ABSTRACT
Diabetes used to be a rare condition among Inuit in Greenland. However, research in recent decades has shown a high prevalence of undiagnosed diabetes. Addressing diabetes in the geographically dispersed population of Greenland presents a challenge to the health care system. In 2008, a new model of diabetes care was introduced in Greenland that included continual monitoring, analysis, and adjustment of initiatives taken. The overall aim of this review was to review the feasibility of the monitoring of an ongoing national diabetes care programme. After ten years of observation it was clear that monitoring of such a programme based on information in electronic medical records in Greenland was feasible. It was found that the majority of the population in Greenland was in contact with the health care system. Increased diagnostic activity resulted in an increased prevalence of diagnosed diabetes. The quality of diabetes care in Greenland and the testing effectiveness of gestational diabetes were improved. Microvascular complications were frequently observed among Greenlandic diabetic patients, except for retinopathy that was as an exception. In summary, this model may improve diabetes care and potentially care for other chronic conditions in Greenland, and may also be helpful in other remote settings where chronic disease care is difficult.
Abbreviations: AD: Anno Domini; ADA: American Diabetes Association; BC: Before Christ; BMI: Body Mass Index; BP: Blood Pressure; CWB: Capillary Whole Blood; EMR: Electronic Medical Record; EASD: European Association for Study of Diabetes; GA: Gestational Age; GDM: Gestational Diabetes Mellitus; FIGO: The International Federation of Gynaecology and Obstetrics; HbA1c: Glycosylated haemoglobin; IDF: International Diabetes Federation; LDL: Low density lipoprotein; NDQIA: National Diabetes Quality Improvement Alliancel; NICE: National Institute for Health and Care Excellence; OECD: Organisation for Economic Co-operation and Development; OGTT: Oral Glucose Tolerance Test; QIH: Queen Ingrid Hospital; RCT: Randomised Controlled Tria;l T1D: Type 1 Diabetes; T2D: Type 2 Diabetes; UACR: Urine Albumin Creatinine Ratio; WHO: World Health Organisation
Executive summary
Diabetes is a common and serious chronic disease associated with premature death and multiple complications. Indigenous people in particular were among those with the highest rate of diabetes and complications, presumably driven by increasing age, changes in lifestyle and living conditions, and genetic susceptibility. Diabetes used to be a rare condition among Inuit in Greenland. However, a population survey from 1999 reported a very high prevalence of diabetes at 10%, and pre-diabetes at 20% among adult Greenlanders aged 35 years or above. The majority of persons (70%) observed with diabetes were not aware of their disease indicating a need for an increased focus on diabetes in Greenland. However, addressing diabetes in the geographically widely spread population of Greenland presents a huge challenge for the health-care system in Greenland.
In 2008, a national diabetes programme was initiated in Greenland. It aimed at increasing the detection of undiagnosed cases, improving care for patients with diabetes and promoting the awareness of diabetes. The programme was developed and adjusted based on health research within the health-care system. Thus, a new method was introduced including continual monitoring, analysis, and the adjustment of initiatives taken in diabetes care. However, it was not known, if it was feasible to use this method over longer time horizon in a country covering more than 2 million km2 where the population was sparsely located in isolated towns and settlements with small health care units often short of health care professionals and expertise.
The overall aim of this review was to summarise the results regarding the feasibility of monitoring an ongoing national diabetes care programme in the geographically dispersed population of Greenland.
From this work six hypotheses were formulated and comprised the purposes for the study: First, the majority of males and females in Greenland are in annual contact with the health care system in Greenland allowing a case-finding strategy to detect new cases of diabetes. Second, diagnostic activity regarding diabetes could be improved in Greenland from 2010 to 2015. Third, the prevalence of diagnosed diabetes could be monitored and increased from 2008 to 2017. Fourth, the quality of diabetes care could be monitored and improved from 2008 to 2017. Fifth, the prevalence of gestational diabetes mellitus (GDM) is increasing and testing effectiveness could be improved from 2008 to 2014. And sixth, the prevalence of microvascular complications among patients with type 2 diabetes is equal among Greenlanders and non-Greenlanders in Nuuk.
These hypotheses were tested using repeated cross-section register studies based on information found in the electronic medical record (EMR) used in Greenland. The diabetes programme and initiatives to improve care were gradually developed and adapted based on the information provided by the studies including organisation of diabetes care and screening for complications, diagnostic strategy, management of gestational diabetes, national guidelines, education of health care professionals, performance feedback, patient information in Greenlandic, promotion of awareness of diabetes and lifestyle factors, and the national registration of diabetes added to the data collection in the EMR.
After 10 years of observation, it was clear that the monitoring of an ongoing national diabetes care programme based on information from the EMR was feasible even in Greenland. More than 80% of the total population had been in contact with the primary health care system within one calendar year. Thus, opportunistic case-finding was a possible strategy in reducing the number of undetected cases of diabetes in Greenland, and was chosen in the new Greenlandic diabetes initiative.
Furthermore, in 2010, HbA1c was introduced as a supplementary diagnostic tool, and the two-year program of diagnostic activity regarding diabetes increased as a result, climbing from 15.6% in 2010–12 to 24.0% in 2014–15.
The prevalence of diagnosed diabetes was monitored continuously within the last decade. A threefold increase was measured in the number of patients. Prevalence increased with age and was higher among Greenlandic females than among Greenlandic males. The highest prevalence was observed among non-Greenlandic males. This was in contrast to observations from among most Indigenous populations in ex-colonial countries where the prevalence of diabetes among Indigenous people in general was reported to be 2 to 4 times higher than among the non-Indigenous populations. The comparison should, however, be evaluated with some caution, because the non-Greenlanders in Greenland may differ from other general populations.
The quality of diabetes care improved from 2008 to 2017, and was comparable to the level reported internationally. The actual quality of care in 2017, was lower than observed in 2010, shortly after the diabetes project was started emphasising the fact that continued focus was crucial in maintaining the level of quality achieved. Major organisational changes, including expanding the scope of the diabetes project with a new lifestyle initiative, the establishment of new regional primary care, and the use of a new EMR system proved challenging to the establishment of the diabetes initiative.
The prevalence of GDM in Greenland was relatively low, but showed a tendency to rise in level. Testing effectiveness improved significantly between 2008 and 2014. However, around a third of the Greenlandic women that should have been tested were not tested. Testing effectiveness was poorer outside Nuuk.
Microvascular complications were observed frequently among both Greenlanders and non-Greenlanders. However, Greenlanders had a much lower prevalence of retinopathy than non-Greenlanders and may thus be less prone to this complication.
The majority of the population in Greenland was in contact with the primary health care system and thus increased diagnostic activity, and opportunistic case-finding resulted in an increased prevalence of diagnosed diabetes. Despite the increased number of patients, the quality of diabetes care in Greenland was still improved from 2008 to 2017. Testing the effectiveness of gestational diabetes was improved, and a trend was observed towards increasing prevalence. Microvascular complications were observed frequently among patients with diabetes in Greenland with retinopathy as an exception among Greenlanders. However, room for improvements was identified too, and continued adjustment is necessary regarding the national strategy. Undetected diabetes prevalence remained high and alternative supplementary testing strategies must thus be considered including providing easier access to testing in settings outside the traditional health care system such as in the workplace and in leisure facilities, targeting of specific subgroups, and testing methods. Although improved since 2008, the quality of diabetes care in 2017, is still suboptimal. Monitoring of the quality of diabetes care in the new electronic medical record system should be further optimised including the registration of data and more sophisticated extraction features.
Testing for gestational diabetes also remained unacceptably low outside of Nuuk, and other testing strategies have to be considered.
In conclusion, the model introduced based on continued monitoring and adjustment of diabetes care in Greenland proved feasible and could thus be used to further improve diabetes care in the near future as well as to include the care for other chronic conditions found in Greenland under its unique conditions. In addition, this methodology may be helpful in other remote settings where diabetes care and chronic disease management are difficult.
Introduction
Diabetes is a common, serious, chronic disease caused by insufficient production of insulin, the ineffectiveness of the body to use insulin or a combination of both leading to elevated blood glucose [Citation1–Citation3]. The condition can lead to premature death and multiple complications. These are classified as either small vascular injury (microvascular disease) typically including retinopathy, nephropathy and neuropathy, or injury to the large blood vessels of the body (macrovascular disease), mainly atherosclerosis [Citation3]. Globally, diabetes is the most frequent cause of preventable adult blindness [Citation4] and chronic renal disease [Citation5]. Between 2000 and 2010 – just one decade – the number of people with diabetes has more than doubled in the world [Citation6,Citation7]. An increasing proportion of diabetes afflicted patients live in low and middle-income countries [Citation6]. In industrialised countries, the highest prevalence of diabetes is observed among the socially and economically disadvantaged groups [Citation6]. Also, amongst Indigenous people, disproportionate high rates of diabetes and complications have been reported as a global phenomenon [Citation8]. Among Inuit in Greenland, diabetes used to be a rare condition [Citation9]. However, in the beginning of this century a population survey indicated that diabetes had become frequent among adult Greenlanders. Surveys showed that around 10% of adults aged 35 years or older were affected. In addition, another 20% had pre-diabetes, a condition associated with increased risk of developing diabetes [Citation10]. The majority (70%) of persons observed with diabetes in the survey were themselves not aware of their condition, and thus the author recommended increased awareness of diabetes in Greenland [Citation10]. However, addressing diabetes in the geographically widely spread population of Greenland represents a huge challenge for the health care system in Greenland.
In 2008, a national diabetes programme was initiated, aiming to improve the detection of undiagnosed cases, improve care for patients with diabetes and promote awareness of diabetes. Short-term evaluation of the project was evaluated in my Ph.D. thesis titled Diabetes in Greenland, in 2011 [Citation9]. The prevalence of diagnosed diabetes and the quality of diabetes care improved in the observation period between 2008 and 2010. In 2011, the diabetes programme was replaced by a lifestyle initiative. However, the methods introduced in the diabetes program were maintained including continued monitoring, analysis, and adjustment of initiatives taken in the diabetes care. Health research within the health care system was thus used as an essential tool and was performed in close interaction with the strategies and actions taken to optimise diabetes management. This method was new in Greenland, and no long-term observations had been performed. Especially, it was unknown if it was feasible to use this method including health research, continued monitoring, analysis, and adjustment of initiatives with a longer time perspective in a country covering more than 2 million square kilometres, sparsely populated with isolated towns and settlements each with small health care units often lacking health care professionals with expertise.
The overall aim of this review was to summarise the results regarding the feasibility of the monitoring of an ongoing national diabetes care programme in the geographically dispersed population of Greenland. The review comprises a background section with special focus on diabetes among Indigenous people, especially Inuit, including prevalence, gestational diabetes, and complications related to diabetes and diabetes care based on the available literature. This part closes with an introduction to the new diabetes initiative, followed by the hypotheses and aims of the present survey. The methods used are explained and evaluated in the next section. Thereafter, the results are presented, discussed and related to the existing studies and knowledge in the field. Conclusions and a summary finish the review.
Background
Greenland: geography, population, history and health care
Greenland is part of the arctic region and covers an area of 2.16 million km2 and is thereby considered to be the largest island in the world. Around 80% of the island is covered with permanent ice, and it is only populated along the estimated 4,000 km of coastline. The 56,000 inhabitants live in 16 towns and approximately 60 smaller settlements [Citation11,Citation12]. Nuuk is the capital, located on the west coast and is by far the largest town with almost a third (17,000) of the national population living there. Around 15% of the population lives in smaller settlements. Towns and settlements are isolated from one another and transportation possibilities include plane, helicopter, boat, snowmobile, and dog sledges, their use depending on season and locality [Citation13]. Around 90% of the population was born in Greenland whereas 10% are immigrants, mainly Danes. In Nuuk, around 20% of the population was born outside Greenland [Citation11]. Greenlanders are of Inuit origin [Citation13], a people indigenous to the circumpolar region in the northern hemisphere. Inuit share a common past and are related geographically, historically and culturally. Thus, Inuit is a genetically distinct Indigenous population that has survived in the Arctic under extreme physical conditions. Outside of Greenland, Inuit live in the United States of America (Alaska), Canada, and Russia [Citation14]. The people of different waves of immigrations of Paleo-Eskimos from Canada settled in Greenland for periods and include the Independence I culture (North and Northeast Greenland) and the Saqqaq culture (West and South Greenland) from around 2500 BC, the Independence II culture (North and Northeast Greenland) and the Dorset culture (West and South Greenland) around 800 BC, and the Later Dorset culture around 800 AD [Citation15]. The Later Dorset culture settled in North Greenland and lived there for around 500 years before they disappeared [Citation15]. The Inuit (former called Neo-Eskimos) arrived from Alaska through Canada around 1200 AD and settled in North Greenland [Citation15]. Also, Vikings from North Europe populated West and South Greenland from around 985 AD to 1450 AD. A recent genetic study has indicated that the Greenlandic Inuit ancestry was consistent with a single immigration wave (the Neo-Eskimoes 1200 AD) settling the island from north to west to south to east [Citation15]. Furthermore, it was documented that the Inuit genome was unique, but also, that more than 80% of present Greenlanders had some degree of European ancestry. Several European expeditions to Greenland have been described [Citation15]. In 1636, Denmark unsuccessfully attempted to claim sovereignty of the sea around Greenland [Citation16–Citation18]. Whalers from England, Holland and other European countries were also in contact with the Inuit population in Greenland. In 1721, the Norwegian priest and missionary Hans Egede, came to Greenland and founded Godthaab (present day Nuuk) [Citation17,Citation18] and Greenland was gradually colonised and Christianised by missionaries primarily from Denmark. “Den Kongelige Grønlandske Handel” (The Royal Trade of Greenland) was established in 1774; trade in Greenland was monopolised by Denmark until 1950. During the 18th century, the population of Greenland increased from around 6,000 to around 11,000 and new strategies to supplement food supplies from hunting were initiated including sheep breeding and fishery. However, the development and changes developed relatively slowly until the end of the Second World War. In 1953, Greenland was internationally accepted by the court in The Hague (Den Haag) as being a part of Denmark, which changed its status to a Danish protectorate [Citation18]. A new strategy was initiated for the development of modern fishery in Greenland. Infrastructure was established including educational institutions and health care. Fish factories and apartment blocks were constructed in the towns, and the percentage of the population living in towns increased rapidly as did the population size. The rapid development was accomplished largely by experts and guest workers, coming mainly from Denmark [Citation18]. Thus, almost 20% of the population of Greenland were immigrants in the 1970s. This rapid socio-cultural change in Greenland has been parallelled by an epidemiological transition characterised by a reduction in some infectious diseases, a higher life expectancy, and an increased prevalence of psychosocial health problems including suicides, domestic violence, alcohol and tobacco abuse, sexually transmitted diseases and abortions [Citation19–Citation22]. Similar health-related effects were observed globally among many other Ingenious people affected by rapid sociocultural transitions and colonialisation [Citation23–Citation25]. Also, lifestyle-related illnesses have increased including cardiovascular diseases, obesity, physical inactivity and diabetes [Citation10,Citation19,Citation21]. Addressing these developing chronic diseases in the geographically widely spread population of Greenland represents a huge challenge for the health care system in Greenland.
