ABSTRACT
Recent reports have found a rise in Hepatitis C virus (HCV) infection in reproductive age women in the USA. Surveillance data suggests one group that is at increased risk of HCV infection is the American Indian and Alaska Native population (AI/AN). Using the National Center for Health Statistics (NCHS) birth certificate and the Indian Health Services, Tribal, and Urban Indian (IHS) databases, we evaluated reported cases of HCV infection in pregnant women between 2003 and 2015. In the NCHS database, 38 regions consistently reported HCV infection. The percentage of mothers who were known to have HCV infection increased between 2011 and 2015 in both the AI/AN population (0.57% to 1.19%, p < 0.001) and the non-AI/AN population (0.21% to 0.36%, p < 0.001). The IHS database confirmed these results. Individuals with hepatitis B infection or intravenous drug use (IDU) had significantly higher odds of HCV infection (OR 16.4 and 17.6, respectively). In total, 62% of HCV-positive women did not have IDU recorded. This study demonstrates a significant increase in the proportion of pregnant women infected with HCV between 2003 and 2015. This increase was greater in AI/AN women than non-AI/AN women. This highlights the need for HCV screening and prevention in pregnant AI/AN women.
Introduction
Despite an overall downward trend in infectious disease mortality in the USA, rates of hepatitis C virus (HCV) diagnosis are on the rise [Citation1]. The best estimates of HCV prevalence in the USA are based on data from the National Health and Nutrition Examination Survey (NHANES) that has been corrected for under-represented populations [Citation2]. Samples from this national, population-based survey have been tested for the presence of HCV antibodies, and RNA and provide a national estimate of HCV prevalence of approximately 1.4% [Citation3–Citation5]. Of concern, information from the 2001–2008 dataset show that 50% of individuals who are infected was not aware of their infection [Citation3].
Individuals born between 1940 and 1965 have the highest prevalence of HCV infection, and national recommendations specifically target this group for testing [Citation6,Citation7]. Recent reports have found that the rate of new HCV infections in other age groups is on the rise, with an increase observed in women of reproductive age [Citation8–Citation11]. This is likely due, at least in part, to the recent opioid epidemic and the resulting increase in intravenous drug use (IDU) [Citation12]. This trend is specifically concerning as HCV can be transmitted at birth from mother to infant [Citation13,Citation14]. For this reason, it is especially important to identify pregnant women who are infected with HCV, as both the mother and the infant require follow up.
Based on surveillance data from the Centers for Disease Control and Prevention, American Indian and Alaska Native populations (AI/AN) appear to be at increased risk of HCV infection [Citation15]. Indian Health Service, Tribal, and Urban Indian (IHS) facilities provide care to eligible AI/AN people who are members of 573 federally recognised Tribes. This system provides care for approximately 2.2 million (59%) of the nation’s estimated 3.7 million eligible AI/AN people [Citation16]. The IHS system maintains a national database, the National Data Warehouse (NDW), where all diagnosis codes related to both inpatient and outpatient visits are recorded [Citation17].
The National Center for Health Statistics (NCHS) maintains a national database of birth certificates, recording greater than 99% of all births nationally [Citation18]. The 2003 revised birth certificate form includes questions regarding the mother’s HCV infection status. A recent publication using this database to evaluate the trend in HCV infection across the country, with a special focus on Tennessee, found that the proportion of women with recognised HCV infection increased nationwide 89% between 2009 and 2014 [Citation10]. This analysis evaluated the trend for white, black and Hispanic individuals in Tennessee, but did not evaluate the trend in AI/AN people.
Given the possible increased risk of HCV infection in AI/AN people and the recent increases in HCV in women of reproductive age, the current study evaluated trends in both HCV testing frequency and the number of reported cases of HCV infection in AI/AN women who gave birth or were pregnant between 2003 and 2015 using two different databases. In addition, multiple demographic factors and co-morbid conditions were evaluated to identify factors that are associated with an increased risk of HCV infection in this population.
Methods
National centre for health statistic’s birth certificate database
State laws require birth certificates to be completed at the birth facility for all births, and NCHS collects and publishes information regarding all births as required by Federal law. Analysis was performed evaluating the proportion of maternal HCV positivity in AI/AN and non-AI/AN women nationwide from 2011 to 2015. A woman was considered AI/AN based on bridged race. Data regarding hepatitis C, hepatitis B, syphilis, chlamydia, gonorrhoea, maternal age, education, cigarette use, and prenatal care were collected. Individuals whose birth certificate was missing infection data were excluded. As some states did not take up the use of the birth certificate form that included HCV infection until recently, the analysis for the country as a whole was performed only using those states that continuously reported throughout the study period (Supplemental table 1).
