ABSTRACT
There is a lack of data about the influence of sunshine hours on the prevalence for gestational diabetes mellitus (GDM). The aim of this study was to evaluate whether the prevalence of GDM varied according to hours of daily sunshine during the first trimester. The study cohort (N = 6189) consists of all primiparous women with a Finnish background who delivered between 2009 and 2015 living in Vantaa city, Finland. Data on births and maternal characteristics were obtained from National Health Registers. Data on sunshine hours were obtained from the Finnish Meteorological Institute. Individual daily sunshine hours during the first trimester of pregnancy were calculated for each woman. Diagnosis of GDM was based on a standard 75-g 2-h glucose tolerance test (OGTT). No relationship was observed between month of conception and GDM. Daily sunshine hours during the first trimester and GDM showed a U-shaped association (adjusted p-value 0.019). In OGTT, a U-shaped association was observed between 0-h glucose value and daily sunshine hours during the first trimester (p = 0.039) as well as with the 1-h glucose value (p = 0.012), respectively. In primiparous women daily sunshine hours during the first trimester showed a U-shaped association with the prevalence of GDM independent of pre-pregnancy risk factors.
Abbreviations: BMI: body mass index; GDM: gestational diabetes mellitus; OGTT: standard 75 g 2-h glucose tolerance test; SD: standard deviation
Introduction
Gestational diabetes mellitus (GDM) is a common disorder in pregnant women. Globally over the last decade, the prevalence of GDM has been rapidly increasing, and it has been estimated that one in six pregnant women are diagnosed with GDM [Citation1,Citation2]. GDM has both short- and long-term adverse effects on the pregnant woman and her offspring [Citation3,Citation4]. Some of the well-known risk factors for GDM include increasing childbearing age, maternal obesity, and genetic predisposition for diabetes [Citation2,Citation5].
The influence of seasonal variation – based on outdoor air temperature – on glucose concentrations during an oral glucose tolerance test or on the prevalence of GDM has been studied with inconsistent findings [Citation6–Citation12]. However, more recent studies have reported a seasonal variation in GDM, based on outdoor temperature [Citation7–Citation12]. The influence of seasonal variation is thought to be due to factors including alterations in blood flow, seasonal variations in food consumption and physical activity, alterations in brown adipose tissue, and vitamin D mediated effects [Citation13–Citation17]. According to recent systematic reviews, low vitamin D levels in pregnant women seem to be associated with GDM [Citation18,Citation19]. Main sources for vitamin D are sunshine and fish oil [Citation20]. By the solar ultraviolet B radiation, vitamin D is produced in the skin [Citation20]. To the best of our knowledge, research data are missing about the prevalence of GDM in varying according to daily sunshine hours.
In Finland, the prevalence of GDM is high (http://urn.fi/URN:NBN:fi-fe2018103146930) and the variation in daily sunshine hours varies substantially by season, offering a unique opportunity to evaluate the impact of sunshine on the risk of GDM. Our aim was to evaluate whether the prevalence of GDM varied according to daily sunshine hours in the city of Vantaa, Finland.
Materials and methods
Study design and participants
This study is a cohort study from the city of Vantaa, Finland (coordinates: 60°17′40″ N 025°02′25″ E). In 2015 the city of Vantaa had 211 000 inhabitants being the fourth biggest city in Finland. The study cohort (N = 6189) consists of all primiparous women with a Finnish background (i.e. born in Finland with Finnish or Swedish as native language) living in Vantaa and without previously diagnosed diabetes mellitus who delivered a living offspring between 1 January 2009 and 31 December 2015. We included only primiparous women with Finnish background in this study to exclude the confounding effects of ethnicity, prior GDM and multiparity on the risk for GDM.
In Finland, the National Institute for Health and Welfare (THL) maintains the Finnish Medical Birth Register. This register contains data from all live births and stillbirths from 22 gestational weeks or 500 g onwards from every delivery hospital in Finland. We obtained data on primiparous women’s age, status of cohabiting, smoking during pregnancy, pre-pregnancy weight and height, number of previous pregnancies (induced abortions, miscarriages, or ectopic pregnancies) and deliveries, use of infertility treatments, and number of foetuses (http://www.thl.fi/en/statistics/parturients). The quality of the Finnish Medical Birth Register is considered to be excellent [Citation21].