To sum up, Greenland, the largest island in the world, is sparsely populated in isolated towns and settlements located along the coastline. A third of the population lives in the capital, Nuuk. The majority of the population is genetically of Inuit origin – Indigenous people of the Arctic – with a unique history and culture of surviving in the extreme arctic environment. For centuries, Greenland has been closely connected to Denmark. Since World War II, within only a few generations, the society and living conditions have changed dramatically in Greenland. Similarly, the health and disease patterns among Greenlanders changed rapidly. The unique geography remains a special challenge to providing health care services in Greenland.
Health problems among indigenous people in a global perspective
Indigenous peoples account for around 5% of the world population. More than 370 million Indigenous people live in more than 70 countries [Citation26]. According to WHO, the definition of Indigenous people can include peoples who: identify themselves and are recognised and accepted by their community as indigenous; demonstrate historical continuity with pre-colonial and/or pre-settler societies; have strong links to territories and surrounding natural resources; have distinct social, economic or political systems; maintain distinct languages, cultures and beliefs; form non-dominant groups of society; resolve to maintain and reproduce their ancestral environments and systems as distinctive peoples and communities [Citation26].
In general, the health of Indigenous people is worse than in the general population [Citation23–Citation25,Citation27]. This is also the case with diabetes [Citation23,Citation24].
Prevalence of diabetes among indigenous people
The International Diabetes Federation has estimated that 425 million adults, aged 20–79, lived with diabetes in 2017, corresponding to a global prevalence of 8.8% [Citation28]. The number was expected to rise to 645 million by 2045 [Citation28]. About 79% of all cases lived in low and middle-income countries [Citation28]. At least one-third of the increase was expected to be a consequence of population growth and advanced age [Citation28]. T2D was the most common form and accounted for around 90% of all diabetes cases. The prevalence was slightly higher for men (9.1%) than for women (8.4%) and increased with age. The highest prevalence, almost 20% for both women and men, was observed in the age group from 65 to 79. Regionally, the highest age-adjusted prevalence was found in North America and the Caribbean (11%), followed by the Middle East and North Africa (10.8%), South East Asian (10.1%), the Western Pacific (8.6%), South and Central America (7.6%), Europa (6.8%) and Africa (4.4%).
Still, around 50% of all cases were undiagnosed (212 million cases in 2017). The highest proportion of undiagnosed diabetes, 76.5%, was found in low-income countries compared to 52.5% in middle-income countries and 37.3% in high-income countries [Citation28]. Globally, the prevalence of diabetes among many Indigenous populations was reported as extraordinarily high [Citation27].
Despite great variations in histories and cultures, the consequences of rapid changes in nutrition and exercise seems to have the same escalating effect on the prevalence of diabetes in Indigenous populations [Citation29]. The magnitude may be determined by genetic susceptibility [Citation29]. Within the last 40 to 50 years, the prevalence of diabetes among many Indigenous groups around the world have been documented to have risen rapidly [Citation27]. The Pima Indian community in Arizona, USA, experienced a rapid increase in the diabetes prevalence, around 42%, within a 10-year period as early as 1967–77 and, since 1980, around 50% of adults aged 35 years or above were affected [Citation27,Citation30,Citation31]. These observations were followed by a dramatic increase among American Indians and Alaskan Natives in all USA. Even among youth below 35 years old, around 9.3% were affected in 1998, an increase of 46% within 10 years [Citation32]. In Canada, in the Sioux Lookout zone, and in Saskatchewan, the prevalence almost doubled from the early 1980s to early 1990s [Citation27]. Today, among First Nations in Canada, around 40–50% of individuals aged 60 or above live with diabetes compared to 20–25% among non-First Nations peoples [Citation32].
The trend of disproportional high diabetes rates among Indigenous people compared to the general populations was observed globally. Indigenous populations of the Pacific Island were among those with the highest rate of diabetes in the world [Citation27]. Among Aboriginals and Torres Strait Islanders – 2% of the population in Australia – around 39% of adults aged 55 or above live with diabetes [Citation33], five to six times higher than in the general population [Citation27]. Maori, Indigenous people of New Zealand, accounting for approximately 15% of population and living in rural areas, have a four times higher rate of diabetes than non-Maori people, 10.7% versus 2.4%, in the age group 20–64 [Citation33]. A lower prevalence, 3.7%, was observed among urban Maori [Citation34]. In Samoa and within three decades, the prevalence of diabetes among adults aged 25 to 64 rose from around 2% to almost 20% along with an increasing prevalence of obesity, now affecting more than 50% of males and 75% of females [Citation35]. However, within the global trend of higher prevalence of diabetes, variations exist among Indigenous peoples. The disproportionately high prevalence of diabetes among Indigenous people was mainly observed in settings where life conditions had changed rapidly forced by colonialism or other external forces [Citation27,Citation29]. In contrast, in areas where the Indigenous populations have maintained traditional lifestyles the prevalence of diabetes has been reported lower [Citation27]. Thus, the prevalence of diabetes is 2 to 5 times lower among Oregan Asli of Malaysia and Chilean Aymara and Mapuche than in their corresponding general populations [Citation27].
In addition to classical health determinants such as income, education, employment, living conditions, social support, and access to health services as listed in the 1986 Ottawa Charter for Health Promotion, a range of cultural factors have to also be included in the understanding [Citation24]. Along with racism, various Indigenous-specific factors, such as loss of language and connection to the land, environmental deprivation, and spiritual, emotional, and mental disconnectedness may influence health too [Citation24]. The increase must be understood in a complex perspective including biological, environmental, and lifestyle changes that have occurred during the last five to six decades [Citation27]. Both organisational and economic risk factors may contribute to the explanation, i.e. health care systems and poverty, and individual factors such as age, family history, gender and genetics [Citation27].
In conclusion, within a few decades the prevalence of diabetes has increased dramatically around the globe. Indigenous populations are among those affected most. Increasing age, rapid change in living conditions and lifestyle, socio-economically factors, and genetic susceptibility to diabetes may be main drivers.
Prevalence of diabetes among inuit in Greenland
Historically, diabetes was a rare condition in Greenland [Citation9]. Only sporadic cases were observed in the beginning of the 19th century. The first case was reported in South Greenland in 1910 [Citation36]. As late as 1940, obesity was not observed among Inuit while few cases were reported among immigrants [Citation36]. The prevalence of diabetes in Greenland has been evaluated in four population surveys listed in [Citation10,Citation21,Citation37,Citation38] and one register study [Citation39].
Table 1. Population surveys estimating the prevalence of diabetes in Greenland.
The first population study was performed in 1962 as a population survey combined with a register study [Citation37]. A total of 4,384 individuals (1,187 age 30 or above) corresponding to 14% of the total population of Greenland or 7% of the Inuit in the world were included. Of those, 4,249 individuals age 3 or above were screened for diabetes. Urinary glucose 2 hours after a meal was used as the screening test, followed by an oral glucose tolerance test (OGTT) in each case of positive urine screen tests. Among 24 persons with positive urine tests, three cases were categorised with possible diabetes corresponding to a prevalence of 0.07% [Citation37]. An additional study was made of diagnosed diabetes based on surveys of medical records from all health care districts in Greenland. Only 10 cases of diabetes were identified, corresponding to a prevalence of diagnosed diabetes of less than 0.001% among the entire population in Greenland (34.312) in 1962, or approximately 0.001% of adults aged 20–79 (assuming 50% of the population in Greenland was adults as gleaned from the observed figures later in the 1970s). Of those 10 cases, three were siblings of mixed Danish-Greenlandic origin [Citation37]. The next study of diabetes in Greenland, a register study, was published 24 years later in 1980 [Citation39,Citation40]. The incidence of diagnosed diabetes in the Upernavik district in Northwest Greenland was studied based on a review of medical records from 1950 to 1974 [Citation39]. Only one case of diabetes was observed in that time period. The expected number of cases at that time was nine according to corresponding incidence in Europe [Citation39].
The second population survey of diabetes was performed in 1999, and included 917 Greenlanders. Of those, 894 were tested for diabetes using an oral glucose tolerance test [Citation10]. A high prevalence of diabetes among Greenlanders was reported. Thus, the age-standardised prevalence of diabetes was 10.8% among females and 9.4% among males. Of those tested 70% were undiagnosed and unaware of their condition. In addition, a very high age-standardised prevalence of impaired glucose tolerance was reported: 14.1% among females and 9.4% among males [Citation10]. A similarly high prevalence was observed among Greenlanders living in Denmark [Citation41]. The high proportion (70%) of undiagnosed cases in Greenland was higher than the 50% level observed globally at that time [Citation42].
Based on the results from the population survey in Greenland, the authors concluded that the prevalence of diabetes was high among the Inuit of Greenland. Heredity was a major factor, while obesity and diet were important environmental factors. The high proportion of unknown cases highlighted a need for increased awareness of diabetes in Greenland [Citation10].
A high prevalence of diabetes, 6–9%, among adult Greenlanders, was confirmed in the third population survey performed from 2005 to 2011 in Greenland [Citation43,Citation44]. Again, the diagnosis was based on an oral glucose tolerance test. Furthermore, the prevalence of diabetes was higher in settlements (9%) and smaller towns (8%) when compared to larger towns (6%) in Greenland [Citation38,Citation45].
The most recent – and fourth population survey – performed in 2014 reported a lower prevalence of diabetes at 6.7% based on measurements of glycosylated haemoglobin (HbA1c t) and not OGTT. Of those, 40% was not diagnosed [Citation21]. Thus, the prevalence of diabetes in the four studies cannot be compared directly due to the different screening methods used.
Today, a urine dip stick is not acceptable as a screening tool for diabetes. The first study also included a register study, and it seems fair to assume that the prevalence of diabetes has increased dramatically from 1960 to 2000. The differences observed in the prevalence of diabetes in the most recent and fourth study compared to the second study from 1999 and the third study from 2005 to 2010 most likely reflect the different diagnostic tests used rather than a real difference in prevalence. The two methods, HbA1c and OGTT, categorise different individuals with diabetes [Citation46,Citation47]. This difference has also been documented among Greenlandic Inuit [Citation48].
Type 2 diabetes was the predominant form of diabetes observed whereas the prevalence of autoimmune diabetes has been reported to be at low levels [Citation49]. In addition to the increasing age of the population, increasing prevalence of overweight and obesity observed within the last three decades has been considered to be a main driver of the increasing prevalence of diabetes [Citation21,Citation43]. Physical activity has also decreased within the last few decades and may contribute to the increase of diabetes among Greenlanders [Citation50,Citation51]. Furthermore, the change in diet from a traditional diet high in marine protein and fat towards increased consumption of imported food high in carbohydrates has been investigated. Surprisingly, intake of traditional diet was associated with increased risk of diabetes [Citation52]. Mercury, which was found in high concentrations in marine mammals and accumulates in liver, kidney, brain, and other organs in humans consuming them [Citation53,Citation54], was weakly associated with diabetes [Citation55]. On the other hand, no association was found between glucose intolerance and dietary exposure to persistent organic pollutants among Greenlanders [Citation56]. Finally, vitamin D, which also was found in rich amounts in the traditional diet [Citation57,Citation58], was not associated with diabetes [Citation59]. Among the most remarkable recent observations was the identification of a new genetic mutation (p.Arg684T in TBC1D4) present in 17% of contemporary Greenlanders [Citation60]. The mutation was associated with severely decreased insulin-stimulated glucose uptake in muscle and postprandial hyperglycaemia in homozygote carriers [Citation60]. In contrast, the mutation was not associated with increasing fasting glucose. Also, interestingly, the level of HbA1c was negatively associated with the mutation [Citation60]. The same effects were observed among heterozygote carriers of the mutation, but to a lesser degree than among homozygote carriers. Follow-up analyses of skeletal muscle biopsies showed lower muscle protein levels of the glucose transporter called GLUT4 among carriers of the mutation [Citation60]. Thus, a population-specific subtype of diabetes seems to exist among Greenlandic Inuit explaining 15% of the diabetes cases observed in the aforementioned population surveys [Citation60].
In conclusion, it is plausible that the prevalence of diabetes among Greenlandic Inuit can be inferred to have increased between 1962 and 2000, despite the different methods applied. The main contributing factors were considered to be increasing age in the general population, increasing overweight, and decreasing physical activity. A genetic susceptibility to a subtype of diabetes also seemed to play an important role.
Prevalence of diabetes among inuit outside Greenland
Inuit are a group of Indigenous people living in Greenland, Canada, USA and Russia [Citation14]. Inuit are connected historically, culturally and genetically and have survived in the extreme Arctic climate for generations [Citation14]. In addition to the Greenlanders, subgroups exist in different geographical locations and are also referred to as Inuvialuit (Mackenzie Delta), Inupiat (Northern Alaska) and Yupik (Central and South-West Alaska in USA and Chukotka in Russia) [Citation14].
Similar to the circumstances of the Greenlandic Inuit, diabetes used to be a rare condition among the Inuit in Alaska affecting less than 0.2% of adults 60 years ago [Citation61–Citation63]. These first observations were based on OGTT. Diabetes was hardly ever observed among full blooded Inuit in Canada in 1950s [Citation64]. In 1968, the prevalence of diabetes among Canadian Inuit was also reported to be comparatively low. Among 13,000 Inuit, a review was performed of the 15 cases of diagnosed diabetes. The diagnosis was only correct in two cases and the prevalence was considered to be quite low [Citation65]. In contrast, glycosuria was observed quite frequently. Among 3,716 Canadian Inuit tested, 4.7% had glycosuria – 10.7% among the Caribou Inuit (former called Eskimoes) of the Central Arctic and 2.4% coastal Eastern Arctic Inuit [Citation65].
In the beginning of the 1980s, the prevalence was still reported as being low (0.4%) among Inuit in Quebec, based on a survey using random glucose measurement [Citation66]. At the same time, no cases of diabetes were observed in a population survey using OGGT among Inuit in Chukotka in Russia [Citation67].
