, intake of marine mammals, smoking and region of residence (); T2D was not significantly associated with FaBMD when BMI was included in the model. Biomarkers (adiponectin, leptin, 25(OH)D), medication or supplement use did not contribute to the regression model for FaBMD. The above-mentioned results were similar to the results obtained when evaluating FaBMD T-scores (data not shown). The odds ratio of having a T-score consistent with osteoporosis () was reduced by having T2D (OR 0.228; 95%CI 0.062, 0.833) and a greater BMI (OR 0.863; 95%CI 0.794, 0.937) and elevated with advancing age (OR 1.209; 95% CI 1.152, 1.270), but not by having IFG (OR 0.467; 95%CI 0.135, 1.605).
Table 2. Bone health variables among Inuit women with impaired fasting glucose (IFG) or diabetes mellitus type 2 (T2D) and a normoglycemic group
Table 3. Beta coefficients and 95% confidence intervals (CI) for distal one-third forearm bone mineral density in Inuit women 40 y of age and over
Discussion
This study addressed the relationship between IFG, T2D and their risk factors with bone health measures among Inuit women ≥40 y in the Canadian Arctic. Overall, FaBMD was not in the osteoporotic range and no adverse associations with either IFG or T2D were observed after accounting for age and BMI. Remarkably, the women with a diagnosis of T2D were in very good glucose control with the median FPG below 7 mmol/L (6.1, IQR 5.5, 6.8), and a lower odds ratio of having osteoporosis in the distal forearm. In contrast, other studies found that T2D associates with higher BMD and T-scores [Citation4,Citation5] and that bone material strength is paradoxically reduced in women with DM2 in association with inadequate long-term glucose control (average HbA1c over 10 years) [Citation27]. Our study adds to the growing body of evidence that reinforces the importance of considering glycemic control in examining the relationships between T2D and bone health and offers new perspectives on the beneficial role of traditional food for Inuit.
Among the traditional food variables, only frequency of intake of marine mammals positively related to FaBMD. The frequency of consuming fish was not different among the IFG, T2D cases and normoglycemic women, the homogeneous sample likely explains the lack of significance of fish in the regression model. Fish was a commonly consumed traditional food in the overall survey (median (IQR) of 0.11 (0.02, 0.30)), with a younger mean age of 42 ± 15 y [Citation28]. Similar to fish, marine mammals are rich in omega-3 polyunsaturated FA [Citation26] and vitamin D [Citation10] that are beneficial to bone health [Citation15,Citation16]. Interestingly, neither serum 25(OH)D or RBC-Omega-3 were related to FaBMD in the current study population, although RBC-Omega-3 approached significance (p = 0.086) in the regression model (data not shown). These biological markers, however, reflect a shorter exposure duration relative to the one year assessment of traditional food intakes. Similar to our findings, in postmenopausal Chinese women BMD was 3.2–6.8% higher in the top quintile compared to lowest quintile of traditional sea fish intake [Citation29] and higher frequency of intakes of fish in a meta-analysis associated with reduced risk of T2D [Citation30]. Traditional food patterns including fish and seafood also associate with lower HbA1c [Citation14]. Our observation that marine mammal intakes were associated with greater FaBMD could be ascribed to the benefits of traditional food intakes on glucose control [Citation30]. Collectively, these studies underscore the benefits of traditional foods, which should be highlighted as a source of omega-3 and vitamin D, and beneficial to bone health.
Insulin is known to promote an increase in BMD [Citation31], likely through suppression of bone turnover, and fasting insulin, as expected [Citation31], was higher among IFG and T2D cases than normoglycemic women in this study. In contrast to previous research where adiponectin inversely relates to BMD [Citation18], adiponectin did not help to explain the variability in BMD in our regression analyses. It is important to mention that BMD, including that of the forearm, is not an indicator of bone quality, as T2D is associated with increased porosity in bone and accumulation of collagen advanced glycation end-products, which may increase the risk of fracture [Citation32,Citation33]. The association between T2D and increased fracture risk is more commonly observed at the hip and spine, compared to the wrist or forearm [Citation8]. Thus, future studies should incorporate assessments of the axial skeleton and femoral neck regions. Further studies are also needed to assess the longitudinal impact of IFG and T2D on bone quality and fracture risk in Inuit women to establish if risk is as elevated as it is in other Aboriginal groups [Citation34].