Pre-pregnancy body mass index (BMI) was calculated as pre-pregnancy body weight divided by height squared (kg/m2). The date of conception was calculated by reducing the duration of pregnancy from the day of delivery. The duration of pregnancy was assessed by an ultrasound examination between 11 and 14 weeks of gestation.
Educational attainment was defined according to years of schooling with a national classification, which was obtained from Statistics Finland (http://www.stat.fi/meta/luokitukset/koulutus/001-2016/kuvaus).
Assessment of gestational diabetes mellitus
In Finland since 2008, GDM has been screened using a 75 g 2-h oral glucose tolerance test (OGTT) between 24 and 28 weeks of gestation in all pregnant women, with the exception of those who are at low risk (www.kaypahoito.fi). Low-risk nulliparous women are defined as women aged under 25 years with BMI 18.5–24.9 kg/m2 and without a first-degree family history of diabetes. The women with high risk should be tested for the first time at 12 to 16 weeks of gestation. High-risk nulliparous women are defined as women with BMI≥35 kg/m2, glucosuria, first- or second-degree family member with type 2 diabetes, continuous use of oral corticosteroids, or polycystic ovary syndrome. One or more pathological glucose value in OGTT with the following diagnostic thresholds lead to GDM diagnosis: fasting plasma glucose ≥5.3 mmol/L, 1-h glucose ≥10.0 mmol/L, and 2-h glucose ≥8.6 mmol/L (www.kaypahoito.fi). GDM screening is mainly made in communal antenatal clinics in primary health-care centres and it is free-of-charge for women. In Finland, 99.7–99.8% of the reviewer pregnant women use the services of communal antenatal clinics (https://thl.fi/fi/web/lapset-nuoret-ja-perheet/peruspalvelut/aitiys_ja_lastenneuvola/aitiysneuvola). Of the total study cohort (N = 6189), 4450 women had complete data from OGTT.
Assessment of sunshine levels
The Finnish Meteorological Institute maintains data on daily sunshine hours and temperature by municipality (en.ilmatieteenlaitos.fi/open-data). Sunshine is reported when the intensity of direct radiation from the sun is at least 120 W/m2 (solar irradiance as watts per square metre). We obtained data from the Finnish Meteorological Institute on daily sunshine hours from the 14th of March 2008 to the 24th of April 2015 in the city of Vantaa, Finland. Daily sunshine hours were calculated for each woman individually from the conception until to the 84th day of pregnancy (during the first trimester of pregnancy).
Primiparous women were divided into five sunshine levels using centiles (levels I to V, 12.5th, 37.5th, 62.5th, 87.5th) corresponding to grades containing 12.5%, 25%, 25%, 25%, and 12.5% of the total distribution, respectively. The respective sunshine hours per day during the first trimester were following: level I – <1 h, level II – 1–<3 h, level III – 3–<6 h, level IV – 6–<8.5 h, and level V – ≥8.5 h.
Statistical analyses
Data are presented as means with standard deviation (SD) or as counts (n) with percentages (%). Statistical significances for linearity across categories of sunshine level and characteristics of the study participants were evaluated by using the Cochran–Armitage test for trend and analysis of variance. The relationship between sunshine and the prevalence of GDM and oral glucose tolerance test was analysed by using general linear models. The model included quadratic terms for sunshine hours or levels adjusted for age, pre-pregnancy BMI, and educational attainment. A U-shaped relationship was tested by using the Lind and Mehlum method [Citation22]. The normality of the variables was tested using the Shapiro–Wilk W test. Stata 15.1 (StataCorp LP; College Station, Texas, USA) statistical package was used for the analysis.
Results
Mean age of the primiparous women was 28.5 (SD 5.2) years and their mean pre-pregnancy BMI was 24.1 (SD 4.6) kg/m2. The women had on average 13.5 (SD 2.6) years of education. Characteristics of the 6189 primiparous women according to sunlight levels are shown in .