In 1987, a population survey performed among Inuit and other Native Americans in Alaska, demonstrated that diabetes prevalence among Inuit had increased to 4.7% of adults aged 40 or above although much lower than the prevalence of 10% reported among the Athabascan Natives [Citation68]. Within the next decade, it became clear that diabetes had become a health problem among Inuit in Alaska. Population surveys performed in 1992 and 1994 among Alaskan Natives based on OGTT demonstrated a high prevalence of diabetes among Siberian Yupik Inuit at around 9% of the adults aged 40 or above. In addition, 12% had impaired glucose tolerance [Citation69]. The prevalence among Siberian Yupik was higher than among Inupiat (3.7%) and Central Yupik (2.8%) [Citation70]. The prevalence of diabetes among Inuit living along the northwest coast of Alaska, mainly Inupiat, was estimated to be between 2000 and 2004. Based on OGTT, the prevalence of diabetes and impaired fasting glucose among adults at or above 18 years old was reported to be 6.9% and 15.6%, respectively [Citation71].
Still, the prevalence of diagnosed diabetes among Inuit reported in register studies, naturally, remained much lower than reported in the population surveys. Obviously, undiagnosed diabetes, which can account for as much as 50% of all diabetes cases, is not portrayed in the registers. Furthermore, not all patients diagnosed may be registered. On the other hand, population surveys reporting diabetes prevalence based on one test, and not followed by a confirmatory test may tend to overestimate the prevalence [Citation9]. In addition, people at risk of diabetes may be more interested in participating in the surveys. A possible overrepresentation of those who are interested cannot be excluded and the prevalence of diabetes could thereby be overestimated.
A circumpolar study performed in 1992 reported age-adjusted prevalence of diabetes among Inuit in Alaska Canada and Russia. The highest prevalence was observed in Alaska, 0.79% compared to 0.36% in North West Territories in Canada, and 0.018% in Chukotka, Russia [Citation72]. A rapid rise in the prevalence of diagnosed diabetes has been observed in Alaska corresponding with the introduction of The Alaska Native Diabetes Programme in 1985 [Citation73–Citation75]. An increase of an alarming 231% in the prevalence of diagnosed diabetes among Alaskan Natives was observed from 1985 to 2006 [Citation75]. The increase was highest among Inuit, for whom the prevalence rose from 2.0% to 3.4% [Citation75]. Also in Canada, the prevalence of diagnosed diabetes more than doubled at that time (from 1987 to 1997), and rose from 1.2% to 2.6% among the First Nations populations [Citation76]. The highest prevalence was observed in the most southerly regions among those with the most contact to western lifestyle [Citation76]. In addition, a trend towards earlier age onset was observed to be related to the increasing prevalence of diabetes [Citation77]. Also, the prevalence of diabetes escalated among Native Americans and Native Alaskans particularly among young people below 35 years. The total number increased by 46% from 4,534 to 7,736 within few years (1990–1998), corresponding to a prevalence of 9.3% in 1998 [Citation62].
To sum up, the high prevalence of diabetes, mainly undiagnosed, among Inuit in Greenland around 2000, seemed much in line with observations made among the Alaskan Inuit a decade earlier. However, the prevalence varies among subgroups, probably reflecting different degrees of western influence on lifestyle. Greenland may be temporarily behind the Alaska Inuit in adapting western lifestyle. However, like in Alaska, an increasing prevalence of diabetes and complications related to diabetes may be expected to occur in Greenland in the near future.
Gestational diabetes among indigenous people
In a global perspective the prevalence of gestational diabetes mellitus (GDM) has been reported to be higher among Indigenous versus non-Indigenous women [Citation78–Citation82]. GDM is defined as hyperglycaemia diagnosed during pregnancy [Citation83]. More than 50 years ago it was hypothesised (The Pedersen Hypotheses named after the Danish obstetrician Jorgen Pedersen) that foetal overgrowth among patients with T1D was a result of maternal hyperglycaemia and increased trans placental transfer of glucose, stimulating foetal insulin production and thereby foetal growth [Citation84]. Although debated recently, this pathogenic pathway has been used in the understanding of GDM for decades [Citation84]. GDM is a serious condition associated with short and long-term complications for both mother and child [Citation85–Citation87]. Increased risk of adverse outcome has been documented, including preeclampsia, caesarean delivery, shoulder dystocia, macrosomia, stillbirth, neonatal hyperglycaemia, and hyperbilirubinemia [Citation85–Citation87]. In addition, GDM was a very strong predictor of later development of T2D. It is observed that around 50% of all mothers diagnosed with GDM develop diabetes later in life GDM [Citation78,Citation88,Citation89]. This risk was even higher among Indigenous people [Citation90]. In a study from Australia among Indigenous and non-Indigenous women with former GDM, progression to T2D within 8 years was observed four times more frequently among Indigenous women than among non-Indigenous women [Citation81]. Furthermore, the offspring of mothers with GDM has an increased risk of developing overweight and diabetes early in life [Citation89,Citation91–Citation93]. Generation after generation the risk of overweight and diabetes may be exacerbated, leading to hyperglycaemia in future pregnancies. This vicious cycle may play an important role in the growing incidence of T2D observed among Indigenous people worldwide [Citation27,Citation94].
Treatment of GDM including lifestyle interventions such as healthy eating, physical activity, self-monitoring of blood glucose concentration, and in some cases the administration of blood glucose lowering drugs has been reported to reduce the risk of some of the short-term outcomes such as shoulder dystocia, foetal overgrowth, caesarean delivery, and hypertensive disorders [Citation95–Citation97]. In addition, treatment was widely reported to be harmless, including treatment with insulin and oral anti-diabetic pharmacological therapies [Citation97,Citation98]. However, evidence of the positive effects on long-term maternal and neonatal outcomes remained poorly evaluated [Citation98]. It was thus important to identify and treat women with GDM to prevent adverse outcomes [Citation95,Citation96]. However, the condition most often presents itself without symptoms and can only be diagnosed using an OGTT [Citation83,Citation99–Citation101]. No worldwide consensus yet exists concerning testing strategy and cut-off values [Citation99]. Testing may be either universal including all pregnant women or selective risk-factor-based testing [Citation99]. Established risk factors of developing GDM are maternal age, pre-pregnancy overweight, number of previous births, and a history of diabetes in the family or a history of GDM, stillbirth or giving birth to an infant large for gestational age in earlier pregnancies [Citation102–Citation105]. Also, excessive weight gain during pregnancy increased the risk of developing GDM [Citation28,Citation106].
Ethnicity was also reported as an independent-risk factor for developing GDM [Citation94,Citation102,Citation107,Citation108]. In the USA, Indigenous people, Asians, Hispanics, and African-Americans showed an increased risk of developing GDM compared to non-Hispanic whites [Citation109]. In Australia and New Zealand, the highest prevalence was observed among Indigenous people, aboriginals in Australia and Maori and Pacific Islanders in New Zealand [Citation79,Citation109–Citation111]. Also in Europe higher prevalence of GDM has been documented among ethnic minorities from Africa and Asia, with people from India having the highest risk [Citation112,Citation113].
Maternal origin or place of birth played also a role for the risk of GDM. In Australia, higher risk was observed among mothers born in India or China compared to mothers born in Europe or Africa [Citation114]. Increased risk of GDM was also observed among mothers in the USA who were born outside the USA and among Black people, Asian Indians, Filipinos, Pacific Islanders, Chinese, Mexicans and non-Hispanic white women [Citation108]. In contrast, lower risk was observed among Japanese and Korean foreign-born mothers [Citation108]. Globally, the prevalence of GDM has increased within the last two to three decades from 10% to 100% in many different ethnic groups and countries [Citation94,Citation99,Citation109].
Comparing the prevalence of gestational diabetes around the world was been complicated due to lack of universally accepted diagnostic criteria, a factor that also influences the management of GDM [Citation99]. In addition, selective risk-factor-based screening compared to universal screening of all pregnant women clearly underestimated the prevalence of GDM as well. Changing diagnostic criteria among Aboriginals in Australia from selective to universal screening resulted in a 40% increase in the prevalence of diagnosed GDM [Citation115]. Screening effectiveness may also have influenced the prevalence estimated and have caused selection bias. A recent study from New Zealand, demonstrated a relative low screening rate at 63% of pregnant mothers [Citation116], lower (56%) among Maori mothers than among European mothers (76%) [Citation116]. A considerable variation has thus been reported in GDM prevalence around the globe.
A comprehensive review of diabetes among Indigenous people including 42 peer review studies reported a higher prevalence of GDM among Indigenous people compared to non-Indigenous people in most studies (65% of them) [Citation117]. The prevalence reported was highest among Canadian Aboriginals (11.5%), followed by Australian Aboriginals (8.4%), the Pacific Islanders (8.1%), Native Americans (7.9%), as compared with the 2–5% levels found either in this study worldwide [Citation117], or in the other global estimates of prevalence at around 5–7% [Citation99]. Recent estimates from Europe indicated a prevalence of GDM of around 2–6%. The prevalence was mostly reported to be less than 4% in the Northern or Atlantic seaboard parts of Europe, while a prevalence above 6% dominated in South and Mediterranean seaboard regions [Citation99]. In Denmark, the prevalence of GDM almost doubled among mothers aged 35–49 within less than a decade from 2.9% in 2004 to 4.7% in 2012 [Citation113]. A study from the USA including more than 200,000 deliveries in the period 1995–2004 estimated the prevalence of GDM in different ethnic groups [Citation108]. The lowest age-adjusted prevalence of GDM was observed among non-Hispanic white women (4.2%), followed by African Americans (4.4%), Hispanics (5.4%) and Japanese (5.5%), Koreans (6.7%), Mexicans (7.1%), Pacific Islanders (7.2%), Chinese (7.9%) and was highest among Southeast Asians (8.8%), Filipinos (9.6%) and Asian Indians (11.1%) [Citation108].
While GDM has not been investigated among Greenlanders before, the prevalence of GDM has been reported as early as in 1988, to be high among Yupik Inuit. Based on OGTT, the prevalence was estimated to be 6.7%, which was twice the rate in the USA in the late 1980s, despite the fact that the Inuit had a lower prevalence of diagnosed diabetes at that time [Citation68]. The authors concluded that undiagnosed diabetes and impaired fasting glucose could be a severe problem among Inuit due to severe lifestyle changes taken place within the last 30 years [Citation118]. In Canada, a somewhat lower prevalence of diagnosed gestational diabetes, 28 per 1000 live births, was observed [Citation72].
To sum up, GDM is a serious condition associated with both short and long-term complications for mother and child. The prevalence has increased globally within the last two to three decades and may influence the prevalence of diabetes in offspring, the coming generations. Indigenous people and other minorities are at particularly high risk of developing GDM. Diagnosing GDM is important because proper management can reduce adverse outcomes. Until now, no studies on GDM among Greenlanders have been performed.
Prevalence of diabetes-related complications among indigenous people
The rising prevalence of diabetes has inevitably led to an increase in diabetes-related complications among Indigenous people [Citation27]. In general, diabetes complications are separated into macrovascular disease, including coronary artery disease, peripheral arterial disease and stroke, and microvascular disease including diabetic nephropathy, neuropathy and retinopathy [Citation3]. In addition, diabetes is associated with several comorbidities and increased risk of death due to liver cancer and other liver disease, renal disease, and infection, falls, mental disorders, and self-inflicted harm [Citation119]. Many complications to diabetes including retinopathy, microalbuminuria, end-stage renal disease, lower limb amputations, cardiovascular disease, and mortality were observed more frequently among Indigenous people in general and among other minorities compared to the population at large (see the next sections) [Citation8].
Retinopathy
Within the two decades from 1990 to 2010, retinopathy due to diabetes increased dramatically (27%) and diabetic retinopathy is now the leading cause of preventable adult blindness and the fifth most common cause (only surpassed by cataract, uncorrected refractive error, macular degeneration, and glaucoma) of global blindness and impaired vision [Citation4,Citation120,Citation121]. In 2010, 0.8 million people were blinded (2.6% of all blindness in the world) due to diabetes [Citation120]. The proportion of blindness due to diabetic retinopathy was lowest (1.1%) in East Asia. Higher levels were observed in high-income regions with older populations as in Europe (3.7–4.2), and North America (3.9%), Australia (4.2%), and in Southern Latin America (5.5%) [Citation120]. The prevalence of signs of retinopathy and vision-threating retinopathy among patients with diabetes has been estimated to be 34.6% and 10.2%, respectively, based on 35 publications which included around 20,000 patients [Citation122]. Regional diabetes shows variations from 5% to more than 40% as have been described around the world [Citation8,Citation123,Citation124]. In the USA, the prevalence of retinopathy among all patients with T2D has been reported to be around 40% among non-Hispanic white people [Citation124]. Prevalence among different minorities and Indigenous people varies. Higher prevalence, around 50%, was observed among African Americans and Hispanics [Citation124,Citation125], while lower prevalence was observed among Oneida Indians (9%). In Canada, a prevalence of around 40% was observed for both native and non-native Canadians [Citation126]. A similar prevalence of retinopathy around 40% has been reported in most European countries. However, a much lower prevalence, around 5%, has been reported in Denmark, Finland, the Netherlands and rural France [Citation124]. Higher prevalence was reported among Asians in England compared to the general population [Citation125]. In Australia and South East Asia, the prevalence has been reported around 35% [Citation124], similar to that in Europe. Around the same level of retinopathy has been reported for Australian aboriginals and non-aboriginals regarding any form of retinopathy. However, almost a third of Aboriginals with retinopathy had vision-threatening retinopathy [Citation127]. Thus, the proportion of Australian Aboriginals with vision impairment was estimated to be 11.2% compared to the 6.5% level among the general population [Citation128]. Likewise, no ethnic difference in the prevalence of any retinopathy was observed in New Zealand, whereas vision-threatening retinopathy was observed more frequently among Pacific Islanders (15.8%) and the Maori (12.9%) compared to people of European origin (12.9%) [Citation124]. Thus, one may conclude that in addition to established risk factors for retinopathy such as duration and severity of hyperglycaemia, hypertension and hyperlipidaemia, ethnicity has can be considered to be and is reported as risk factors as well [Citation8,Citation123,Citation124,Citation129].
Neuropathy
Peripheral neuropathy is a common complication related to diabetes. It may be asymptomatic. Symptoms can be classified as negative, including loss of sensation and loss of strength, or positive, including pricking sensation or pain [Citation130,Citation131]. The prevalence of neuropathy has been reported at around 8% among newly diagnosed patients to above 50% for patients with the long-standing disease [Citation132]. Despite great variations in the prevalence of neuropathy around the world, ethnicity has not been established as a contributing risk factor [Citation125,Citation129]. Prevalence has been reported as being high for some Indigenous populations like the Australian Aboriginals (41.2%) [Citation133], Sandy Lake First Nations (46.3%) [Citation134], Navajo (28.4%) [Citation135], and low among others as, for example the Oneida (16.0%) [Citation136], First Nations and Metis in Manitoba (12%) [Citation53,Citation137], and among the James Bay Cree (9.6%) [Citation138]. However, the comparisons should be taken with clear reservations due to differences in the methods used in the different studies and the age distribution, including the duration of diabetes of participants.
