ABSTRACT
The goal of the Norwegian Ministry of Health and Care Services is to offer an equal health-care service with the same outcomes wherever people are living within the country. The aim of this study was to evaluate whether this was true for patients diagnosed with metastatic prostate cancer (mPC) and living in Nordland County, a region with a challenging geography and climate and having, several small and remote communities and only 1 department of oncology. The latter is located in the main city, Bodø. We also compared a subgroup living in communities having lower average annual income (less than NOK 240,000 (equivalent to USD 28,600)) with patients living in Bodø (NOK 285,000 (USD 33,900)). Overall 288 patients were included and stratified into 3 subgroups (favourable distance and income, unfavourable distance and income, and unfavourable distance and favourable income). No statistically significant differences were observed regarding patient characteristics. There was no indication towards under-treatment among patients from the distant regions or the lower income region. Given that disparities were not observed, it was not surprising to see comparable survival outcomes (p=0.35). In conclusion, these results suggest that the health-care system in Nordland County successfully delivers state-of-the-art oncology care to patients with mPC.
Introduction
Equal access to affordable health care is not guaranteed for all cancer patients in Europe, North America and the circumpolar region, where a variety of different health-care systems exist and where several studies have described disparities that explain variations in survival outcomes not only between countries but also within some countries’ populations [Citation1–Citation7]. For example, rural patients and those from low average annual income regions have been reported experiencing reduced access to high-quality oncology care [Citation8–Citation10]. The publicly funded Norwegian health-care system aims to avoid disparities [Citation11,Citation12] and financial barriers to oncology care, e.g., by providing travel and accommodation. The goal of the Norwegian Ministry of Health and Care Services (HOD) is to provide good and equal health and care services to the population of Norway. Even within the rural, arctic region of North Norway, with several small islands, long fjords and remote small communities, not all patients have large travel distances to the main and local hospitals [Citation13]. Norway has been known for a policy aiming to minimize poverty and offer public health insurance to all inhabitants. Consequently, differences in average income between various subgroups of the population have been restricted. However, the average annual income varies by community and has been reported to be less than NOK 240,000 (USD 28,600) in several small communities in our county (Nordland) [Citation14]. In contrast, the average annual income among inhabitants of Bodø (the capital of Nordland County, approximately 50,000 inhabitants) was NOK 285,000 (19% higher). The region’s main hospital, and the only 1 with a department of oncology, is also located in Bodø. Many patients have to travel more than 200 km (often by air or by sea) to personally consult with an oncologist or receive radiotherapy. Systemic treatment is also administered at local hospitals, which consult with an oncologist via weekly virtual, web-based meetings. There are a total of 6 local hospitals in Nordland County and 5 of them are connected to the Nordland hospital trust. Due to these circumstances, regular monitoring of pattern of care and outcome is necessary to ensure the health-care system is well aligned to its political and social framework. Therefore, we studied the pattern of care and survival among a sample consisting of men with metastatic prostate cancer (mPC), stratified by home community within the county of Nordland.
Material and methods
This retrospective study included 288 consecutive men (all Caucasian) with mPC who received oncology care at the Nordland hospital Bodø (academic teaching hospital in rural North Norway). Some patients presented with metastases at diagnosis, others later during the disease trajectory. In all cases, metastatic disease was diagnosed some time between 2003 and 2015. National clinical pathways ensure that diagnostic procedures, after a suspected cancer diagnosis, eventually lead to initiation of treatment within specified time frames. Systemic treatment was given according to the National guidelines. It did not include early docetaxel during the hormone-sensitive stage in this study. Palliative radiotherapy was utilized for different indications, e.g. painful bone metastases or metastatic spinal cord compression. We arbitrarily defined a potentially favourable subgroup of patients, which included those who lived in the higher income area close to the Nordland hospital Bodø. An unfavourable subgroup included patients who had larger travel distance and lived in communities with lower average annual income. All remaining patients formed the intermediate group. The regional electronic patient record (EPR) system, named DIPS®, was used to collect all follow-up, treatment and baseline data. Patient relocation was also identified in the EPR and led to exclusion from further analysis. Actuarial survival from the diagnosis of metastatic disease and from first cancer diagnosis was calculated with the Kaplan–Meier method and compared between subgroups with differing baseline characteristics with the log-rank test. Fourteen patients were censored at the time of last follow-up (22–64 months, median 36 months). Associations between different variables of interest were assessed with the chi-square or Fisher exact probability test (2-tailed). A p-value ≤0.05 was considered statistically significant. Based on the number of patients who lived in Bodø (n = 76), we estimated that we were able to detect a difference in 2-year survival of 22% if the other group of patients had the same size (alpha 0.05, power 80%, estimated 2-year survival 50%).