This study is among very few studies that characterise BMD of Inuit women. In a study by Andersen et al. [Citation35], BMD was assessed in the distal forearm (and calcaneus) and was found to be higher than the FaBMD values in the present study. However, their participants were younger (30–49 y) and the authors did not address glucose metabolism and its impact on BMD. To our knowledge, there are no other studies on the association between IFG or T2D and BMD in Inuit. A meta-analysis that pooled many studies, regardless of ethnicity which was mainly Caucasian, found no association between FaBMD and T2D, but a 0.04 g/cm2 increase in BMD at femoral neck and a 0.06 g/cm2 increase in BMD at the total hip and lumbar spine was observed [Citation36]. Similar to our odds ratio analyses, a study among Chinese women 40 y or older showed that the rate of BMD-based assessments of osteoporosis is lower in women with T2D relative to age-matched controls [Citation37]. Conversely, a longer duration (>10 y) of T2D associates with greater risk of fracture in women over 40 y of age [Citation34], and a recent systematic review of Indigenous populations suggests that Canada is among the few countries with an elevated risk of hip fracture that associates with T2D [Citation38]. This study included some women with newly diagnosed T2D, and combined with the challenges in obtaining an accurate medical history, this precluded assessment of risk of fractures according to duration of T2D. Hence, more studies are needed to further explore the association between T2D, BMD and fracture rates in Inuit over time.
Of the other lifestyle variables explored in this study, smoking was fairly common with 37% of the women reporting being a current smoker. After accounting for well-known covariates (age, BMI, height), smoking was weakly yet inversely related to FaBMD. The rate of smoking was aligned with 43% reported for women >55 y in the Aboriginal Peoples Survey [Citation39]; the odds ratio of smoking among Inuit women was lower among those with a postsecondary education and residence within food-secure homes. Smoking is reported to increase the risk of fracture regardless of frequency [Citation40,Citation41], whereas the impact on radial BMD is minimal [Citation42,Citation43]. Future studies that prospectively capture incident fracture will be required to clarify the adverse effects of smoking in this population.
Strengths and limitations
The IPY-HIS was designed to provide baseline data for longitudinal assessments of Inuit health [Citation19]. It is however not without limitations including the small sample size for those with IFG and T2D, inclusion of women 40 y of age and older without a healthy young reference group specific to Inuit, and without data regarding menopausal status. Having women above and below 50 y of age challenged our assessment of osteoporosis based on WHO guidelines where T-scores are usually applied only to those 50 y of age and over. In addition, the only assessment for bone that was feasible was FaBMD owing to the remote regions studied; assessment at other sites is needed to build upon the results of this study. Moreover, the food frequency questionnaire assessed the frequency of traditional food over a year based on recall and did not assess market food dietary patterns. Further research regarding actual age at menopause, and other lifestyle variables not captured (alcohol, exercise) in this report, are also needed since these major factors are likely to confound the association between dysglycemia and markers of osteoporosis. The assessment of other biomarkers of bone metabolism and long-term biomarkers of glucose control (HbA1c) would have improved our understanding of the relationship between bone health and IFG and T2D. Finally, data on the duration of previously diagnosed T2D and complete fracture history were not always available.
Conclusions
In summary, in this study we found that IFG and T2D did not have adverse associations with FaBMD, and FaBMD T-scores in Inuit women. Osteoporosis, as defined by a T-score of −2.5 or less, was low (8.5%) overall. This is potentially important since recently, BMD of the radius has emerged as protective against all-cause mortality in women with T2D [Citation44]. Frequency of marine mammal intakes was positively associated with FaBMD. Fish intake is protective from T2D in other populations [Citation30], and based on our results, marine mammals may confer protection to bone as well [Citation15]. The intake of traditional foods should be encouraged among Inuit women.
Acknowledgments
The authors would also like to express their gratitude to all of those involved in the Inuit Health Survey of 2007–2008; steering committee members from Inuit organisations, researchers and participants. This manuscript was reviewed and approved by the National Inuit Health Surveys Working Group, Inuit Tapiriit Kanatami.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Leslie WD, Rubin MR, Schwartz AV, et al. Type 2 diabetes and bone. J Bone Miner Res. 2012;27(11):2231–8.
- Vestergaard P. Discrepancies in bone mineral density and fracture risk in patients with type 1 and type 2 diabetes–a meta-analysis. Osteoporos Int. 2007;18(4):427–444.