Microalbuminuria
The global prevalence of microalbuminuria among T2D patients has been estimated to be around 39% based on a large cross-sectional study including 32,208 patients from 33 countries [Citation125]. The highest prevalence (55%) was observed among south Asians and Hispanics. An even higher prevalence has been observed among some Indigenous groups. Prevalence of microalbuminuria among different groups has been summarised by Naqshbandi et al. in a comprehensive review [Citation8]. The highest prevalence was reported for Zuni in America (62%), followed by The Torres Strait Islanders in Australia (54.2%), First Nations in Canada (44.0%), Cherokee (41%), Pima and Tohono O’odham (36.5%), Maori (30.7%), Pacific Islanders of New Zealand (22.5%), and Oneida (8.0%) [Citation8]. Other risk factors of microalbuminuria include the severity of HbA1c, systolic blood pressure, retinopathy, duration of diabetes, kidney function, body weight and smoking [Citation139].
End-stage renal disease
Diabetes has been reported to be the biggest risk factor for developing end-stage renal disease accounting for around a third of all cases [Citation140]. The incidence of end-stage renal disease due to diabetic nephropathy disease varies across the globe. The lowest incidence, 17 per 100,000 patients per year, was reported among the Basque in Spain 2003 [Citation141]. In 2010, the rate was reported to be 60 per 100,000 patients per year in Spain compared to 200 in the general USA population [Citation141,Citation142]. A much higher rate, 558 per 100,000 patients per year, was reported among Native Americans in 2001 [Citation143]. Also, among African Americans (327.7 per 100,000 patients per year in 2006) and Hispanic (254.3 in 2006), higher rates were observed when compared to white people in the USA in 2006 [Citation141]. Higher rates have also been observed among Indigenous populations compared to their non-Indigenous counterpart outside the USA. The incidence of end-stage renal disease among Aboriginal people in Canada was many times higher than among non-Aboriginals, 560 versus 240 per 100,000 patient per year in 1990 [Citation141,Citation144]. Also, among Pacific Islanders (330 per 100,000 patient per year) and the Maori (470) compared to the level of 30 of diabetes found in the general population of New Zealand [Citation145]. Other risk factors associated with increased risk of developing diabetic end-stage renal disease include T1D, male gender, age, duration of diabetes, blood pressure, HbA1c, and any history of atherosclerosis [Citation140]
Lower extremity amputations
Diabetes has been associated with an increased risk of lower extremity amputation. Globally, the rates of major amputation ranged from 5.6 to 600 per 100,000 in the population with diabetes, and from 3.6 to 68.4 per 100,000 in the total population [Citation146]. For all lower extremity amputations, the figures were 46.1 to 9,600 per 100,000 among patients with diabetes compared with 5.8–31 per 100,000 in the total population [Citation146]. The variations could partly be explained by varying ethnicity and levels of social deprivation. However, diabetes and complications to diabetes were the most important risk factors [Citation146]. In the USA, the lower extremity amputation rates among Native Americans varied among different tribes ranging from 30 to 170 per 100,000 patient per year compared to 5.8 per 100,000 in the general diabetes population in 1990 [Citation8,Citation142]. Very high rates were reported among Canadian Aboriginals (360 per 100,000 patient years) whereas lower rates have been observed among the Maori and Pacific Islanders, 28 and 10 per 100,000 patient per year, respectively, [Citation8].
Cardiovascular disease
Cardiovascular disease including angina, myocardial infarction, stroke, peripheral artery disease, and congestive heart failure has been reported as common and to be the cause of the death of around 50% of patients with T2D [Citation147]. The risk of cardiovascular disease increased with age, smoking, abnormal blood lipids and hypertension [Citation147].
Globally, the prevalence of the cardiovascular disease has been estimated to be 32.2% of patients with T2D with a mean duration of 10.4 years [Citation148]. However, higher prevalences have been reported among different Indigenous populations including Australian Aboriginals affecting (47.7%), Native Americans (34.7%), Indigenous people of Canada (34%), Pacific Islanders (36.0%), and the Maori (34.0%) and in New Zealand [Citation8,Citation133,Citation135,Citation149].
Mortality
Mortality due to diabetes was generally reported to be higher among Indigenous people. Among Native Americans and Indigenous people in Canada, mortality rates at 36.2 and 19.5 per 100,000 inhabitants have been observed compared to 16.7 and 13.3 per 100,000 in their respective general populations [Citation150]. The highest mortality rate reported was among the Australian Aboriginals at 85.4 per 100,000 compared to 8.7 per 100,000 in the general population of Australia [Citation150].
The reasons for the higher diabetes mortality and morbidity among Indigenous populations with diabetes are complex. Genetic susceptibility may be a part of the explanation [Citation29]. Also, earlier onset, intergenerational risk associated with GDM, greater severity of disease and reduced access to health care are among the important factors [Citation27]. Poverty and harsh living conditions among Indigenous people, including limited food supply in earlier times, may have also played a role too. The thrifty phenotype hypothesis, as proposed by Hales and Barker, suggested an association between poor foetal and infant growth and the subsequent development of T2D as an effect of poor nutrition in early life, which again produced permanent changes including a reduced capacity for insulin secretion and insulin resistance [Citation151]. In combination with changing lifestyle conditions towards more westernised food and sedentary lifestyle, the risk of obesity and diabetes may have been aggravated [Citation151].
In general, Indigenous people have increased risk factors related to general health determinants [Citation27]. The prevalence of smokers among patients with diabetes in many Indigenous population in Canada, Alaska, Australia, and New Zealand was around 40%, three to four times higher than among patients with diabetes in the general population [Citation27]. Initiatives to improve diabetes and chronic disease care and management in Indigenous populations within the primary health care system have been recently proposed [Citation27].
Reduced rates of complications in some high-income countries
In high-income countries such as in USA, Canada, Australia and some countries in Europe and Asia, the mortality and rates of complications including myocardial infarction, stroke and lower extremity amputation have been reduced within the last two decades [Citation142]. Reduction in diabetes complications has been driven by improvements in lessening different risk factors including control of blood pressure, blood lipids, smoking and hyperglycaemia. In addition, earlier diagnosis, better delivery of preventive care, self-management, and the organisation of diabetes care have all played a role [Citation142]. Between 1990 and 2010, in the USA, the incidence of myocardial infarction declined by 68% (from 141 to 45 per 10,000 people with diabetes per year) and the incidence of stroke declined by 53% (from 112 to 53 per 10,000 patients per year) [Citation142]. Also in the USA, a reduction was observed in the rate of lower extremity amputation by 52% (from 58 to 28 per 10,000 patients per year) and rate of end-stage renal disease by 29% (from 28 to 20 per 10,000 patients per year) [Citation142]. Smoking rates among patients with diabetes were reduced from 41% in the early 1970s to 17% at around 1992. The proportion of patients with blood pressure below 130/85 mmHg, HbA1c below 53 mmol/mol (7.0%) and LDL cholesterol below 2.5 mmol/l was increased from 10% to 20% from 1999 to 2010 [Citation142].
Prevalence of complications among the Inuit
In Greenland, only one study has evaluated the prevalence of complications of diabetes among Greenlanders. Sixty-eight per cent of Greenlandic patients suffered from at least one complication. Neuropathy was observed among 51% of the patients followed by microalbuminuria (43%), retinopathy (14%), and macrovascular complication (10%) [Citation152]. In addition, 90% of the patients were overweight or obese and 43% were current smokers [Citation152]. However, only 81 Greenlanders were included in the study and the results have to be confirmed in a larger study.
In a study among Alaskan Natives diagnosed with diabetes, the Inuit had a lower prevalence of death due to ischaemic heart disease (2.6/1,000 patient per year in the time period 2002–2006) compared to Aleut people (3.73/1,000) and Native Americans (4.2/1,000) [Citation75]. On the other hand, Inuit had a higher risk of cerebrovascular complications (2.2/1,000) compared to Aleut people (1.75/1,000) and Native Americans (1.45/1,000) [Citation75]. Both mortality and complication rates decreased among Alaskans in the period 1985–2006. In the beginning, the overall amputation rate for all Native Alaskans was 6/1,000 patient per year, lowest among Inuit (3/1,000 person year). In 2006, the amputation rate was reduced to 2.6/1,000 patient per year. Also, renal replacement was reduced from 3.3 to 1.2/1,000 persons per year. Mortally rates were reduced from 41.7 to 33.2/1,000 patients per year [Citation75]. The rates were lower than observed in the USA at that time, but the small number of patients, shorter time of duration of diabetes, earlier mortality and low frequency of renal replacement therapy complicated the comparison [Citation75]. Eight (31%) out of 21 patients with end-stage renal failure died without renal replacement in the beginning of the 1990s [Citation153]. The reduction in mortality and complications may be a result of the Alaska Native Diabetes Programme initiated in 1994 and further described in the next section [Citation75].
To sum up, microvascular complications including retinopathy, microalbuminuria, and neuropathy were frequently observed among Indigenous people around the globe. Ethnicity seemed to be an independent risk factor for retinopathy and microalbuminuria whereas this was not the case with neuropathy. Thus, great variation was observed in the prevalence of complications among different Indigenous and ethnic groups. In Alaska, the prevalence of ischaemic heart disease was lower among Inuit with diabetes compared to Aleuts and Indians with diabetes, while Inuit had higher rates of cerebrovascular disease. In general, the prevalence of both microvascular and macrovascular complications and even mortality among Indigenous people was disproportionately high compared to the non-Indigenous populations due to complex factors. In some high-income countries, and also among Alaskan Natives, the rates of complications have been reduced within the last few decades, driven by improvements in different risk factors, earlier diagnosis and diabetes care.
Diabetes care among indigenous people
Diabetes can have a major impact on the health and quality of life of the individuals affected [Citation154]. In addition to increased risk of premature death and diabetes-related complications, diabetes has a negative impact on social and psychological well-being [Citation154]. However, controlling HbA1c, blood pressure and LDL cholesterol can reduce complications related to diabetes [Citation155–Citation157], which underscores the need for high-quality diabetes care. However, diabetes care is complex reflecting the many aspects of the disease. Consequently, measuring the quality of diabetes care remains challenging [Citation155,Citation158].
Within the last two decades several sets of quality indicators have been developed in different settings around the world in order to measure and benchmark diabetes care within healthcare systems, at locations including the Centre for Medicare and Medicaid, the National Committee on Quality Assurance, the American Diabetes Association, and the National Diabetes Quality improvement Alliance in USA, the European Union Diabetes Indicator Project in Europe, and the National Heath Priority area in Australia, to mention a few [Citation155,Citation159–Citation161]. These quality indictors captured different elements of diabetes care. However, due to the diversity in the indicators both between and within countries, it has been difficult to compare diabetes health care globally [Citation161]. In 2006, a selection of indicators was published to measure the quality of diabetes care using health care systems in OECD countries [Citation162]. Four process indicators were selected including a proportion of patients with diabetes tested annually for HbA1, LDL cholesterol, nephropathy and retinopathy. Furthermore, two proximal outcome indicators including control of HbA1c (most recently HbA1c measured below 53 mmol/mol or <7%), and LDL control (most recently LDL measured below 3.5 mmol/l) were chosen. Finally, three new distal outcome indicators were proposed, i.e. rate of major amputations, rate of end-stage renal disease, and cardiovascular mortality. The indicators were selected based on three core elements proposed by the US Institute of Medicine including relevance, scientific soundness, and feasibility [Citation160,Citation162]. Credible evidence was thus required, linking process measures to important clinical outcomes, and requiring modifiability of the clinical outcome measures. Furthermore, the task of collecting accurate and reliable data at a reasonable cost was crucial. Finally, variability across health care settings was essential, thereby providing an opportunity to learn from and be inspired by other health care systems regarding ways to improve diabetes care [Citation162,Citation163].
The implementation of diabetes care programmes has been associated with improved diabetes care in many settings even when using different combinations of quality of diabetes care indicators, feedback to clinicians, public reporting and financial incitements [Citation155]. However, many programs tend to change specific aspects of diabetes care, which have been measured and or paid for, but not necessarily applying the change to all aspects of diabetes care [Citation155]. Diabetes indicators cannot be used uncritically to measure quality diabetes care. Additional indicators may be needed that are not only dichotomised as good or bad (fx.HbA1c below 53mmol/mol or not) but also provide the opportunity to capture relevant clinical actions. Thus, for example prescribing angiotensin-converting-enzyme inhibitors in the case of microalbuminuria, and avoiding harmful treatment outcomes (for example the rate of hypoglycaemia) may be considered and developed in the future [Citation155]. Some of these indicators are already used in some settings as found in the national indicator project in Denmark [Citation164]. Several efforts have been made to evolve instruments to measure patients’ reported outcomes including auditing of Diabetes-Dependent Quality of Life, Diabetes Health Profile, Diabetes Impact Measurement Scale, Questionnaire on Stress in Diabetes-Patients, and several others [Citation154]. With the increasing use of EMR and standardised recording – including possibilities to glean laboratory data linked to patients with diabetes – more effective measurements will probably arise in the near future including patients’ reported outcomes [Citation155].
Obviously, around the world diabetes management and quality of diabetes care vary considerably [Citation165]. The OECD indicators have been used to compare the performance of diabetes care in the USA, Canada, Australia, New Zealand and UK [Citation161]. Although diabetes care has improved in the USA within the last decades [Citation142,Citation156], the quality of care was still suboptimal. Annual HbA1c and LDL cholesterol testing were performed on 70–80% of patients in the USA and the UK [Citation161]. For New Zealand and Australia, the figures were 50–60% [Citation161]. Furthermore, even within countries, great variation has been reported in diabetes care. A study performed in the UK documented that lower quality of diabetes care was associated with conditions of deprivation and to foreign ethnicity [Citation165]. Also, among Indigenous people, Native Americans and Alaskan Natives in the USA, and the Maori and Pacific Islanders in New Zealand, diabetes care has been reported to be poorer than in the general population [Citation27,Citation161]. Geographical disparities have been reported in diabetes care within countries showing lower-quality care in rural and remote settings compared to urban areas [Citation166–Citation168].