Table 1. Relationship between characteristics of the primiparous women (N=6 189) and sunshine levels
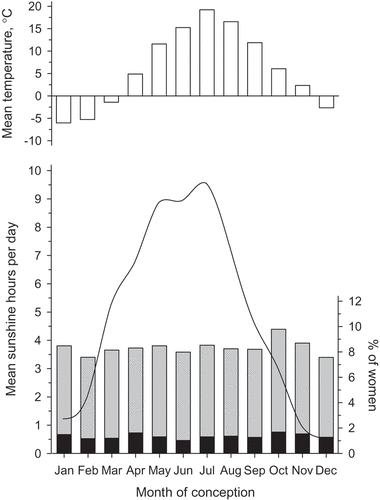
In the city of Vantaa, Finland, the highest number of sunshine hours per day was reported in July (mean 9.6 [SD 4.8] hours per day) and the lowest in December (mean 0.5 [SD 1.1] hour per day). illustrates the mean temperature and mean daily sunshine hours during different months. Further, shows the percentage of pregnancies and those with GDM according to the month of conception. No relationship was observed with the month of conception and GDM (p = 0.34).
Figure 1. Mean daily temperatures and hours of sunshine by the month and percentages of pregnancies (columns in the bottom part of figure) and gestational diabetes mellitus (black part of the column) according to the month of conception among 6189 primiparous women. Solid line in the bottom part of figure illustrates mean daily sunshine hours during different months
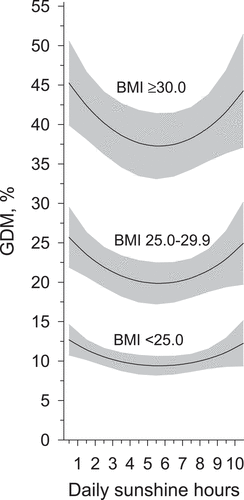
Of the primiparous women, 16.2% were diagnosed with GDM. During the first trimester daily sunshine hours and GDM showed a U-shaped association () (p-value 0.019, adjusted for age, pre-pregnancy BMI, and educational attainment). A similar U-shaped relationship between daily sunshine hours and GDM, was observed in women with pre-pregnancy BMI<25.0 kg/m2, in women with pre-pregnancy BMI 25.0–29.9 kg/m2, and in women with pre-pregnancy BMI≥30 kg/m2 (). The lowest prevalence of GDM was observed in the group with estimated daily 5.5 sunshine hours during the first trimester.
Figure 2. Prevalence of gestational diabetes mellitus in percentages (%) in primiparous women (N = 6189) according to both daily sunshine hour levels and on a continuous scale (adjusted for age, pre-pregnancy body mass index, and educational attainment) during the first trimester
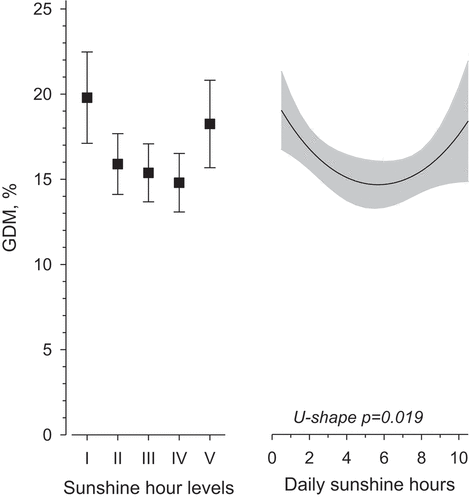
Figure 3. Prevalence of gestational diabetes mellitus in percentages (%) in primiparous women (N = 6 189) divided into body mass index categories (<25.0 kg/m2, 25.0–29.9 kg/m2, and ≥30 kg/m2) according to daily sunshine hours during the first trimester as a continuous scale, adjusted for age and educational attainment. Shaded area represents 95% confidence interval
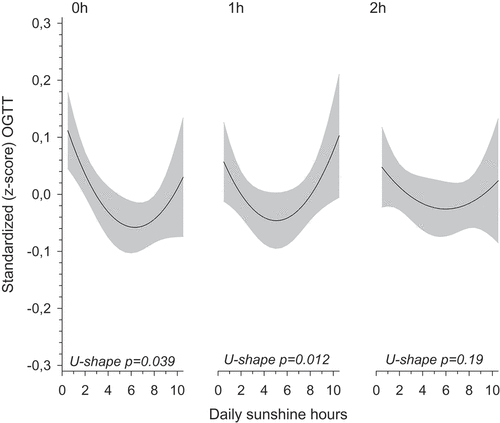
In OGTT, mean fasting glucose concentration was 4.79 (SD 0.48) mmol/L, mean 1-h glucose concentration 7.44 (SD 1.76) mmol/L, and mean 2-h glucose concentration 6.36 (SD 1.45) mmol/L, respectively. Fasting glucose (p-value 0.012), 1-h glucose concentrations (p-value 0.039) and 2-h glucose concentrations (p-value 0.19) showed a U-shaped association with daily sunshine hours during the first trimester ().