- Zhou Y, Li Y, Zhang D, et al. Prevalence and predictors of osteopenia and osteoporosis in postmenopausal Chinese women with type 2 diabetes. Diabetes Res Clin Pract. 2010;90(3):261–269.
- Oei L, Zillikens MC, Dehghan A, et al. High bone mineral density and fracture risk in type 2 diabetes as skeletal complications of inadequate glucose control: the Rotterdam study. Diabetes Care. 2013;36(6):1619–1628.
- Siddapur PR, Patil AB, Borde VS. Comparison of bone mineral density, T-Scores and serum zinc between diabetic and non diabetic postmenopausal women with osteoporosis. J Lab Physicians. 2015;7(1):43–48.
- Leslie WD, Morin SN, Majumdar SR, et al. Effects of obesity and diabetes on rate of bone density loss. Osteoporos Int. 2018;29(1):61–67.
- Kruger MC, Wolber FM. Osteoporosis: modern paradigms for last century’s bones. Nutrients. 2016;8(6):E376.
- Leslie WD, Derksen SA, Metge C, et al. Demographic risk factors for fracture in First Nations people. Can J Public Health. 2005;96(Suppl 1):S45–S50.
- Pratt WB, Holloway JM. Incidence of hip fracture in Alaska Inuit people: 1979-89 and 1996-99. Alaska Med. 2001;43(1):2–5.
- El Hayek J, Pronovost A, Morin S, et al. Forearm bone mineral density varies as a function of adiposity in Inuit women 40-90 years of age during the vitamin D-synthesizing period. Calcif Tissue Int. 2012;90(5):384–395.
- Jakobsen A, Laurberg P, Vestergaard P, et al. Clinical risk factors for osteoporosis are common among elderly people in Nuuk, Greenland. Int J Circumpolar Health. 2013;72:19596.
- Egeland GM, Cao Z, Young TK. Hypertriglyceridemic-waist phenotype and glucose intolerance among Canadian Inuit: the International Polar Year Inuit Health Survey for Adults 2007-2008. CMAJ. 2011;183(9):E553–E558.
- Thorseng T, Witte DR, Vistisen D, et al. The association between n-3 fatty acids in erythrocyte membranes and insulin resistance: the Inuit Health in Transition Study. Int J Circumpolar Health. 2009;68(4):327–336.
- Batis C, Mendez MA, Gordon-Larsen P, et al. Using both principal component analysis and reduced rank regression to study dietary patterns and diabetes in Chinese adults. Public Health Nutr. 2016;19(2):195–203.
- Harris TB, Song X, Reinders I, et al. Plasma phospholipid fatty acids and fish-oil consumption in relation to osteoporotic fracture risk in older adults: the Age, Gene/Environment Susceptibility Study. Am J Clin Nutr. 2015;101(5):947–955.
- Weaver CM, Alexander DD, Boushey CJ, et al. Calcium plus vitamin D supplementation and risk of fractures: an updated meta-analysis from the National Osteoporosis Foundation. Osteoporos Int. 2016;27(1):367–376.
- Ley SH, Harris SB, Connelly PW, et al. Adipokines and incident type 2 diabetes in an Aboriginal Canadian [corrected] population: the Sandy Lake Health and Diabetes Project. Diabetes Care. 2008;31(7):1410–1415.
- Biver E, Salliot C, Combescure C, et al. Influence of adipokines and ghrelin on bone mineral density and fracture risk: a systematic review and meta-analysis. J Clin Endocrinol Metab. 2011;96(9):2703–2713.
- Saudny H, Leggee D, Egeland G. Design and methods of the Adult Inuit Health Survey 2007-2008. Int J Circumpolar Health. 2012;71.
- Abdul-Ghani MA, DeFronzo RA. Pathophysiology of prediabetes. Curr Diab Rep. 2009;9(3):193–199.
- Canadian Diabetes Association Clinical Practice Guidelines Expert, C., Goldenberg R, Punthakee Z. Definition, classification and diagnosis of diabetes, prediabetes and metabolic syndrome. Can J Diabetes. 2013;37(Suppl 1):S8–S11.
- Kanis JA, Gluer CC. An update on the diagnosis and assessment of osteoporosis with densitometry. Committee of Scientific Advisors, International Osteoporosis Foundation. Osteoporos Int. 2000;11(3):192–202.