Results
Patient characteristics
While 76 patients (26%) lived in Bodø, i.e. closest to the department of oncology (favourable distance and income area), 28 (10%) lived in 7 distant small communities with average annual income below NOK 240,000 (unfavourable distance and income area). These communities included Nesna, Leirfjord, Hemnes, Hamarøy, Tysfjord, Øksnes and Bø (1,800–4,500 inhabitants). The remaining 184 patients (64%) lived at variable distances from Bodø (<100 km and also >200 km) in small- or medium-sized communities (smallest: Røst (500 inhabitants), largest: Rana (26,000)). These communities had higher average annual incomes than NOK 240,000 but did not exceed the Bodø income. Further patient characteristics are shown in . None of the differences was statistically significant. However, the patients from the unfavourable distance and income area tended to be younger and healthier.
Table 1. Patient characteristics, n = 288
Treatment details
Systemic treatment for castration-resistant disease included docetaxel, cabazitaxel, enzalutamide and abiraterone acetate. Some patients received bone-targeting agents (often zoledronic acid or denosumab) alone or in combination with other systemic treatment. The proportion of patients without any such treatment was highest in Bodø (46%, p = 0.075, ). Almost all patients who received no systemic treatment did so as a result of physician choice (poor performance status, frailty, comorbidities). After exclusion of patients who received bone-targeting drugs alone, the proportion of untreated patients remained higher in Bodø (66%, p = 0.16). The proportion of patients who received at least 2 sequential lines, e.g. docetaxel plus cabazitaxel or enzalutamide plus docetaxel, was lowest in Bodø (14%, p = 0.048). Comparable to the utilization of systemic treatment, there was no indication towards under-treatment with palliative bone radiotherapy in the distant/lower income region. Actually, the rate of 80% was highest among all 3 regions.
Table 2. Pattern of care, n = 280 (exclusion of patients who were alive and not yet treated with the approaches mentioned below)
Overall survival
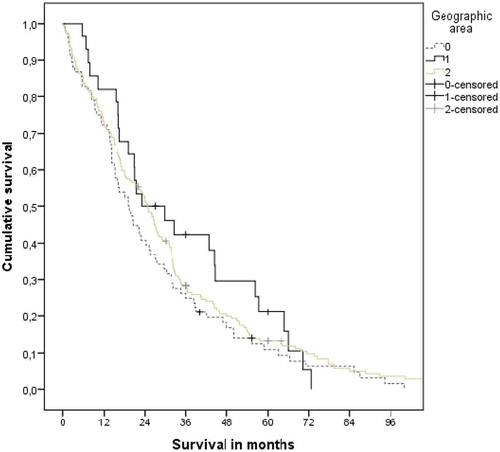
Median survival from diagnosis of distant metastases is shown in . It was shortest in the subgroup from Bodø (19.2 months as compared to 23.1 and 24.7 months, p = 0.35). And 41%,, 46%, and 51% of the patients were alive after 2 years. Also, median survival from initial diagnosis of prostate cancer was shortest in the Bodø group (60.5 vs 70.3 and 68.2 months, p = 0.68, Kaplan–Meier curves not shown).
Figure 1. Actuarial Kaplan–Meier survival curves for patients from 3 different geographic areas (Bodø: favourable distance and income (coded “0”); 7 small communities: unfavourable distance and income (coded “1”); other communities: unfavourable distance, favourable income (coded “2”)). The median was 19.2, 23.1 and 24.7 months, respectively (p = 0.35)
An additional analyses of 2 subgroups were performed. The first one included all patients who lived in communities with average annual income < NOK 240,000 and the second one those who lived in communities with income ≥ NOK 240,000 (range 240,000–285,000). The median income for these 2 subgroups was NOK 250,000 and 230,000, respectively. Median survival from diagnosis of distant metastases was 23.6 (higher income) and 22.2 months (lower income), respectively (p = 0.86).
Discussion
We performed a comprehensive analysis of pattern of treatment and survival in men with metastatic prostate cancer. The study cohort consisted mainly of elderly, retired men (median age >70 years, most of them married or partnered) with bone-only metastases. Typically, metastatic disease developed after an initial period of locally or locoregionally confined cancer. Most patients (76%) did not live close to the county’s only department of oncology, located in Bodø. We decided to stratify for travel distance (Bodø vs. other communities) and, since previous research from other parts of the world identified lower average annual income as a source of disparities that may cause shorter survival [Citation3,Citation7,Citation15], we also analysed a subgroup with both longer travel distance and lower community income. The latter patients lived in 7 different small communities (<5,000 inhabitants), each without a local hospital. As a limitation of this methodology, it should be noted that information about the patients’ actual income and personal economy was not available. In addition, the difference of 19% in average annual income between the lower income communities and the city of Bodø was modest.