Figure 4. Fasting, 1- and 2-h glucose concentrations calculated as z-scores from a 75-g 2-h oral glucose tolerance test in primiparous women according to hour of daily sunshine during the first trimester as a continuous scale (adjusted for body mass index, age and educational attainment). Shaded area represents 95% confidence interval
Discussion
Daily sunshine hours during the first trimester showed a U-shaped association with the prevalence of GDM and the association was observed regardless of the pre-pregnancy degree of adiposity. No association was observed between the month of conception and GDM. As expected, the prevalence of GDM was high, 16%.
We observed that during the first trimester daily low or high sunshine hours increased the risk for GDM. A similar observation was made in all women regardless of the pre-pregnancy degree of adiposity. In OGTT, fasting and 1-h glucose showed a U-shaped association with daily sunshine hours during the first trimester. To the best of our knowledge, this is the first study to evaluate whether there is an association between hours of daily sunshine and GDM.
There might be several explanations for the observed association. Synthesis of vitamin D in the skin is initiated by absorption of solar ultraviolet B radiation [Citation20]. If the solar ultraviolet B radiation is low, production of vitamin D in the skin will remain low exposing the individual to lower blood vitamin D levels. Further, low blood vitamin D levels have been shown to be a risk factor for GDM [Citation18,Citation19]. It has been suggested that vitamin D mediates the onset of GDM by regulating the immune system and inflammatory responses, oxidative stress, blood calcium level, hepatic metabolism, as well as with the function and development of pancreatic islets [Citation23–Citation26]. Beyond the production of vitamin D, the physiological responses to sunshine also include factors such as regulation of biological circadian and circannual rhythms, beta-endorphins which avail a feeling of well-being, and control of circulating melatonin [Citation27,Citation28]. However, we have no explanation for the observation that high daily hours of sunshine during the first trimester increased the risk for GDM. Possibly, to some extend lifestyle factors such as seasonal changes in physical activity and in food consumption may explain our findings [Citation14,Citation29]. It has been shown that the amount of physical activity seems to decrease if the outdoor temperature is especially high or cold [Citation14]. A large Finnish study reported that among pregnant women dietary changes were observed: in the autumn and winter, roots and tubers were favoured, whereas in the spring and summer, vegetable fruits and leafy vegetables formed a larger part of the diet [Citation29]. Further, during summertime, pregnant women had an increased consumption of barbecued food, ice cream, and soft drinks [Citation29].
According to our study findings, there was no association between the month of conception and GDM. In contrast, a large Australian study observed that if conception took place during rainy months the risk for GDM was increased [Citation9]. Perhaps, highly different weather conditions in Finland and Australia explain some of the disparate between the study findings.
Our study finding of a GDM prevalence of 16% in primiparous women is in line with Finnish nationwide findings. In 2017 the nationwide GDM prevalence in Finland – including both primiparous and multiparous women – was 19% (http://urn.fi/URN:NBN:fi-fe2018103146930). However, commonly in Europe, the prevalence of GDM with an estimate 6% is lower [Citation30]. These differences in prevalence rates could at least partly be due to the fact that in Finland since 2008 there has been a comprehensive universal screening for GDM (www.kaypahoito.fi).