Improving diabetes care in remote areas has been reported to be a special challenge and the quality initiatives have to be designed to the specific setting [Citation169]. A comprehensive review has been recently published evaluating different strategies to improve diabetes care in remote areas [Citation166]. Nine overall intervention strategies were identified including three which target the patients themselves (patient education, promotion of self-diabetes management, reminder systems), two targeting health care providers (feedback on performance and education), and four targeting the health care system (case management, team changes, electronic patient registers, and facilitated dissemination of information to clinicians). The majority of interventions (50%) were single strategy quality initiatives. Of these interventions, 80% included patient education. Several initiatives were associated with better diabetes care. However, the highest improvement in diabetes care was associated with the use of multiple strategies. It was concluded that efforts to improve diabetes care in rural communities should focus on interventions with multiple strategies targeting clinicians and health care systems, rather than on traditional patient-oriented interventions [Citation166].
This conclusion is much in line with recommendations from the American Diabetes Association [Citation156]. Four recommendations were published including (1) a patient-centred communication style that incorporates patients’ preferences, assesses literacy and numeracy, and addresses the requirement that cultural barriers should be addressed, (2) treatment decisions should be made timely and based on evidence-based guidelines that are tailored to individual patient preferences, prognosis and comorbidities, (3) care should be aligned with the component of the Chronic Care model to ensure productive interactions between prepared proactive practice team and an informed activated patient, and (4) when feasible, care systems should support team-based support tools to meet patient needs.
Diabetes care among Inuit
Among Inuit, the special diabetes programme from Alaska represented an example of a multifaceted intervention strategy targeting the organisational aspects of diabetes care, health care professionals, and patients.
Initially, the Indian Health Service was responsible for diabetes care. However, the special diabetes programme was initiated in 1994 and fully implemented in 1999 [Citation73–Citation75]. The programme included a strengthening of health care infrastructure, national guidelines, training of local health care professionals, a central register allowing follow-up on patients, and performance feedback [Citation73]. Both processes of care and proximal diabetes outcomes were improved from 1994 to 2006 [Citation74]. In Canada, a 5-year national strategy, Canadian Diabetes Strategy (CDS) was launched in 1999 to address the increasing prevalence of diabetes. The Aboriginal Diabetes Initiative was one of the four elements in the strategy. The other three were Prevention and Promotion of Diabetes, National Diabetes Surveillance System and National Coordination [Citation170]. The Aboriginal Diabetes Initiative focused on workforce training and capacity building among diabetes prevention workers and health-care providers who managed diabetes with Aboriginals [Citation170]. Also, the development of national diabetes guidelines, healthy food promotion programs targeting families, schools and other institutions, increased access to the screening of diabetes and its complications, the Screening for Limb, Eye, Cardiovascular and Kidney Project (SLICK), mobile units (SLICK Vans) equipped with necessary laboratory and ophthalmological equipment were all founded by the initiative [Citation170]. Also in Canada, a better control of hypertension was obtained by a programme among the Indigenous population living in remote areas [Citation161–Citation163]. This initiative was based on home care blood pressure monitoring by nurses in cooperation with primary care physicians. Among Inuit in Greenland, efforts were taken to improve diabetes care including the use of a quality database in 2006 in two towns: Nuuk and Aasiaat [Citation171]. However, because we did not perform any evaluations before the new diabetes initiative was taken, details will be described in the next sections.
To summarise, high-quality diabetes care is highly important in reducing the burden of diabetes-related morbidity. Globally, diabetes care remains suboptimal in many settings. Disparities in diabetes care are related to deprived areas, ethnic minorities, Indigenous people, and other isolated populations. Measuring the quality of diabetes care is complex and still not optimal. Improving diabetes care in remote settings is highly challenging and several strategies have to be combined. Increased use of EMR may be an important step on the way towards better monitoring and the delivering of high-quality diabetes care. Increased diabetes care among Inuit and Native Americans in Alaska was the result of a multifaceted diabetes initiative.
Health care system in Greenland
In 1992, the Home rule Government of Greenland took over responsibility for the health care system in Greenland from Denmark. Thus, the health care system today is a publicly financed governmental responsibility [Citation13]. Health care service including dental health care and prescribed medicine is delivered free of charge. The health care system aims at delivering equal health care service to every citizen in Greenland regardless of the place of residence [Citation13]. This was an overwhelming challenge in a sparsely populated nation covering more than 2 million square metres with many individuals living in remote sites. In addition the extreme weather conditions in arctic Greenland contribute to making the population even more isolated because transportation is not always possible. Limited economic resources in combination with an increased demand for specialised, advanced treatment presented fundamental challenges [Citation13]. The economic burden imposed by around-the-clock service in several remote health sites, and unique requirements like expensive airplane evacuations of patients with need of specialised care, referrals to advanced treatment abroad, and difficulties in recruiting and retaining health care professionals all provided profound challenges [Citation13]. Also, cultural and linguistic barriers exist between local patients and recruited short-term health care professionals from outside Greenland. This was a special challenge for chronic conditions that need continued medical care and service. While acute medical conditions may be obvious and handled by the health care system, the real risk of paying less attention to delivering long term, continued and preventive health care existed as well.
Fundamental changes in society, living conditions, and lifestyle, and the accompanying changes in disease patterns and epidemiology have raised the need for new solutions. Focusing on the advantages and resources within the existing health care system has been fundamental for decades.
The health care system is one united health care system. In comparison with other national health care systems, it was relatively small and has traditionally been flexible and adaptable to new challenges. The incidence of tuberculosis was reduced from around 2,000 per 100,000 inhabitants after World War II to less than 100 hundred per 100,000 inhabitants in the 1970s partly due to changed living conditions and partly due to a national strategy within the health care system [Citation172]. Also, a dramatic decrease of venereal diseases was observed from 1970s to the beginning of the 1990s [Citation173]. Prenatal screening programmes for Greenlandic specific mutations related to severe diseases like cholestasis-familiaris-groenlandica and propionic-acids-anaemia have been implemented – the only place in the world to have done so. Also, the inclusion of hepatitis B immunisation in the general child vaccine programme in 2010, as a consequence of the endemic prevalence of hepatitis B among Greenlanders reflected the adaptability of the health care system [Citation174]. As a consequence of the small population, the absolute number of patients was also limited, increasing the possibility of providing high-quality health care for all. The health care system in Greenland has been in the forefront with the implementation of digital solutions. Some primary health care sites have used electronically medical records (EMR) since the mid-1990s. In 2007, the same EMR (Æskulap®) was implemented in all primary health care sites all over Greenland. Only dental care and secondary care were not digitalised [Citation9]. Also, the X-ray (chilli web®) system and the laboratory system (BBC lab®) were fully implemented by the year 2007. Since 2013, a new mutual EMR including all parts of the health care system has been under implementation in the secondary health care system, and since 2015, is gradually replacing the former EMR used in the primary health care system. It had thus replaced the former EMR in Nuuk by March 2015. During the end of 2016 and the first half of 2017, the EMR was fully implemented in the rest of Greenland except for parts of East and North Greenland (in the cities of Tasiilaq and Upernavik) where limited internet capacity limited its use. Also, telemedicine has been used for decades in Greenland, which has gradually adapted to new technological options [Citation175–Citation177].
In brief, the public health care system is publicly financed and free of charge for the inhabitants of Greenland. Severe challenges existed in delivering high-quality health care to the geographically dispersed population. Limited economical resources and difficulties in recruiting and retaining health care professionals were important issues too. The health care system strived to address these challenges. New solutions and technical options were continuously considered and adapted in a flexible manner.
Organisation of health care
Primary health care is provided in all towns and settlements in Greenland. In 2011, 16 local medical districts managed by a chief district physician were merged into larger geographical regions [Citation178]. Today the health care system in Greenland is divided into five health care regions (). Each region consisted of a number of towns and settlements. A regional health care centre was located in the largest towns of the region (in Nuuk, Sisimiut, Ilulissat, Qaqortoq and Aasiaat) while local, regional health care centres existed in all other towns [Citation178] (). Depending on the number of inhabitants and degree of geographical isolation, the regional and local health care clinics were staffed with physicians, nurses, midwives and other health care workers. In addition to primary health care, some secondary health care and inpatient care took place at the regional and local clinics. Smaller health stations (58 in total) in settlements are staffed with a nurse or a health care worker depending on the number of inhabitants in the settlement were the smallest health care units in Greenland are found [Citation178].
Specialised and secondary health care was offered at Queen Ingrid Hospital located in Nuuk or by travelling specialist coming from Nuuk or Denmark [Citation13,Citation178]. Three main departments – including internal medicine, psychiatry, and surgery – offered specialised health care within most areas for all patients in Greenland. In addition, the only central laboratory, department of radiology, and intensive care units are located at Queen Ingrid´s Hospital.
Patients in need of advanced treatment not available in Greenland were offered treatment in hospitals in Copenhagen, Denmark.
The basic treatment and follow-up of patients with diabetes are a responsibility of the local health care clinics and stations. After referral from the local physician, they might be seen by travellinga specialist in internal medicine, orthopaedics, or ophthalmology. Screening for gestational diabetes was a risk factor-based and part of prenatal care. Prenatal care for pregnant women in Nuuk, including the first prenatal care visit, was performed by midwives working at Queen Ingrid’s Hospital. Outside of Nuuk, prenatal care was performed by midwives in some larger towns. In settlements and towns without midwives, prenatal care was performed by other healthcare professionals, by visiting midwives or by facilitating the pregnant women to be able to visit towns with midwives [Citation179]. Pregnant women at risk of complications were referred to Nuuk for delivery while the remaining births took place in the local clinics in the larger towns.
To sum up, primary health care is delivered by five regional health care centres, 11 local clinics, and 58 health care stations. Secondary health care is delivered by Queen Ingrid’s Hospital in Nuuk, and by travelling specialist from the hospital or from Denmark. Some advanced treatments are delivered to patients at hospitals in Denmark.
A new diabetes initiative
In order to address the lack of awareness regarding diabetes in Greenland, a mutual pilot project between the primary health care clinics in Nuuk and Aasiaat focusing on the quality of diabetes care was initiated in November 2006. The diabetes care was monitored using an electronic database and a number of selected process indicators. However, in 2007, before any evaluation had taken place a large donation with no conditions attached was received from Novo Nordisk A/S, and earmarked to the health care system in Greenland in order to increase awareness of diabetes in Greenland. In February 2008, a three year diabetes project was initiated aiming to improve detection of undiagnosed cases, improving care for patients with diabetes and promoting awareness of diabetes.
The prevalence of diagnosed diabetes and the quality of diabetes care improved in the observation period between 2008 and 2010. In 2011, the diabetes program was replaced by a lifestyle initiative. However, the methods introduced in the diabetes program were maintained including continued monitoring, analysis, and adjustment of initiatives taken in the diabetes care. Health research within the health care system was thus used as an essential tool, which performed in close interaction with the strategies and actions taken to optimise diabetes management. This method was new in Greenland, where no long-term observations had been performed. Especially, it was unknown if it was feasible to use this method including health research, continued monitoring, analysis, and adjustment of initiatives given the geographically dispersed population of Greenland. Would it be possible to monitor and to increase the diagnostic activity of diabetes in a health care system facing such severe challenges, which also included a lack of permanently employed health care professionals? Could the prevalence of diabetes and quality of care be monitored and would increase diagnostic activity affect the prevalence? Furthermore, gestational diabetes and the management including testing effectiveness were unexplored. Would knowledge about prevalence and testing effectiveness change management of gestational diabetes in Greenland? Also, the prevalence of microvascular complications among Greenlanders had only been sparsely evaluated. Ethnicity may influence the prevalence of some complications. Thus Greenlanders may have a different tendency to develop microvascular complications than non-Greenlanders after receiving the same quality of care for diabetes.
Hypotheses and aims
The overall hypothesis here states that monitoring of an ongoing national diabetes care programme in the geographically dispersed population of Greenland is feasible.
The specific hypotheses are that the:
majority of males and females in Greenland are in annual contact with the health care system in Greenland allowing a case-finding strategy to detect new cases of diabetes
diagnostic activities related to diabetes could be improved in Greenland from 2010 to 2015
prevalence of diagnosed diabetes can be monitored and increased during the period from 2008 to 2017
quality of diabetes care could be monitored and improved from 2008 to 2017
prevalence of GDM is increasing and testing effectiveness could be improved from 2008 to 2014
prevalence of microvascular complications among patients with type 2 diabetes are equal among Greenlanders and non-Greenlanders in Nuuk
The overall aim of this thesis is to report the monitoring of an ongoing national diabetes care programme in Greenland.
The specific objectives were to estimate and compare the
contact with the health care system in Greenland among males and females in different age groups in 2010–2011
diagnostic activity between 2010–12 and between 2014–15
prevalence of diagnosed diabetes from 2008 to 2017
quality of diabetes care from 2008 to 2017
prevalence of gestational diabetes and testing effectiveness between 2008 and 2014
prevalence of microvascular complications among Greenlanders and non-Greenlanders with type 2 diabetes in Nuuk in 2016
Methodological considerations
A donation from Novo Nordisk A/S provided the impulse enabling the Health care system to initiate a three-year national diabetes project. I was employed as a project manager from 2008 to 2011. In cooperation with one registered nurse, one bachelor in nutrient and human health, and one state registered chiropodist, the diabetes group was established to fulfil the project in 2008.
National diabetes care program
Initial analysis of existing diabetes care
Before any initiatives were taken, an initial analysis was performed. This included evaluation of earlier Greenlandic experiences in using a diabetes quality of care database in Nuuk and Aasiaat; of baseline prevalence and quality of care in all Greenland; from interviews with physicians and other health care workers in Greenland and Denmark concerning barriers in delivering diabetes care, and; a review of initiatives taken elsewhere in the world to improve diabetes care in sparsely populated settings. Several lessons were learned from this analysis.
In the beginning of 2008, the prevalence of diagnosed diabetes in Greenland was low, around 2.2% among adults aged 40 or above [Citation171]. This confirmed a need for increased detection of new cases as reported earlier [Citation10]. The quality of diabetes care compared to the rest of Greenland was much better in the clinics in Nuuk and Aasiaat, where a database was used as a quality monitoring tool [Citation171,Citation180]. The quality of care was estimated for 140 patients who had T2D and were affiliated with one of the two clinics using this quality database and these estimations were used and then compared to 245 patients with T2D affiliated with 1 of the 10 other clinics not using the database. Annual screening rates of HbA1c, blood pressure, and blood lipids were around 95% among patients affiliated with clinics using a quality database compared to lower than 70% among the remaining patients [Citation171]. Also, screening for microalbuminuria within the last 2 years was performed much more often in the clinics using the database compared to the other clinics without a database, 76% versus 24% [Citation171]. Only screening for retinopathy within 2 years was performed less well in the clinics with a database compared to the rest, 32% and 48% [Citation171]. However, this was a result of delayed response regarding results of retinal photography from the ophthalmologist rather than a difference in the quality of care [Citation171]. The clinical overview of important health care factors facilitated delivery of health care information such as that dealing with smoking habits, weight, height, body mass index (BMI), glycosylated haemoglobin (HbA1c), blood pressure, level of cholesterol, screening for retinopathy, nephropathy and neuropathy provided by the quality database in each consultation. In addition, the database provided an overview of the quality of care among all patients in the clinic. However, using a database outside the normal EMR represented a double registration that preferably should be avoided [Citation171]. Similarly, a diabetes register was also part of the diabetes program used in Alaska, where diabetes care improved from 1994 to 2004 [Citation73,Citation74]. The higher quality of care observed in clinics with databases compared to usual care in clinics without a database in Greenland was also in line with information from the international literature at that time. Thus, a comprehensive review, which included 41 controlled trials, documented that multifaceted interventions targeting health care professionals and the structure of health care organisation were effective in improving the quality of care in primary health care settings [Citation181]. This has been further documented in the recent review mentioned previously in the background section [Citation166]. Efforts to improve diabetes care in rural communities should focus on interventions with multiple strategies targeting clinicians and health care systems, rather than on traditional patient-oriented interventions [Citation166].