- Barzilay JI, Davis BR, Pressel SL, et al. The impact of antihypertensive medications on bone mineral density and fracture risk. Curr Cardiol Rep. 2017;19(9):76.
- Liu J, Li X, Fan L, et al. Proton pump inhibitors therapy and risk of bone diseases: an update meta-analysis. Life Sci. 2019;218:213–223.
- Folch J, Lees M, Sloane Stanley GH. A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem. 1957;226(1):497–509.
- Zhou YE, Kubow S, Egeland GM. Highly unsaturated n-3 fatty acids status of Canadian Inuit: international Polar Year Inuit Health Survey, 2007-2008. Int J Circumpolar Health. 2011;70(5):498–510.
- Farr JN, Drake MT, Amin S, et al. In vivo assessment of bone quality in postmenopausal women with type 2 diabetes. J Bone Miner Res. 2014;29(4):787–795.
- Jamieson JA, Kuhnlein HV, Weiler HA, et al. Higher n3-fatty acid status is associated with lower risk of iron depletion among food insecure Canadian Inuit women. BMC Public Health. 2013;13:289.
- Chen YM, Ho SC, Lam SS. Higher sea fish intake is associated with greater bone mass and lower osteoporosis risk in postmenopausal Chinese women. Osteoporos Int. 2010;21(6):939–946.
- Muley A, Muley P, Shah M. ALA, fatty fish or marine n-3 fatty acids for preventing DM?: a systematic review and meta-analysis. Curr Diabetes Rev. 2014;10(3):158–165.
- Tonks KT, White CP, Center JR, et al. Bone turnover is suppressed in insulin resistance, independent of adiposity. J Clin Endocrinol Metab. 2017;102(4):1112–1121.
- Nilsson AG, Sundh D, Johansson L, et al. Type 2 diabetes mellitus is associated with better bone microarchitecture but lower bone material strength and poorer physical function in elderly women: a population-based study. J Bone Miner Res. 2017;32(5):1062–1071.
- Comptston, J.Type 2 diabetes mellitus and bone. J Intern Med. 2018;283(2):140–153.
- Majumdar SR, Leslie WD, Lix LM, et al. Longer duration of diabetes strongly impacts fracture risk assessment: the Manitoba BMD cohort. J Clin Endocrinol Metab. 2016;101(11):4489–4496.
- Andersen S, Boeskov E, Laurberg P. Ethnic differences in bone mineral density between Inuit and Caucasians in north Greenland are caused by differences in body size. J Clin Densitom. 2005;8(4):409–414.
- Ma L, Oei L, Jiang L, et al. Association between bone mineral density and type 2 diabetes mellitus: a meta-analysis of observational studies. Eur J Epidemiol. 2012;27(5):319–332.
- Shan PF, Wu XP, Zhang H, et al. Age-related bone mineral density, osteoporosis rate and risk of vertebral fracture in mainland Chinese women with type 2 diabetes mellitus. J Endocrinol Invest. 2011;34(3):190–196.
- Brennan-Olsen SL, Vogrin S, Leslie WD, et al. Fractures in indigenous compared to non-indigenous populations: A systematic review of rates and aetiology. Bone Rep. 2017;6:145–158.
- Bougie E, Kohen DE. Smoking correlates among Inuit men and women in Inuit Nunangat. Health Rep. 2018;29(3):3–10.
- Kanis JA, Johnell O, Oden A, et al. Smoking and fracture risk: a meta-analysis. Osteoporos Int. 2005;16(2):155–162.
- Thorin MH, Wihlborg A, Akesson K, et al. Smoking, smoking cessation, and fracture risk in elderly women followed for 10 years. Osteoporos Int. 2016;27(1):249–255.
- Hermann AP, Brot C, Gram J, et al. Premenopausal smoking and bone density in 2015 perimenopausal women. J Bone Miner Res. 2000;15(4):780–787.
- Tian L, Yang R, Wei L, et al. Prevalence of osteoporosis and related lifestyle and metabolic factors of postmenopausal women and elderly men: A cross-sectional study in Gansu province, Northwestern of China. Medicine (Baltimore). 2017;96(43):e8294.
- Lenchik L, Register TC, Hsu FC, et al. Bone mineral density of the radius predicts all-cause mortality in patients with type 2 diabetes: diabetes heart study. J Clin Densitom. 2018;21(3):347–354.