The strengths of the study
In Finland, every person has a unique personal identification number, which enables the combination of national register data from several administrative registers on a personal level. Further, the individual daily sunshine hours during the first trimester of pregnancy were calculated for each woman individually. Our study observations endorse previous study findings that seasonality with different amounts of sunshine has an association with well-being and health [Citation31,Citation32].
Study limitations
Because this is a register-based study we are missing information on the study participants’ dietary and physical activity habits, family history of diabetes, use of vitamin supplements, blood vitamin D levels, time spent outside as well as time spent in the city of Vantaa, Finland.
The time spent outside is also influenced by the occupation of the mother and whether she is at work or on vacation: we do not have this information. Moreover, we are missing information on the dress style of pregnant women. The meaning of missing information of blood vitamin D levels is emphasised because in Northern countries like Finland, the amount of UVB radiation is satisfactory to cause noteworthy vitamin D synthesis in the skin from April to September [Citation33]. Further, all study participants were women with a Finnish background; thus, the generalisability of our study observations is limited.
Conclusions
In primiparous women daily sunshine hours during the first trimester showed a U-shaped association with the prevalence of GDM. Further studies, with individual information on the amount of sunshine exposure, if possible, are needed to show whether our study findings are reproducible and to investigate the underlying mechanisms of the association.
Ethical approval
The ethics committee of the Hospital District of Helsinki and Uusimaa, Finland (356/13/03/03/2015, 2 November 2015), and the health authority of the city of Vantaa, Finland, have approved the study. National Institute for Health and Welfare (THL) and Statistics Finland have given permission to use register data in the study.
Informed consents were not required because this is an observational register-based cohort study and no study participants were contacted.
Availability of data and materials
Data cannot be shared for both legal and ethical reasons. Data from the National Institute for Health and Welfare, Statistics Finland, and the Finnish Social Insurance Institution can only be used for the purpose stated in the license granted, scientific research on society by the license applicant, and can therefore not be shared with third parties.
Acknowledgments
We thank Jalmari Kautiainen for excellent statistical advice.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Lavery JA, Friedman AM, Keyes KM, et al. Gestational diabetes in the USA: temporal changes in prevalence rates between 1979 and 2010. BJOG. 2017;124:804–7.
- American Diabetes Association. 13. Management of diabetes in pregnancy: standards of medical care in diabetes 2018. Diabetes Care. 2018;41:S137–143.
- Malcolm J. Through the looking glass: gestational diabetes as a predictor of maternal and offspring long-term health. Diabetes Metab Res Rev. 2012;28:307–311.
- Mitanchez D, Yzydorczyk C, Siddeek B, et al. The offspring of the diabetic mother – short- and long-term implications. Best Pract Res Clin Obstet Gynaecol. 2015;29:256–269.
- Zhang C, Rawal S, Chong YS. Risk factors for gestational diabetes: is prevention possible? Diabetologia. 2016;59:1385–1390.
- Schmidt MI, Matos MC, Branchtein L, et al. Variation in glucose tolerance with ambient temperature. Lancet. 1994;344:1054–1055.
- Moses RG, Wong VCK, Lambert K, et al. Seasonal changes in the prevalence of gestational diabetes mellitus. Diabetes Care. 2016;39:1218–1221.
- Katsarou A, Claesson R, Ignell C, et al. Seasonal pattern in the diagnosis of gestational diabetes mellitus in Southern Sweden. J Diabetes Res. 2016. DOI:https://doi.org/10.1155/2016/8905474.
- Verburg P, Tucker G, Scheil W, et al. Seasonality of gestational diabetes mellitus: a South Australian population study. BMJ Open Diabetes Res Care. 2016;4:e000286.
- Booth GL, Luo J, Park AL, et al. Influence of environmental temperature on risk of gestational diabetes. CMAJ. 2017;189:E682–689.
- Vasileiou V, Kyratzoglou E, Paschou SA, et al. The impact of environmental temperature on the diagnosis of gestational diabetes mellitus. Eur J Endocrinol. 2018;178:209–214.