The five infectious diseases listed above are recorded in the NCHS birth certificate database. The response options include “Yes” for each individual disease, and “none of the above” for the entire category. Information in the database recorded as “No” or “Unknown or not stated” with regard to a specific infection were considered indicative of a lack of confirmed maternal infection and combined for analysis. Data for prenatal care was dichotomised into late to prenatal care (3rd trimester, “No Prenatal Care”, and “Unknown or not stated”) and earlier initiation of care (1st and 2nd trimester) based on the month prenatal care began. Maternal education was dichotomised into those women who completed high school and those who did not. Maternal smoking was categorised as smoker and non-smoker based on current smoking status. Maternal age was categorised as 10–19, 20–29, 30–39, and over 40 years. Parity was divided into two categories; four or fewer versus five or more previous term pregnancies based on the standard definition of grand multiparity.
Sub-analysis was performed to evaluate the average proportion of maternal HCV positivity in from two states with a high number of AI/AN resident, Alaska and Arizona, starting the year they reported data (2013 and 2014, respectively). No trends were reported for these two states due to the limited number of cases and years of data.
IHS national data warehouse (NDW)
The NDW database includes diagnosis codes and basic demographics from all inpatient and outpatient visits to IHS facilities between 2001 and 2014. The first year of analysis was 2003, as two years of data prior to pregnancy were needed for analysis. 2007 was evaluated as an intermediate year and 2011–2014 were analysed as they matched with the years available in the NCHS birth certificate database. A woman was considered pregnant in the NDW database if she had two International Classification of Diseases, 9th Revision, Clinical Modification (ICD) codes related to pregnancy (supplemental table 2) within a calendar year of interest and 8 months prior [Citation18]. If a woman had multiple pregnancy codes in two consecutive years, this woman was excluded from the earlier year’s pregnancy cohort and counted in the later year’s cohort. This was done to avoid double counting women whose pregnancy spanned two calendar years.
Within the NDW database, a woman was considered HCV positive if she had two ICD codes related to HCV (supplemental ) in the year of or the two years preceding pregnancy. A woman was considered to have a new diagnosis of HCV related to prenatal HCV testing if she had an ICD code for HCV during her pregnancy period but did not have an ICD code for HCV in the previous two years. A woman was considered to have a history of injection drug use (IDU) if she had any of the ICD codes for IDU (supplementary table 2) during pregnancy or in the previous two years.
Table 1. Reported hepatitis C in pregnant women or women who recently delivered from the NCHS birth certificate database* and the IHS national data warehouse, 2011–2015
Table 2. Injection drug use (IDU) and documentation of hepatitis C among pregnant American Indian/Alaska Native (AI/AN) women; IHS national data warehouse, 2003, 2007, 2011–2014
Statistical analysis was performed using SAS software v. 9 · 4 (SAS Institute Inc., USA). The rate of change over the study period was compared between AI/AN and non-AI/AN in the NCHS dataset using generalised linear regression. Univariate comparisons were made by use of the likelihood ratio chi-square test or the Cochran Armitage test of trend. Variables with a univariate p-value < 0.25 were considered in the multivariable logistic regression models [Citation19]. All variables were entered into the model and a backwards elimination with re-entry was used to select the final statistical model. Variables were considered confounders and remained in the model if their exclusion changed the value of the coefficient(s) of interest by >15%. A p-value < 0.05 was considered statistically significant.