The initial analysis of the quality of diabetes care in Greenland made it evident that a possibility to analyse HbA1c and test for microalbuminuria in health care settings outside Nuuk was desirable as an alternative to sending blood and urine samples to the central laboratory at Queen Ingrid’s Hospital. Use of point-of-care devices has been used to improve the quality of care in other sparsely populated settings having limited access to central laboratories [Citation182].
No clinical guidelines were available to adapt to Greenlandic conditions, neither was there information about diabetes in Greenlandic. Also, the diagnostic setup represented a challenge. The recommended use of fasting venous plasma glucose as a diagnostic tool [Citation83] was not available in Greenland. Instead, capillary whole blood (CWB) glucose was used, but the reference of normal values in the lab card was below 6.6mmol/l and not below 6.1 mmol/l as recommended internationally [Citation83]. Also, oral glucose tolerance tests were sparsely used. Continuity in diabetes care was challenging to deliver because of short-term physicians and other health care workers in the many health care settings outside Nuuk. Other diabetes programmes applied in remote areas with Indigenous people were investigated to help overcome some of these challenges and to get inspiration. The Special Diabetes Programme for Alaskan Natives initiated in 1994 in Alaska, comprised elements and addressed challenges similar to those observed in Greenland. Improved diabetes care was observed among patients with diabetes in rural Alaska within the first 10 years after the implementation of the programme. As mentioned earlier, the program was based on an enhanced health care infrastructure, a national diabetes register, standardised guidelines for care, and annual evaluation of and feedback from clinics [Citation74]. Also, a unified EMR and free health care service including medicine were considered important factors [Citation74].
To sum up, the initial analysis confirmed a low prevalence of diagnosed diabetes in Greenland. The quality of diabetes care was higher in clinics in Nuuk and Aasiaat, compared to usual care in the remaining clinics in Greenland. Technical and other obstacles were identified concerning delivering high-quality diabetes care including the lack of possibilities to measure HbA1c and U-ACR outside of Nuuk, misleading values for normal CWB glucose concentrations in the lab card, the lack of continuity in diabetes care with many physicians holding only short-term positions, and, a lack of local health professionals dedicated to diabetes care. Lack of national guidelines for diabetes care and patient information in Greenlandic was also observed as a problem. It was concluded that a diabetes programme in Greenland should be multifaceted including initiatives targeting both the organisation of diabetes care, health care professionals, and patients with diabetes. Furthermore, it was evident that a diabetes register or quality database providing a possibility to monitor diabetes care was essential in a diabetes programme, preferably integrated in the EMR to avoid double registration procedures. Specific issues obviously needed special attention including possibilities to test for HbA1c, microalbuminuria, retinopathy and neuropathy outside of Nuuk, and to increase awareness of diagnostic criteria for diabetes.
A new diabetes care concept based on integrated health research continued monitoring analysis and adjustment
The initial analysis was followed by an innovation period and the implementation of a new diabetes concept begun in the autumn of 2008, with a diabetes course for health care professionals from 17 different health care localities in Greenland. The evaluation of the implemented initiatives then a re-analysis followed by adjustments of the initiatives were considered important processes from the beginning. Continued monitoring and health care research within the health care system was thus given high priority as a part of the diabetes concept. The model represented a new concept in planning and monitoring diabetes care in Greenland both on local and national levels. The model is illustrated in . The principle idea of having the model include analysis, innovation, implementation, and evaluation may not be new itself. However, the continued use of health research in order to continuously address clinical challenges had not before been done in Greenland and the feasibility had not been tested.
Figure 2. The Greenlandic model based on continued monitoring, analysis and adjustment of initiatives taken to improve diabetes care.
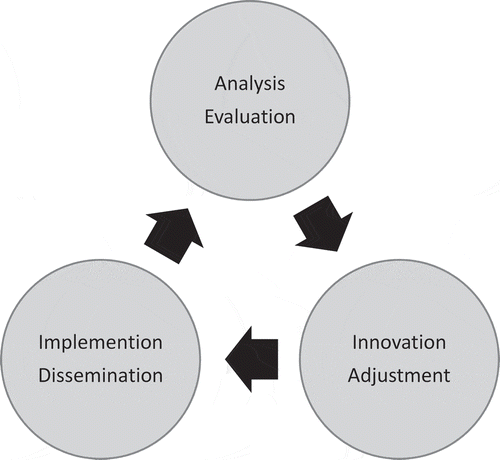
The diabetes care concept included the development of a special diabetes profile integrated in the EMR providing an overview at three different levels. The overview was provided in the clinical consultation with each patient thus facilitating the clinical decision-making process. Based on the statistical module within the EMR, the quality of diabetes care could be assessed and monitored continuously in each town as well as on a national level. Measurements of quality of care indicators were introduced, and annual feedback from the diabetes group to the clinics was established. Local, key health care professionals with responsibility for following up on patients were educated with annual courses on diabetes.
National guidelines were developed and continuously updated and gradually simplified. The guidelines were based on Danish and internationally accepted guidelines from the American Diabetes Association (ADA), European Association for the Study of Diabetes (EADS), European Society of Cardiology (ESC), and The Danish College of General Practitioners [Citation183–Citation186]. Patient information in Greenlandic and Danish was developed and printed. Movies with patient information focusing on special topics like insulin treatment were produced and distributed throughout the health care system.
Local health care clinics were equipped with a DCA Vantage® Analyser (Siemens Healthcare Diagnostics) [Citation187], allowing local point-of-care testing of HbA1c and U-ACR. Acceptable precision and accuracy have been demonstrated for point-of-care devices when using DCA Vantage® Analyser, Axis-Shield AfinionTM AS100 Analyser (Alere, Holstebro, Denmark), and HemoCue® Glucose 201+ System (AB, Ängelholm, Sweden) [Citation182,Citation187–Citation192] as compared to utilising central laboratory services.
Point-of-care testing has also been the cornerstone in diabetes care among different Indigenous peoples such as the Australian Aboriginal and Torres Strait Islander peoples living in remote areas [Citation182]. However, point-of-care testing devices are not recommended for diagnosis of T2D [Citation193] and they were thus used mainly to monitor the treatment. Although inferior to central laboratories, the point-of-care devices provide good, reliable testing opportunities that can be performed locally with the potential to increase the number of patients tested.
To sum up, a new model of the diabetes programme was designed to include continued monitoring, analysis, and adjustment to initiatives taken to improve diabetes care. The initial analysis was used to design a multifaceted initiative targeting (1) the organisation of diabetes care including the establishment of a diabetes group bearing national responsibility for diabetes care, the education of local, key diabetes health care professionals, providing technical solutions including point-of-care devices and other tools to increase the feasibility of providing diabetes care and guidelines of care, (2) health care professionals in a program including providing national guidelines in accordance with international recommendations, annual courses in diabetes, performance feedback, and (3) patients including educational initiatives to provide information in Greenlandic and Danish. Further actions to increase detection of new cases, to facilitate the screening of microvascular complications, to improve the management of gestational diabetes, and to promote advocacy for diabetes are described in the following sections.
Screening for microvascular complications
Screening for retinopathy used to be performed by travelling ophthalmologists from Denmark using direct ophthalmoscopy. This method is inferior to retinal fundus photography [Citation194]. In 2007, a retinal fundus camera was installed in Nuuk. Initially, it was used by the travelling ophthalmologist. Later on, the nurse in the diabetes group was educated in the procedures of use and has since 2008, performed the retinal fundus photography on patients in Nuuk. The analysis of the photo was performed by an ophthalmologist in Denmark. For years the retinal fundus photography was performed on dilated pupils. In 2015, a Daytona Fundus Camera from Optos® [Citation195] facilitated non-mydriatic wide-field digital photograph of the fundus and replaced the former camera.
In addition, Daytona Optos® cameras were installed in nine towns and key, local diabetes staff were trained in the procedure, making fundus screening available in these locations. Fundus photography was, however, still prescribed by an ophthalmologist in Denmark. Patients in need of this treatment for retinopathy were referred to an ophthalmologist and treated in Greenland or Denmark.
Prior to the diabetes project screening for microalbuminuria and nephropathy used to be performed on a 24-hour urine sample, which could only be performed at the central laboratory at Queen Ingrid´s Hospital in Nuuk. However, testing of microalbuminuria can also be measured by the albumin to creatinine ratio (UACR) in a random spot urine sample [Citation196]. This method was part of standard diabetes care as recommend by ADA [Citation196,Citation197]. This method was much more feasible for both patient and health care professionals and provides sufficient accuracy [Citation196]. Therefore, this method was introduced as the preferred test of microalbuminuria in Greenland at the beginning of 2008. The examinations of UACR on spot urine samples were performed on the Architect c8000 Clinical Chemistry Analyser® [Citation198] at the central laboratory at Queen Ingrid´s Hospital in Nuuk. Outside of Nuuk the collected urine samples were either sent to Nuuk for analysis or analysed locally using the DCA Vantage® Analyser. A guideline was established so that elevated values of 2.5mg/mmol or above should be confirmed by examining new sterile urine. If UACR was measured above 25mg/mmol or above, a 24-hour sterile urine sample should be examined for content of protein.
All analyses of venous blood were accompanied by testing HbA1c, cholesterol, alanine transaminase, creatinine, sodium, potassium, cobalamin concentrations using an Architect 8000 Analyser® [Citation198] at the central laboratory at Queen Ingrid´s Hospital in Nuuk. The laboratory is a member of the Danish quality system for laboratories (DEKS).
Screening for neuropathy was initially performed by the chiropodist from the diabetes group while travelling around in Greenland. She tested foot sensation modalities including pressure (10 g monofilament), thermal (using Tip Therm®), and vibration (using Biothesiometer, Rova Company®). A threshold of 25 mV was used to define the presence of neuropathy. A threshold of 24.5 mV has been documented to provide diagnostic accuracy to detect distal neuropathy (area under a receiver operating characteristic (ROC) curve at 0.81) including sensitivity (82%) and specificity (70%) [Citation199]. All clinics were equipped with 10 gram monofilaments to facilitate the possibility of identifying feet at risk for ulceration at local clinics [Citation197]. In time these procedures were gradually supplemented with biothesiometers in all towns, allowing local, key health care professionals to perform an annual assessment of distal neuropathy as is internationally recommended [Citation197].
In addition to the testing of sensation, a foot examination included gathering a careful history, inspection of the feet and shoes, and assessment of blood pulse [Citation197]. This was in an ideal environment performed by a trained chiropodist. However, only one chiropodist could be included in the diabetes group. Thus, local, key health professionals were also trained to make a basic foot examination. This might not be as good as that performed by a chiropodist, but surely better than no screening at all for neuropathy. Awareness and education of people with diabetes about the need to look after their feet have been reported to improve people’s foot care knowledge and behaviour [Citation200]. Furthermore, the education of local health care professionals to included foot examination in their diabetes care may increase their own awareness of foot problems among patients with diabetes. This combined strategy targeting both health care professionals and patients may be more effective in preventing foot problems and ulcers than a single strategy although this has not yet been evaluated in high-quality studies [Citation201].
No single test was used to screen for macrovascular complications. Instead, risk factors were evaluated and recorded annually including smoking status, blood pressure, cholesterol level, any history of premature death in the family, and any presence of microalbuminuria [Citation202]. The aim was to manage the risk factors according to national guidelines.
In conclusion, the strategy for screening of microvascular complications was changed to increase quality and the feasibility of implementation. Retinal photography was introduced in Nuuk and gradually established in clinics in larger towns, replacing the inferior direct ophthalmoscopy. Testing for microalbuminuria and nephropathy was implemented with measurements of UACR on a spot urine sample rather than using the more cumbersome 24-hour urine sample test. Furthermore, the feasibility of using point-of-care testing was proven, making screening possible in settings outside of Nuuk. Finally, increased focus on screening of neuropathy was initiated by the travelling chiropodist. Later, the foot examination including use of biothesiometers and monofilaments became part of the local, key health care professional´s skill set. Thus, the possibilities of improved screening for microvascular complications were introduced.
Actions to increase the detection of undiagnosed diabetes in patients
The strategy for testing for diabetes was developed based on case-finding among patients whom had themselves sought out the primary health care system. Awareness of risk factors for diabetes such as family history of diabetes, age, physical inactivity, overweight, hypertension and dyslipidaemia was already highlighted in the national guidelines. Also, the presence of or symptoms related to cardiovascular disease, neuropathies, and recurrent infections were mentioned as indications for performing diabetes testing.
To ensure detection of new diabetes cases the normal values for fasting capillary whole blood glucose concentration in the lab card was changed in 2008, from below 6.6mmol/l to below 6.1mmol/l and thereby aligned in accordance with the international accepted values according to WHO [Citation83]. In addition, an oral glucose tolerance test (OGTT) was recommended in cases of fasting capillary whole blood concentration at 5.6 or up to 6.0mmol/l. A 2 hour whole blood glucose concentration at 11.1 mmol/l or above was considered to be diagnostic evidence of diabetes [Citation83].
In January 2010, the American Diabetes Association published new diagnostic guidelines allowing diabetes to be diagnosed based on the measurement of HbA1c. Thus, a confirmed value at or above 48 mmol/mol (6.5%) was included as a diagnostic criteria in Greenland. Measuring HbA1c can be done on a non-fasting individual and represents a more feasible diagnostic feature that potentially could decrease the number of undetected cases of diabetes in Greenland. In addition, the lack of the possibility to use venous plasma glucose as well as the use of the less valid whole blood capillary glucose concentration made it clear that the time had come to change diagnostic strategy. By June 2010, measuring of HbA1c was therefore introduced as a supplementary diagnostic tool in Greenland.