- Wainstock T, Yoles I. Pregnant women may be sweater in the summer: seasonal changes in glucose challenge tests results. A population-based study. Diabetes Res Clin Pract. 2019;147:134–137.
- Moses RG, Patterson MJ, Regan JM, et al. A non-linear effect of ambient temperature on apparent glucose tolerance. Diabetes Res Clin Pract. 1997;36:35–40.
- Tucker P, Gilliland J. The effect of season and weather on physical activity: a systematic review. Public Health. 2007;121:909–922.
- Watson PE, McDonald BW. Seasonal variation of nutrient intake in pregnancy: effects on infant measures and possible influence on diseases related to season of birth. Eur J Clin Nutr. 2007;61:1271–1280.
- Lagunova Z, Porojnicu AC, Lindberg F, et al. The dependency of vitamin D status on body mass index, gender, age and season. Anticancer Res. 2009;29:13–20.
- Lee P, Smith S, Linderman J. Temperature-acclimated brown adipose tissue modulates insulin sensitivity in humans. Diabetes. 2014;63:3686–3698.
- Amraei M, Mohamadpour S, Sayehmiri K, et al. Effects of vitamin D deficiency on incidence risk of gestational diabetes mellitus: a systematic review and meta-analysis. Front Endocrinol (Lausanne). 2018;9:7.
- Zhang Y, Gong Y, Xue H, et al. Vitamin D and gestational diabetes mellitus: a systematic review based on data free of Hawthorne effect. BJOG. 2018;125:784–793.
- Lucas RM, Norval M, Neale RE, et al. The consequences for human health of stratospheric ozone depletion in association with other environmental factors. Photochem Photobiol Sci. 2015;14:53–87.
- Gissler M, Teperi J, Hemminki E, et al. Data quality after restructuring a national medical registry. Scand J Soc Med. 1995;23:75–80.
- Lind JT, Mehlum H. With or without U? The appropriate test for U shaped relationship. Oxford Bull Econ Stats. 2010;72:109–118.
- Nikooyeh B, Neyestani TR. Oxidative stress, type 2 diabetes and vitamin D: past, present and future. Diabetes Metab Res Rev. 2016;32:260–267.
- Leung PS. The potential protective action of vitamin D in hepatic insulin resistance and pancreatic islet dysfunction in type 2 diabetes mellitus. Nutrients. 2016;8:147.
- Sung CC, Liao MT, Lu KC, et al. Role of vitamin D in insulin resistance. J Biomed Biotechnol. 2012;2012:1–11.
- Luong KVQ, Nguyen LTH, Nguyen DNP. The role of vitamin D in protecting type 1 diabetes mellitus. Diabetes Metab Res Rev. 2005;21:338–346.
- Haljas K, Hakaste L, Lahti J, et al. The associations of daylight and melatonin receptor 1B gene rs10830963 variant with glycemic traits: the prospective PPP-Botnia study. Ann Med. 2019;51:58–67. published on line 14. 2.2019. .
- Holick MF. Biological effects of sunlight, ultraviolet radiation, visible light, infrared radiation and vitamin D for health. Anticancer Res. 2016;36:1345–1356.
- Prasad M, Lumia M, Erkkola M, et al. Diet composition of pregnant Finnish women: changes over time and across seasons. Public Health Nutr. 2010;13:939–946.
- Zhu Y, Zhang C. Prevalence of gestational diabetes and risk of progression to type 2 diabetes: a global perspective. Curr Diab Rep. 2016;16:7.
- Friborg O, Rosenvinge JH, Wynn R, et al. Sleep timing, chronotype, mood, and behavior at an Arctic latitude (69°N). Sleep Med. 2014;15:798–807.
- Shivaprakash JM, Jokelainen J, Sebert S, et al. Vitamin D status and components of metabolic syndrome in older subjects from Northern Finland (Latitude 65° North). Nutrients. 2019. DOI:https://doi.org/10.3390/nu11061229.
- Edvardsen K, Brustad M, Engelsen O, et al. The solar UV radiation level needed for cutaneous production of vitamin D3 in the face. A study conducted among subjects living at a high latitude (68 degrees N). Photochem Photobiol Sci. 2007;6:57–62.