Results
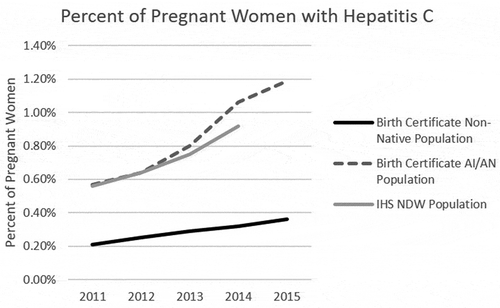
In 2011 3,391,659 birth certificates were available in the NCHS database for analysis, increasing to 3,978,497 in 2015. When limited to only those 38 regions that reported consistently throughout the time period the numbers decreased to 3,233,726 in 2011 to 3,266,257 in 2015 (). Limiting to the consistently reporting states resulted in an elimination of 10.0% of non-AI/AN mothers and 15.2% of AI/AN mothers from the overall time period. The larger loss of AI/AN records was due to the elimination of Alaska and Arizona, states with high AI/AN populations that did not start recording hepatitis C infection until after 2011. Records lacking information regarding the presence of maternal infections accounted for less than 0.64% of individuals in any given year. Within this dataset, 34,208 (2011) and 33,343 (2015) were from women who identified as AI/AN. The percentage of women who were known to have HCV infection increased from 2011 to 2015 in both the AI/AN population (0.57% to 1.19%, p < 0.001) and the non-AI/AN population (0.21% to 0.36%, p < 0.001, ). The rate of change was significantly different for the two populations with the AI/AN population experiencing an increase of 0.12% per year and the non-AI/AN population a 0.03% per year on average (p < 0.0001, ).
The sub-analysis from Alaska found that 0.71% of the AI/AN women and 0.60% of the non-AI/AN women reported HCV infection when averaged over 2013 to 2015. For Arizona, 0.25% of the AI/AN women and 0.19% of the non-AI/AN women reported HCV infection when averaged over 2014 to 2015.
When a similar time period was analysed using the NDW database the percentage of pregnant women who had an ICD code for HCV increased from 0.56% in 2011 to 0.92% in 2014 (p < 0.0001, , ). Between 56% and 66% of women had the first appearance of an HCV ICD code during their pregnancy window suggesting that it was first diagnosed during this time period. NDW data showed an increase in documented hepatitis C over the long term, with 0.38% of pregnant women with documented hepatitis C in 2003, increasing to 0.49% in 2007 (p = 0.048) and 0.92% in 2014 (p < 0.0001; , ).
Multivariable analysis with the birth certificate data showed documented hepatitis C was correlated with all the other recorded infections, including chlamydia (OR 1.5, CI: 1.3–1.9), gonorrhoea (OR 2.0, CI: 1.3–3.2), hepatitis B (OR 16.4 CI: 10.7–25.0) and syphilis (OR 2.7, CI: 1.1–6.2) (). The odds of having documented hepatitis C were increased in women without a high school diploma by 1.5 times (CI 1.3–1.7), who smoked during pregnancy by 6.5 times (CI:5.7–7.4), who had five or more children by 1.8 times (CI:1.6–2.1), and who presented late for prenatal care by 2.5 times (CI:2.1–2.8) (). Within the NDW dataset, documented IDU increased the odds of women having documented hepatitis C by 17.6 (95% CI: 15.0–20.7). Of the 659 women with documented hepatitis C in NDW between 2011 and 2014, 407 (61.8%) did not have any IDU documented in the previous three years.
Table 3. Odds* of hepatitis C associated with other infections or demographic factors among American Indian/Alaska Native (AI/AN) women in the NCHS birth certificate database+, 2011–2015
In the long-term analysis using the NDW database, the number of women with documented IDU in the two years leading up to pregnancy increased from 1.8% in 2003 to 4.3% in 2014 (p < 0.0001; ). Within this group of women, the number with documented HCV infection increased from 6.7% in 2003 to 9.1% in 2014 (p < 0.001).
Discussion
This analysis used two different databases to estimate the prevalence of documented hepatitis C in women who were pregnant or gave birth. Data from the NCHS birth certificate database show that the prevalence of documented hepatitis C in pregnant AI/AN women increased from 0.57% to 1.19%. This prevalence was higher in the AI/AN population compared to the non-AI/AN population and demonstrated a significantly more rapid increase over the time period studied. In the NDW dataset, an ICD code for hepatitis C first appeared in the majority of women’s records during their pregnancy window, suggesting these women were diagnosed secondary to prenatal testing. The percent of women with ICD codes documenting IDU was found to increase significantly between 2003 and 2014, from 2% to 4%. Documented hepatitis C prevalence was most strongly associated with hepatitis B and tobacco use, but also associated with chlamydia, gonorrhoea and late presentation to prenatal care. In addition, the percent of women with a history of IDU with documented hepatitis C increased significantly from 6.7% in 2003 to 9.1% in 2014.