Our publicly funded health-care system provides free travel and accommodation to patients referred to specialist care (except for a minor share covered by the patients). Primary care is provided by each individual community (physicians responsible for general care and, if needed, referral to specialist care; home care provided by nurses and oncology nurses; nursing homes; rehabilitation; cancer care coordinator). In contrast to larger cities, small communities often experience difficulties in recruiting staff, resulting for example in unstable access to primary care physicians. Moreover, many of these communities are unable to offer a cancer coordinator and/or oncology nurses. Unlike other systems, individual insurance status does not cause any bias (faster access, more advanced treatment, etc.), as private health-care insurance is rare.
Further limitations of this study include the number of patients, statistical power of subgroup analyses, and retrospective design. In a larger cohort of patients, the different baseline characteristics, e.g., age, might have reached the level of statistical significance. Information about time to diagnosis was not available in our database. Thus, we cannot exclude the possibility that delay caused by the primary care sector may have influenced outcomes, and that such delay may vary between the subgroups examined. Regarding time to oncology clinic assessment, we have always ensured equal access with typical waiting times of 1–2 weeks and emergency access when needed. A strength of our study is the completeness of data, ensured by the fact that the electronic patient record also includes information from all local hospitals in the county. The absence of other oncology care providers further enhances the data quality.
We wanted to confirm that all patients in our region have equal access to systemic therapy and radiotherapy, and that our health-care system achieves comparable survival outcomes irrespective of distance to the department of oncology. Access to smaller and less specialized local hospitals, which can provide systemic treatment and participate in video-streamed multidisciplinary tumour boards and virtual meetings with oncologists, provides a framework for quality care also in the most remote areas of our sparsely populated county. In general, adherence to national guidelines is high in North Norway and the outcomes for patients with mPC are comparable to those reported from other parts of the world [Citation16]. Patients without medical contraindications are eligible for sequential treatment with docetaxel, cabazitaxel, enzalutamide and abiraterone acetate, i.e. all drugs endorsed by the committee that decides on availability of systemic treatments in Norway. Ra-223 was available, too. These drugs are identical to those utilized in other high-income countries [Citation17,Citation18]. The present results confirm the efficacy of the regional structures and treatment pathways, because the reference patients who lived in the surrounding of the main hospital in Bodø, where the average annual income is relatively high, did not receive significantly more intense treatment. Survival was actually numerically better in the other regions (no statistical significance), meaning that there is no reason to be concerned about clinically meaningful disparities. The fact that survival was better in the other regions is plausible, because more patients had received systemic treatment, a prerequisite for prolongation of survival. Moreover, utilization of more than one line of systemic treatment also was more common and favoured patients from other regions. The reason for differences in nature and intensity of treatment is eligibility (and safety), i.e. the oncologist’s assessment of performance status, symptom burden, organ function, comorbidity and contraindications. Imbalances in some of these baseline parameters likely existed in the present retrospective study.
Even if our study was performed in North Norway, the findings cannot be translated to all other circumpolar regions, due to socio-economic and population differences (different indigenous groups and overall population heterogeneity, variable prostate cancer incidence), variable travel distance and infrastructure, and different health-care systems. In general, lung and colorectal cancer were identified as important public health concern [Citation10]. In Greenland, colorectal cancer stage distribution, provision of oncological treatment and 5-year survival were comparable to patients diagnosed and treated in Denmark [Citation19]. In contrast and based on an earlier publication, Yukon cancer mortality rates were elevated compared with national, provincial, urban, and southern-rural jurisdictions [Citation20]. In an ideal world, the transfer of our current oncology care model to less well-served regions outside of Norway may be considered, yet the economical consequences and difficulties in recruiting qualified staff must not be underestimated.
Conclusions
The present results suggest that the health-care system of the Nordland County successfully delivers state-of-the-art oncology care to patients with metastatic prostate cancer who live in a rural region with challenging climate and geography.
Authors’ contributions
CN participated in the design of the study and performed the statistical analysis. AD, EH and CN collected patient data. CN, JN, EH and AD conceived of the study and drafted the manuscript. All authors read and approved the final manuscript.
Availability of data and materials
Data will not be shared, but a copy of relevant baseline parameters can be provided to researchers attempting to pool data from several institutions for large-scale analyses.