At the same time, a Tosoh G8 HPLC Analyser [Citation203] was installed at the central laboratory at Queen Ingrid’s Hospital in order to analyse Hba1c levels in venous blood, chosen because this method had been reported to be the state of art for testing [Citation203].
In summary, clarification and implementation of the diagnostic criteria for diabetes treatment were initiated in 2008. In 2010, HbA1c was introduced as a supplementary diagnostic tool facilitating the detection of new cases in line with international recommendations.
Actions taken to address gestational diabetes in Greenland
GDM is a responsibility of the department of Gynaecology and Obstetrics at Queen Ingrid’s Hospital. Guidelines for testing and diagnosis of GDM existed as part of the national perinatal guidelines. Testing for GDM was risk factor based. Thus, pregnant women were offered tests of GDM, if they had a history of former GDM, former birth of a child with birth weight above 4,500 g, diabetes among first degree relatives or grandparents, pre-pregnancy BMI above 27 kg/m2, and in the event of a positive urine glucose dip-stick test. The diagnosis was based on the result of a 75 g OGTT performed on fasting women. CWB glucose concentration was examined before and 2 hours after the administration for glucose using a portable Hemocue Glucose 201® System (AB, Ängelholm, Sweden). The test was performed early in pregnancy (gestational week 18) on women with two or more risk factors or on women with a history of former GDM. If the first OGGT was normal, a re-test was performed in late pregnancy (gestational week 28). For women with one risk factor, OGTT was only offered late in pregnancy (gestational week 28). However, the diagnostic cut-off used in Greenland at that time was 2 hours CWB glucose concentration at 11.1mmol/l or above. This was much higher than defined by the WHO 1999 guidelines [Citation83]. The WHO 1999 diagnostic cut-off values for GDM were fasting CWB glucose 6.1mmol/l or above or 2 hours CWB glucose at 7.8mmol/l or above. However, no worldwide consensus existed concerning testing strategy and cut-off values [Citation99]. In 2010, a new guideline for GDM was introduced based on the criteria used in Denmark. However, testing for GDM continued to be selective and risk factor-based using the same criteria as in Denmark at that time, i.e. history of former GDM, former birth of a child with birth weight above 4,500 g, diabetes among first degree relatives or grandparents, pre-pregnancy BMI above 27 kg/m2, and in the case of a positive urine glucose dip-stick test. Only 2 hour measurements of glucose were included and not fasting CWB glucose concentration. Thus, 2 hour glucose concentrations at or above 9.0mmol/l were chosen as a new diagnostic cut-off value according to the clinical guidelines from the Danish Society of Obstetrics and Gynaecology [Citation171]. The Danish criteria were chosen because Denmark represents the major (and nearest) co-operator for the health care system in Greenland. The Danish cut-off was at the time higher than most international recommendations. In 2010, International Association of Diabetes and Pregnancy Study Groups (IADPSG) lanced cut-off values for venous plasma glucose concentrations at or above at 5.1mmol/l (fasting), 10.0mmol/l (1 hour), and 8.5mmol/l (2 hour) after 75 g OGTT [Citation101]. The same criteria was adapted by WHO in 2013 replacing the former 1999 criteria [Citation204]. In 2015, in the UK, cut-off values were introduced by the National Institute of Health and Excellence (NICE, UK) for venous plasma glucose concentrations at or above 5.6mmol/l (fasting) or 7.8mmol/l (2 hours) after 75 g OGTT [Citation205]. Also in Denmark, the cut-off value (9.0mmol/l, 2 hours after OGTT) was based on venous plasma glucose measurements. In Greenland by contrast CWB glucose concentrations were used assuming an acceptable agreement between glucose measured in capillary whole blood and venous plasma [Citation206]. Thus, diagnosis of GDM based on CWB analysis using handheld glucometers, for example the Hemocue Glucose 201® System, has been accepted by the International Federation of Gynaecology and Obstetrics (FIGO) in remote settings where laboratory support is limited [Citation207]. The high 2 hours cut-off value, excluding fasting glucose concentrations, and the use of selective risk factors based screening, rather than universal screening as in Denmark, obviously, would result in a less sensitive diagnostic setup in Greenland compared to many other countries. On the other hand, no solid information about GDM in Greenland was available at that time to support the decision. Choosing a diagnostic testing strategy and cut-off values had to be a delicately balanced decision and had to include both possible health effects on pregnancy outcome (with the possible negative effects coming from pregnant women being labelled “sick” with diabetes) and the capacity limits of treatment within the health care system. According to FIGO, national guidelines and management should be in accordance with available national resources and infrastructure even if the specific diagnostic and treatment strategies are not supported by high-quality evidence [Citation207]. In 2010, a workshop GDM was arranged by the diabetes group in Nuuk in order to increase awareness of GDM in Greenland and to optimise treatment. Participants included Danish experts, local obstetrics, midwives, paediatric and internal medicine physicians, and other key persons. In 2014, the guideline was further updated to include screening of all pregnant women for overt diabetes in the first trimester using HbA1c as a screening tool as was recommended internationally [Citation101,Citation208]. The treatment guideline was further clarified. Women diagnosed with GDM were offered lifestyle intervention including dietary and exercise counselling, monitoring of blood glucose using Freestyle Lite® (Abbott Laboratories A/S, Copenhagen, DK) and exercise monitoring pedometers, and insulin in order to reduce perinatal morbidity if indicated [Citation97,Citation209]. As a follow-up to the workshop, information in Greenlandic was offered to patients with GDM. Still, only 2 hours CWG glucose at or above 9.0mmol/l was considered a diagnosis of GDM using the same cut-off levels as in Denmark.
In conclusion, no written information about GDM existed in Greenland in 2008. To increase awareness of GDM, health care guidelines were updated in 2010 and 2014 including information in Greenlandic, which targeted women diagnosed with GDM. The screening strategy and cut-off values were chosen based on the Danish guidelines accepting a lower sensitivity than in some other settings awaiting further data on GDM in Greenland.
Expansion of the diabetes project with a national lifestyle initiative
In 2011, the diabetes project was replaced by a lifestyle initiative. In addition to diabetes care, the scope was also to improve the management of hypertension and chronic obstructive lung disease in all Greenland. The diabetes group was changed to a lifestyle group and the physician became the head of the lifestyle initiative. The research-based approach with continued monitoring, evaluation, analysis and adjustment of the initiatives taken, used in the diabetes project, was adapted in the new lifestyle concept. Approximately, at the same time DCA vantageTM (Siemens Healthcare Diagnostics) was replaced in the local clinics with Axis-Shield AffinonTM AS100 Analyser (Alere, Holstebro, Denmark) that in addition to HbA1c and U-ACR also could analyse CRP (C-reactive protein). In order to facilitate home blood pressure measurements, all clinics were equipped with a large number of automatically blood pressure machines. Out-of-office monitoring of blood pressure has been recommended in both American and European guidelines for diagnosis and management of hypertension using a lower cut-off for the diagnosis of hypertension (BP above 135/85 mmHg) compared to in-office blood pressure measurements (BP above 140/90 mmHg) [Citation210,Citation211].
Also, the number of spirometers was increased in order to facilitate the performance of lung function test locally. National guidelines for hypertension and chronic obstructive lung disease were developed in addition to a lifestyle book about chronic obstructive lung disease in Danish and Greenlandic, including information about lifestyle factors such as smoking, physical activity, diet, and lifestyle-related diseases such as diabetes, hypertension and chronic obstructive lung disease.
As a consequence of the replacement of the diabetes project with the lifestyle initiative, the diabetes profile within the EMR was expanded to be a lifestyle profile also containing information on lung function and other relevant parameters relevant to chronic obstructive lung disease. Also, the standard lifestyle blood profile lab requisition was gradually extended in to include HbA1c, lipid profile, cobalamin, creatinine, estimated glomerular filtration rate (eGFR), sodium, potassium, and alanine aminotransferase. Electrocardiography (ECG) and urine examinations were still on separate lab requisitions.
In 2015, implementation of the new united EMR for the whole health care in Greenland was started at Queen Ingrid Health Care Centre in Nuuk, and the lifestyle profile in the former system was replaced by a lifestyle table in the new system. It contains information about general lifestyle factor such as smoking, physical activity, weight, height, BMI, abdominal circumference, blood pressure, diabetes parameters like duration of diabetes, HbA1c, UACR, result of screening for neuropathy and retinopathy, obstructive lung disease such as duration and stratification (Global Initiative for Chronic Obstructive Lung Disease, GOLD), lung function, saturation, and heart failure like classification (New York Heart Association, NYHA), and ejection fraction measured by echocardiography [Citation212,Citation213]. Statistical extraction of data from the new EMR was still under development. However, since 2016, parameters registered in the lifestyle table could be extracted allowing the former monitoring of diabetes and other lifestyle factors to be continued.
Baseline analysis and evaluation of other conditions than diabetes have included health research too [Citation177,Citation214–Citation216]. However, more detailed description of this was considered beyond the scope of this thesis.
In summary, the diabetes group was expanded to a lifestyle group in 2011. In addition to maintain focus on diabetes care, the scope of the new initiative was extended to cover management of hypertension and chronic obstructive lung disease too. The model was sustained with continued analysis, monitoring and adjustment of the management of diabetes, but the perspective was extended with initiating an increased focus on measuring blood pressure and lung functions. In 2015, the former EMR used in the primary health care system was replaced by a new united EMR in Nuuk. A lifestyle table was integrated in the new EMR allowing monitoring of diabetes care to be continued, although data could not be extracted initially.
Advocacy and additional initiatives to increase awareness of diabetes
The original diabetes group, and now the life style group, have provided awareness of diabetes in different settings including the health care system, the general population, politicians, and scientific settings. These initiatives have been described in more detail elsewhere [Citation9]. In brief, annual teaching and information have been provided for healthcare professionals. Information about diabetes and prevention has been provided in different settings including primary school, high school, at university and other in public and private institutions and companies. Presentation of the new diabetes care initiative in Greenland has been done in several workshops, political and scientific meetings including West Nordic Minister Meeting, Iceland Greenlandic Science Days, Expert Meeting on Indigenous Peoples, Diabetes and Development, and on NunaMed conferences held every third year in Nuuk. Several public lectures have been given within the context of Society of Greenlandic Medicine. Publicly available papers have been published in Nakorsanut (a journal published by the local organisation of physicians in Greenland). The diabetes group and the lifestyle group have participated in many interviews about diabetes and lifestyle in radio, television and newspaper. Annual health care events like national working initiatives (Pisutta), culture night with lifestyle events, and so called monthly lifestyle cafes giving the public access to lifestyle intervention and diabetes screening at local health care clinics have been performed.
Study populations and variables
The study populations included in this thesis were recruited for observational cross-sectional designs based on information available in the EMR [Citation179,Citation217–Citation223]. This method had advantages as well as limitations.
The major strength of the study was that the whole population in Greenland or selected groups, like all Nuuk inhabitants, could be included in the study minimising the risk of selection bias. Also, the data used in the study could be withdrawn from the EMR. Thus, no participants had to be examined or put at any risk of harm. Furthermore, the study evaluated initiatives in the actual clinical setting in which the results should be interpreted and disseminated. This provided realistic data compared to an idealised research setting as an RCT. The connection between initiatives taken and outcome measured influenced the implementation process positively. On the other hand, limitations existed and interpretation of the results must be done with some reservations. Due to the observational, uncontrolled design, no certain causality between initiatives taken and the outcome can be claimed. However, the task of the diabetes group was to increase awareness and quality of management in all Greenland. A standard randomised trial including only parts of Greenland was not an option. The evaluation of initiatives taken was performed as repeated before-and-after analyses providing indications of effect. Also, data from the EMR could have been misleading. Although standards for clinical measurement existed in the guidelines, health care workers may practice this differently making a comparison of data problematic. However, this effect was more likely to occur randomly than in a systematic way, and the risk of misinterpretation was considered small. Also, the majority of data included in this study represented quite simple measurements of registrations as, for example if blood pressure had been measured or not. Thus, the interpersonal variability in measurements was considered a minor problem in the interpretation of the results.
In conclusion, the observational design was used in this thesis. An RCT was not estimated to be feasible. However, the repeated measurements before-and-after initiatives taken provided indications of effects that could be used to adjust strategies in the actually clinical setting.
Contact with health care system in Greenland 2010–2011
A statistical extraction from the EMR was performed in order to estimate and compare the age-specific proportion of males and females, who had been in contact with the primary health care system within 1 year. Thus, all persons were identified who had been in contact with the primary health care system from 1 June 2010 to 31 May 2011. The population in Greenland as of 1 January 2011 available from Greenland Statistics [Citation224] was used as a base, background population.
The major strength of this study was that the whole population in Greenland was included minimising the risk of selection bias. In addition, the data could be extracted electronically reducing the risk of manual errors. On the other hand, the contact could only be included if the health care professionals had recorded the contact in the EMR. Failure to include a contact in EMR would result in an underestimation of the proportion of patients with contact to the health care system. However, all contacts to the health care system had to be recorded in the EMR by law, and the risk of this underestimation was regarded of less importance. The outcome was only dichotomised in patients with or without contact with the health care system, which is another limitation of this study. Thus, the number of contacts per person could not be estimated. However, the main scope was to estimate the proportion of persons in Greenland in contact with the health care system. This information could contribute to the decision about which testing strategy should implemented to increase the detection of undiagnosed diabetes in Greenland.
In brief, the inclusion of all Greenlanders in Greenland, the electronically gathered and withdrawn data, and the validation of the extracted data represented a major strength in this study that insured a reliable conclusion.
Diagnostic activity from 2010 to 2012 and from 2014 to 2015
Diagnostic activity measured as the proportion of the population tested for diabetes using HbA1c as a diagnostic tool was estimated in two observational periods. The first period of observation was from 1 June 2010 to 31 August 2012 (2 years and 3 months). The first observational period started on the day where HbA1c was introduced as a diagnostic tool in Greenland. A subsample of inhabitants in Greenland at or above 35 years was identified. Thus, only adults born on the first day of a month were included in the subsample. The medical record was reviewed in order to check if they had been tested with HbA1c within the observation period. The proportion of the population tested in the observation was calculated using the population in Greenland as of 1 January 2012 as the background population. The second observational period was from 1 January 2014 to 31 December 2015 (2 years). Thus, all persons who had been tested at least once with an HbA1c measurement during the observation period were identified and the age and gender-specific proportion of the population tested was estimated using the population of Greenland as of 1 January 2015 as the background population. Only, HbA1c tests were included, when they were performed at the Tosoh G8 HPLC analyser at the central laboratory at Queen Ingrid Hospital, whereas HbA1c measured locally on point-of-care devices were not included.