These results show a concerning trend in the AI/AN community. In 2015, AI/AN mothers were three times more likely than non-AI/AN mothers to have hepatitis C documented on their child’s birth certificate. This disparity was apparent in 2011, the beginning of the investigation period, and increased significantly by the end of the investigation period, 2015. During a similar time period, a significant increase in the number of pregnant AI/AN women who had documented IDU increased from 1.8% in 2003 to 4.3% in 2014. The Substance Abuse and Mental Health Services Administration (SAMHSA) documents that 3.3% of the total US population over 18 years of age report illicit drug use other than marijuana in 2013–14 [Citation20]. It is difficult to compare the numbers reported by SAMHSA to those collected from medical record ICD codes, but it is concerning that the number found in this study is higher than the national estimate.
While the presence of IDU was found to be significantly related to HCV in the NDW dataset, it is important to recognise that 62% of women with documented hepatitis C did not have IDU documented in their IHS administrative record in the previous 3 years. Current national guidelines recommend testing only individuals who have risk factors for HCV infection. It is likely this study underestimated the relationship between IDU and hepatitis C. It is possible someone used injection drugs in the past and was infected with HCV at that time, but has since recovered from IDU. Given the limited time frame used to evaluate for IDU ICD codes, these cases would be considered to have had hepatitis C but IDU negative in the analysis. In addition, the relationship between documented IDU and HCV infection is likely underestimated due to inaccurate reporting by patients to physicians [Citation21].
There are a number of limitations to this study. With regard to the NDW data, ICD codes were used to identify individuals who were pregnant, who had a history of IDU, and who were diagnosed with hepatitis C. ICD codes are intended to be used for billing purposes, not as medical records. In addition, it is not possible to know if a person with an ICD code for hepatitis C has an active infection or only exposure. As such, this data must be considered estimates instead of precise numbers. Reassuringly, the NDW and NCHS birth certificate databases provided very similar estimates regarding the proportion of AI/AN women who had documented hepatitis C and showed similar trends over time. A second limitation regarding the NDW database is that this data only represents a subpopulation of the AI/AN population, as 59% of AI/AN individuals receive care outside the IHS system [Citation22]. The population that receives care within IHS is skewed toward individuals living in the rural area [Citation23]. However, as above, the similarity in numbers between the data from the NDW and NCHS birth certificate databases is reassuring and indicates the NDW database provides a good estimation of the prevalence of known HCV infection in the AI/AN population. A third limitation is the restricted time frame that was included in the analysis. ICD codes related to hepatitis C and IDU were evaluated in the year of pregnancy and the preceding two years. A sub-analysis was performed extending the evaluation time to five years. Five additional cases were found using the expanded analysis time, resulting in a 0.02% increase in the estimated percent with hepatitis C. Therefore, while this report is likely to under-identify individuals exposed and at risk, it is only by a small fraction. A third limitation is that 20 out of 56 states and territories did not consistently report hepatitis C on their birth certificates during the analysis period and were excluded from the analysis. Sub-analysis was performed to evaluate the average percent of women who were known to have HCV in Alaska and Arizona, as these states have large AI/AN populations. It was not possible to evaluate trends or significance in these two states due to the limited number of cases and years, but the numbers reported in these states are similar to those in the larger analysis.
A final limitation is that this analysis evaluated the number of women with known hepatitis C; however, it was not possible to determine the number of women tested. Current Procedural Terminology (CPT) codes are available for hepatitis C testing, however, an in-depth analysis of the CPT database maintained by IHS found that these CPT codes were not consistently reported from all facilities. For this reason, it was only possible to evaluate the number of women known to have hepatitis C, not calculate the prevalence of the disease. In 2013 NCHS added a question regarding the history of hepatitis C testing to their National Health Interview Survey (NHIS). Between 2013 and 2015, 12.4% of non-AI/AN and 16.9% of AI/AN women of reproductive age reported a history of testing (data not shown). Given unknown testing rates, it is impossible to calculate an accurate prevalence for hepatitis C, but given the lack of high rates of testing, it is likely the numbers reported here are a low estimate.