Consent for publication
Not applicable.
Ethics approval
As a retrospective quality of care analysis, no approval from the Regional Committee for Medical and Health Research Ethics (REK Nord) was necessary (national policy in Norway). This research project was carried out according to our institutions’ guidelines and with permission to access the patients’ data.
Acknowledgments
The publication charges for this article have been funded by a grant from the publication fund of UiT The Arctic University of Norway.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Baade PD, Yu XQ, Smith DP, et al. Geographic disparities in prostate cancer outcomes–review of international patterns. Asian Pac J Cancer Prev. 2015;16(3):1259–5.
- Temkin SM, Rimel BJ, Bruegl AS, et al. A contemporary framework of health equity applied to gynecologic cancer care: A Society of Gynecologic Oncology evidenced-based review. Gynecol Oncol. 2018 Apr;149(1):70–77.
- Carrera PM, Kantarjian HM, Blinder VS. The financial burden and distress of patients with cancer: understanding and stepping-up action on the financial toxicity of cancer treatment. CA Cancer J Clin. 2018 Mar;68(2):153–165.
- Jacobsen MM, Silverstein SC, Quinn M, et al. Timeliness of access to lung cancer diagnosis and treatment: A scoping literature review. Lung Cancer. 2017 Oct;112:156–164.
- Højgaard L, Löwenberg B, Selby P, et al. The European cancer patient’s bill of rights, update and implementation 2016. ESMO Open. 2017 Jan 6;1(6):e000127.
- Yedjou CG, Tchounwou PB, Payton M, et al. Assessing the racial and ethnic disparities in breast cancer mortality in the USA. Int J Environ Res Public Health. 2017 May 5;14(5) pii:E486.
- Dreyer MS, Nattinger AB, McGinley EL, et al. Socioeconomic status and breast cancer treatment. Breast Cancer Res Treat. 2018 Jan;167(1):1–8.
- Charlton M, Schlichting J, Chioreso C, et al. Challenges of rural cancer care in the USA. Oncology (Williston Park). 2015 Sep;29(9):633–640.
- Nieder C, Dalhaug A, Haukland E, et al. Contemporary radiooncological management of bone metastases from breast cancer: factors associated with prescription of different fractionation regimens (short or long course) in a rural part of North Norway with long travel distance. Int J Circumpolar Health. 2017;76(1):1270080.
- Young TK, Kelly JJ, Friborg J, et al. Cancer among circumpolar populations: an emerging public health concern. Int J Circumpolar Health. 2016 Jan 12;75: 29787.
- Norum J, Heyd A, Hjelseth B, et al. Quality of obstetric care in the sparsely populated sub-arctic area of Norway 2009-2011. BMC Pregnancy Childbirth. 2013 Sep 14;13: 175.
- Norum J, Nieder C. Socioeconomic characteristics and health outcomes in Sami speaking municipalities and a control group in northern Norway. Int J Circumpolar Health. 2012 Aug 20;71: 19127.
- Nieder C, Norum J, Spanne O, et al. Does distance to treatment centre influence the rate of palliative radiotherapy in adult cancer patients? Anticancer Res. 2009 Jul;29(7):2641–2644.
- www.nrk.no/norge/rikeste-og-fattigste-kommuner-1.11991815
- Shen Y, Guo H, Wu T, et al. Lower education and household income contribute to advanced disease, less treatment received and poorer prognosis in patients with hepatocellular carcinoma. J Cancer. 2017 Sep 2;8(15):3070–3077.
- Nieder C, Haukland E, Mannsåker B, et al. Impact of intense systemic therapy and improved survival on the use of palliative radiotherapy in patients with bone metastases from prostate cancer. Oncol Lett. 2016 Oct;12(4):2930–2935.
- Norum J, Nieder C. Treatments for metastatic prostate cancer (mPC): A review of costing evidence. Pharmacoeconomics. 2017;35:1223–1236.
- Nuhn P, De Bono JS, Fizazi K, et al. Update on systemic prostate cancer therapies: management of metastatic castration-resistant prostate cancer in the era of precision oncology. Eur Urol. 2019 Jan;75(1):88–99.
- Odgaard M, Lohse N, Petersen AJ, et al. Oncological treatment and outcome of colorectal cancer in Greenland. Int J Circumpolar Health. 2018 Dec;77(1):1546069.
- Simkin J, Woods R, Elliott C. Cancer mortality in Yukon 1999-2013: elevated mortality rates and a unique cancer profile. Int J Circumpolar Health. 2017;76(1):1324231.