During the investigation period the percentage of women who were known to have hepatitis C increased in both the AI/AN and the non-AI/AN population, with the AI/AN population experiencing an increase of 0.12% per year and the non-AI/AN population a 0.03% per year increase on average. It is likely that part of this observed increase in hepatitis C is a result of increased testing practices. According to the NHIS, the proportion of non-AN/AN women of reproductive age reporting ever having been tested increased by 12% between 2013 and 2016 (data not shown). In contrast, the proportion of AI/AN women of reproductive age reporting ever having been tested fluctuated between 2013 and 2016 between 13% and 24% with no overall trend (data not shown). A more general study in a large insured population found a 2.5-fold increase in the percentage of antibody tests performed between 2005 and 2014 [Citation24]. It is not possible to conclude how much of the increase observed here is due to increased testing versus an increase in the fraction of women with new HCV infection.
Two interesting risk factors for hepatitis C were found in this analysis. The first of which is a very strong association with hepatitis B. The increased odds of hepatitis C were similar for those with IDU (17.6) and hepatitis B (16.4) compared to those without those factors. Not many individuals had documented hepatitis B; however, they would be an easy population to target for HCV testing. A second risk factor of interest was the late presentation to prenatal care. Women who did not receive prenatal care or who did not present for prenatal care until the 3rd trimester had 2.5 times the odds of having hepatitis C compared to those who presented earlier. This observation could be useful to clinicians when they are trying to identify women who should be questioned about risk factors and tested.
Hepatitis C is known to be a transmitted mother to child around the time of delivery, but no prophylaxis is available to prevent it. Transmission rates vary based on a number of factors, but overall estimates suggest approximately 4% to 7% of infants born to HCV-positive mothers become infected [Citation25]. Applying a 6% estimate to our population, we would expect over 70 AI/AN and 2,200 non-AI/AN infants born between 2011 and 2015 to be infected with HCV. One study evaluated the frequency of positive HCV tests in pregnant women, comparing it to the number of reported HCV-infected infants and found that there were significantly fewer reported cases of infant infection than expected [Citation8]. This indicates either an under-reporting of infant HCV infection or a lack of recognition of the infants at risk. Two recent reports support the second option, as these studies found that less than half of infants born to HCV-positive mothers were appropriately followed [Citation26,Citation27]. This lack of infant testing and follow up are concerning as it suggests many HCV infected children are going unrecognised and unmonitored.
This report highlights the disparity in documented hepatitis C between AI/AN and non-AI/AN pregnant women. Not only did pregnant AI/AN women have a higher percent of documented HCV cases than non-AI/AN women, but they also had a more rapid increase in known cases over time. IDU is the most significant risk factor for HCV infection; however, a majority of women who had documented HCV exposure did not have recent documentation of IDU. Further investigation needs to be performed to understand testing practices, risk populations and create a more accurate prevalence estimate in order to best provide resources for treatment and prevention in AI/AN individuals.
Supplemental Material
Download MS Word (19.4 KB)Disclosure statement
No potential conflict of interest was reported by the authors.
Supplementary material
Supplemental data for this article can be accessed here.
Correction Statement
This article has been republished with minor changes. These changes do not impact the academic content of the article.
References
- Ly KN, Hughes EM, Jiles RB, et al. Rising mortality associated with hepatitis C virus in the USA, 2003-2013. Clinl Infect Dis. 2016;62(10):1287–7.
- Edlin BR, Eckhardt BJ, Shu MA, et al. Toward a more accurate estimate of the prevalence of hepatitis C in the USA. Hepatology. 2015;62(5):1353–1363.
- Denniston MM, Klevens RM, McQuillan GM, et al. Awareness of infection, knowledge of hepatitis C, and medical follow-up among individuals testing positive for hepatitis C: National health and nutrition examination survey 2001-2008. Hepatology. 2012;55(6):1652–1661.
- Denniston MM, Jiles RB, Drobeniuc J, et al. Chronic hepatitis C virus infection in the USA, National health and nutrition examination survey 2003 to 2010. Ann Internal Med. 2014;160(5):293–300.
- Udompap P, Mannalithara A, Heo N-Y, et al. Increasing prevalence of cirrhosis among U.S. adults aware or unaware of their chronic hepatitis C virus infection. J Hepatol. 2016;64(5):1027–1032.
- Smith BD, Morgan RL, Beckett GA, et al. Hepatitis C virus testing of persons born during 1945-1965: recommendations from the centers for disease control and prevention. Ann Intern Med. 2012;157(11):817–822.
- Hepatitis C Online. HCV Epidemiology in the USA. 2017. [cited 2018 Jun 1]. Available from: https://www.hepatitisc.uw.edu/pdf/screening-diagnosis/epidemiology-us/core-concept/all
- Ly, K.N., Jiles RB, Teshale EH, et al. Hepatitis C virus infection among reproductive-aged women and children in the USA, 2006 to 2014. Ann Int Med; 2017;166:775–782.
- Koneru A, Nelson N, Hariri S, et al. Increased Hepatitis C Virus (HCV) detection in women of childbearing age and potential risk for vertical transmission - USA and Kentucky, 2011-2014. Morb Mortal Wkly Rep. 2016;65(28):705–710.
- Patrick SW, Bauer AM, Warren MD, et al. Hepatitis C virus infection among women giving birth - Tennessee and USA, 2009-2014. Morb Mortal Wkly Rep. 2017;66(18):470–473.
- Mori K, Provo G. Increase in Hepatitis C cases among young adults —Alaska, 2011–2015. 2016. Available from: http://epibulletins.dhss.alaska.gov/Document/Display?DocumentId=1839.
- Zibbell JE, Asher AK, Patel RC, et al. Increases in acute hepatitis C virus infection related to a growing opioid epidemic and associated injection drug use, USA, 2004 to 2014. Am J Public Health. 2018;108(2):175–181.
- Mast EE, Hwang L-Y, Seto DSY, et al. Risk factors for perinatal transmission of hepatitis C virus (HCV) and the natural history of HCV infection acquired in infancy. J Infect Dis. 2005;192(11):1880–1889.
- Garcia-Tejedor A, Maiques-Montesinos V, Diago-Almela VJ, et al. Risk factors for vertical transmission of hepatitis C virus: a single center experience with 710 HCV-infected mothers. Eur J Obstet Gynecol Reprod Biol. 2015;194:173–177.
- Surveillance for viral Hepatitis, – USA, 2018. Atlanta, GA: centers for disease control and prevention. [cited 2018 Jul 1]. Available from: https://www.cdc.gov/hepatitis/statistics/2016surveillance/index.htm.
- Whittaker R, Economopoulou A, Dias JG, et al. Epidemiology of invasive haemophilus influenzae disease, Europe, 2007-2014. Emerg Infect Dis. 2017;23(3):396–404.
- Trends in Indian health 2014 edition. [cited 2019 Jun 1]. Available from: https://www.ihs.gov/dps/includes/themes/responsive2017/display_objects/documents/Trends2014Book508.pdf
- Martin JA, Hamilton BE, Osterman MJ, et al. Births: final data for 2015. National Vital Stat Rep. 2017;66(1):1.
- Kleinbaum DG, Klein M, Pryor ER. Logistic regression a self-learning text, Statistics for biology and health. 2002;2. NewYork, NY: Springer Publishing.
- SAMHSA. Center for Behavioral Health Statistics and Quality, National Survey on Drug Use and Health, 2012, 2013, and 2014. [cited 2018 Jun 7]. Available from: https://www.samhsa.gov/data/sites/default/files/NSDUHsaeShortTermCHG2014/NSDUHsaeShortTermCHG2014.htm
- Monte AA, Heard KJ, Hoppe JA, et al. The accuracy of self-reported drug ingestion histories in emergency department patients. J Clin Pharmacol. 2015;55(1):33–38.
- Indian health service: quick look. [ cited 2017 Dec 31]. Available from: http://www.ihs.gov/newsroom/factsheets/quicklook.
- Boccuti C, Swoope C, Artiga S, The role of medicare and the Indian health service for American Indians and Alaska natives: health, access and coverage. 2014. [cited 2019 Mar 4]. Available from: https://www.kff.org/report-section/the-role-of-medicare-and-the-indian-health-service-for-american-indians-and-alaska-natives-health-access-and-coverage-report/
- Isenhour CJ, Hariri SH, Hales CM, et al. Hepatitis C antibody testing in a commercially insured population, 2005-2014. Am J Prev Med. 2017;52(5):625–631.
- Roberts EA, Yeung L. Maternal-infant transmission of hepatitis C virus infection. Hepatology. 2002;36(5 Suppl 1):S106–S113.
- Towers CV, Fortner KB. Infant follow-up postdelivery from a hepatitis C viral load positive mother. J Matern Fetal Neonatal Med. 2018;1–81.
- Chappell CA, Hillier SL, Crowe D, et al. Hepatitis C virus screening among children exposed during pregnancy. Pediatrics. 2018;141:e20173273.