The major strength in these studies was that the diagnostic activity was estimated in all Greenland. The first analysis was performed on a quite large representative sample of adults aged 35 years or above (around 1000 persons or more than 3% of all adults aged 35 years or above). The second sample included all persons tested. Another strength indicated in the second sample was that the data could be extracted electronically from the laboratory card. However, limitations exist. The diagnostic activity estimated in this study only described diagnostic activity using HbA1c. Thus, no information was included about fasting glucose measurements or OGTT. This would tend to underestimate the diagnostic activity. However, the size of this underestimation was considered of minor significance due to the superior feasibility of HbA1c testing compared to fasting blood glucose measurements or OGTT [Citation225]. Also, the scope of the analysis was mainly to evaluate the implementation of the new diagnostic method (HbA1c). On the other hand, some persons already diagnosed with diabetes were tested with HbA1c measurement to monitor their disease rather than as a diagnostic test. This resulted in an overestimation of the diagnostic activity. However, the prevalence of diagnosed diabetes was still low and this overestimation was considered of minor significance due to the low prevalence of diagnosed diabetes. Finally, the two samplings were not performed in the same way. The first sample included only a subsample whereas the whole population was examined in the second sample. This represented a limitation in the comparison of the two estimates. However, the subsample was performed as a relatively large and representative sample. Thus, the estimate was considered reliable and the comparison relevant.
In conclusion, limitations exist including both overestimation and underestimation. However, the size of both under- and overestimation was considered of minor significance. The estimated diagnostic activity was regarded as representative for the whole population in both samples and consequently comparable with each other.
Prevalence of diagnosed diabetes from 2008 to 2017
The prevalence of diagnosed diabetes was estimated five times during the last 10 years using different methods, in 2008, 2010, 2012, 2014 and 2017 [Citation218,Citation219,Citation221]. In 2008, all health care districts in Greenland were asked to make a list of patients diagnosed with diabetes – a manual registration [Citation218]. In 2010, 2012 and 2014 all patients diagnosed with diabetes were identified electronically by extracting patients coded with a “D” (diabetic) in the former EMR [Citation218,Citation219,Citation221]. In 2017, all patients diagnosed T89 (T1D) or T90 (TD2) according to International Classification of Primary Health [Citation226] were identified through a statistical extraction from the new EMR (this being data not published previously).
Estimates of prevalence
The age and gender-specific prevalence of diabetes were estimated using the population in Greenland as of 1 January in the respective years as the background population. In addition, the prevalence among Greenlanders and non-Greenlanders was estimated in the 2014 sample. Patients born in Greenland were considered Greenlanders while patients born outside Greenland were considered non-Greenlanders.
In 2008 and 2010, the prevalence was estimated for adults aged 20 or above [Citation218]. In the following samples, the prevalence was also estimated for the age group 20–79 years old, a standard used to compare the prevalence of diabetes across the globe by the International Diabetes Federation [Citation28]. Both patients with T1D and T2D were included in the estimations included in this thesis in accordance with the International Diabetes Federation estimates [Citation28]. Thus, the initial prevalence of T2D prevalence estimated in 2008 and 2010 [Citation218] has been recalculated by including patients with T1D.
Strength and limitations
The major strength of these analysis was the electronically identification of persons diagnosed with diabetes so that the prevalence could be estimated among the whole population of Greenland. Only in 2008, the data was collected manually and only 90% of the population could be included due to lack of information from two minor clinics. Obviously, a risk of missing patients diagnosed with diabetes exited in the 2008 sample since it relied on local health care professionals’ knowledge about patients diagnosed with diabetes. Thus, 78 persons diagnosed with diabetes were identified for the first time in the 2010 sample despite having been diagnosed prior to 2008, underlining the strength in the electronically withdrawn data. This means that the estimated prevalence was underestimated for 2008. However, underestimation of the prevalence was also a risk in the subsequent samples. Thus, some patients may actually have been diagnosed with diabetes, but not coded with a “D” in the former EMR or with a diabetes diagnosis (T89 or T90) in the new EMR. However, the diabetes/lifestyle group validated the extraction persons by cross-checking persons annually using prescription of anti-diabetic medicine, diabetes code, and lab card information about HbA1c to counteract this possible lack of registration. On the other hand, some patients may wrongly have been coded with a diabetes diagnose without having diabetes. This would tend to overestimate the prevalence of diagnosed diabetes. However, this failure was seldom observed. It was corrected and identified using review annually of patients with a diabetes diagnosis.
In conclusion, the prevalence of diagnosed diabetes could be estimated electronically, with the exception of the first sample performed in 2008, ensuring reliable and comparable estimations of the prevalence of diagnosed diabetes in Greenland.
Quality of diabetes care from 2008 to 2017
Quality of diabetes care was estimated in 2008, 2010, 2014, and 2017 [Citation218,Citation221]. No estimation of the quality of care was included in the 2012 analysis that primarily focused on diagnostic activity.
Information about the most recent measure of blood pressure, HbA1c, blood lipids, and the last screening for retinopathy, neuropathy, and microalbuminuria was provided by reviewing medical records 2 years back in time among all patients diagnosed with diabetes [Citation218,Citation221]. In 2008 and 2010, only patients with T2D were included in the analysis, whereas both T1D and T2D patients were included in the analysis in 2014 and 2017, since both T1D and TD2 patients need regular diabetes care. Thus, patients diagnosed at the age of 29 years old or below treated with insulin within the first half year of diagnosis were considered having T1D (22 in the 2008 sample and 30 in the 2010 sample) and were excluded in the quality of care analysis in 2008 and 2010 [Citation218].
Thus, the population in 2008 and 2010 differed slightly from the population in 2014 and 2017. However, the number of patients with T1D was less than 5% in all samples and the impact on the overall health care indicators limited. Also, the classification of patients as having T1D or T2D was not always obvious and the risk of misclassification existed in the clinical setting. Therefore, in order to simplify the monitoring process the combined quality of care was preferred in the later studies.
Quality of care indicators
Quality of diabetes care was described using the selected process of care and proximal outcome indicators proposed by the Organisation of Economic Cooperation and Development (OECD) including annual testing of HbA1c, LDL, retinopathy, microalbuminuria, HbA1c control (HbA1c < 53 mmol/mol), and LDL control (LDL < 3.5 mmol/l) [Citation159]. Furthermore, annual testing of blood pressure, blood pressure control (BP<140 mmHg), and poor metabolic control (HbA1c > 75 mmol/mol) as proposed by the National Diabetes Quality Improvement Alliance (NDQIA) was included [Citation227]. In addition, in reporting annual testing rate for retinopathy, microalbuminuria, and LDL cholesterol, also 2 year testing rates were used as in the Danish National Indicator Project Diabetes [Citation66]. The OECD and NDQIA indictors were selected in the present study since they have been used to compare diabetes care internationally [Citation161]. The selected Danish indicators were included, because 2 year testing rates add important information in some cases. Testing for retinopathy was only offered every second year in Greenland, unless signs of retinopathy were present or in cases were HbA1c or blood pressure was poorly regulated in accordance with guidelines from the ADA [Citation228]. Also, measuring LDL cholesterol annually in all patients with diabetes may not be an essential part of diabetes care. According to recent ADA recommendations, it is reasonable to obtain a lipid profile at the time of diagnosis of diabetes and at least every 5 years thereafter. Also, lipid testing should also be obtained before initiating statin therapy. Furthermore, once a patient was taking a statin, testing for LDL cholesterol may be considered on an individual basis [Citation202]. Thus, the 2 year rather than 1 year proportion of patients tested for LDL may provide more useful information on diabetes care. Proximal outcome indicators were calculated as the proportions of patients fulfiling the control criteria (Blood pressure < 140/90 mmHg, HbA1c<53mmol/mol) among all patients tested.
In addition to annual testing rates of HbA1c, blood pressure, and LDL cholesterol, also 2 year testing rates were included in 2017, to provide a more detailed picture for these parameters.
Choice of indicators
The choice of proximal indicators represented dilemmas. A target level of HbA1c below 53mmol/mol for most patients with diabetes was in accordance with internationally accepted guidelines from the American Diabetes Association (ADA), International Diabetes Federation (IDF), and European Association for Study of Diabetes (EASD) and thus not problematic [Citation183,Citation229,Citation230]. On the other hand, the target level of blood pressure control has been debated. A decade ago, a target level of 130/80 mmHg was internationally accepted. However, recently a target level of diastolic blood pressure below 80 mmHg and systolic blood pressure below 130–140 mmHg was reported by International Diabetes Federation. The most recent American recommendation reported a target level below 140/90 mmHg for most patients in accordance with the included indicator in this study [Citation183,Citation230]. Also, the OECD indicator for LDL control (LDL below 3.5 mmol/l) represented a challenge since the target for LDL varies internationally [Citation230]. A target level of LDL below 2.5 mmol/l for most patients with T2D and below 1.8 mmol/mol for high-risk patients has been recommended [Citation230]. Different target levels could have been chosen. However, in this thesis the possibility of using an accepted OECD indictor designed for comparison internationally was weighted the most.
Limitations
No OECD distal outcome indicators including lower extremity amputations rates, kidney disease in persons with diabetes, and cardiovascular mortality in persons with diabetes were included in the present study due to lack of data. Furthermore, no patient reported, outcome data was available. Thus, not all parts of diabetes care could be evaluated in this study.
Another limitation in the present study was the overestimation of the quality of diabetes care in 2008, making it a suboptimal baseline. The formerly mentioned 78 persons identified in 2010, although diagnosed before 2008, were not followed regularly with a process of care applied indicators from 0% to 22% in 2008 [Citation171]. These persons were not included in the quality assessment for 2008, and consequently, the quality of care was overestimated. Furthermore, the estimated national 2008 diabetes quality represented a mix of very high level of quality performance (testing rates above 90% for HbA1c, blood pressure, and LDL cholesterol) in the clinics that have used a quality database for 2 years from 2006 to 2008, and quite poor performance (testing rates below 70% for HbA1c, blood pressure, and LDL cholesterol) for clinics without a database. Finally, excluding quality of care for 10% of the population, the two minor clinics without diabetes data in 2008, with an assumed lower quality of care further contributed to the overestimation of the real baseline quality of care.
The estimation of the quality of care on a national level in 2017 was challenged by the implementation of the new EMR in clinics outside Nuuk at the end of 2016 and spring 2017. The burden with transferring data and information from the former EMR to the new EMR was performed manually along with the implementation of the new EMR. The resources used on this procedure and the energy used on implementation of the new EMR could have affected the resources left to perform diabetes care in some settings.
In conclusion, the selected indicators of quality of diabetes care used in this study were internationally accepted. Since 2010, the indicators were measured based on electronically extracted data from the EMR. The 2008 national quality of care assessment was overestimated and did not represent a true baseline, since two clinics had already achieved very high diabetes quality using a quality database for 2 years, and not all patients diagnosed with diabetes in 2008 were included. However, no alternative existed and the overestimated quality in baseline must be included in the interpretation of the results.
Prevalence of gestational diabetes and testing effectiveness between 2008 and 2014
The prevalence of GDM and testing effectiveness was estimated in 2008 and 2014 [Citation179,Citation222].
All women who gave birth to a singleton in Nuuk during 2008 [Citation222] and in all Greenland during 2014 [Citation179] were included in this study. Only Greenlanders were included in this thesis. Women with pre-gestational diabetes and women treated with oral steroids were not included in the study. Information about maternal age, country of birth, parity, gestational age (GA), mode of delivery, off-spring sex, birth weight, birth length, self-reported pre-gestational maternal weight and height, result of urine for glucose, former pregnancies, family history of diabetes, any smoking during pregnancy, results of OGTT were obtained from a review of medical records, lab cards and register information from the chief Medical Officer.
In 2014, information about maternal address was included too, and the women were categorised as either Nuuk or outside-Nuuk residents. BMI was based on the women’s self-reported weight and height before pregnancy. The women were considered to be smokers if they reported any tobacco smoking at the first prenatal visit. Delivery was considered vaginal unless a caesarean section had been performed. GA was based on the women’s self-reported last menstrual period. If unknown, and in cases where an ultrasound-based GA calculation deviated 2 weeks or more from the last menstrual period-based GA, the ultrasound-based GA was used.
Diagnostic criteria, calculation of prevalence, and testing effectiveness
OGTT was performed in fasting, pregnant women with the administration of 75 g glucose diluted in water. Capillary whole blood (CWB) glucose concentration was examined before and 2 hours after the administration of glucose. Measurement of capillary whole blood glucose concentration was performed using a portable Hemocue® Glucose 201+ System (AB, Ängelholm, Sweden). GDM was defined according to the WHO 1999 criteria [Citation83]. These criteria were selected because CWG cut-off values were included in contrast to the more recent criteria, which only including venous plasma glucose cut-off values published by International Association of Diabetes and pregnancy Groups [Citation101], WHO 2013 [Citation204], National Institute of [Citation205]. Thus, all women with fasting CWB glucose concentration at or above 6.1mmol/l or a 2 hours CWB glucose concentration at or above 7.8 mmol/l after administration of 75 g glucose where considered having GDM. In 2014, only 2 hour CWB glucose was included. If more than one OGTT had been performed during pregnancy, the highest level of fasting and 2 hour CWB glucose concentration was used.
The prevalence of GDM was calculated as a proportion of women with GDM among women tested as well as among all women included. The testing effectiveness calculated was as the proportion of women meeting the screening criteria that were actually tested.
Strengths and limitations
The major strength in this analysis was that all women who delivered in Nuuk in 2008, and all women who delivered in Greenland 2014, were included. However, limitations were identified as well. Information about all risk factors could not be completed for all women since some of the medical records were not available, presumably due to the archive system used at that time. The perinatal information was recorded on paper that was kept in a manual archive. There were, however, no indications of a systematic bias. Thus, the testing effectiveness estimated was considered representative. Another limitation in the analysis was that the 2008 and 2014 samples were not fully comparable. In 2008, only women who gave birth in Nuuk were including in contrast to all women in Greenland 2014. The proportion of women with an increased risk of adverse birth outcome could be higher in the 2008 sample compared to 2014. Thus, women who gave birth in Nuuk were either Nuuk residents or residents outside Nuuk that had been referred to deliver in Nuuk due to increased risk of adverse birth outcome. This could include severe maternal pre-pregnancy overweight, gestational diabetes, pre-eclampsia, and other factors. This could influence the estimated prevalence of GDM. On the other hand, the larger proportion of women living outside Nuuk in 2014 may also affect the prevalence of GDM, since severe overweight has been reported more frequent among women living outside Nuuk compared to Nuuk citizens [Citation21]. In 2014, only 2 hours values of CWB glucose concentrations after OGTT and not fasting CWB glucose concentrations could be included in contrast to the 2008 sample, where both values were included. Thus, the estimated prevalence of GDM in 2014 (only based on elevated 2 hours glucose) was underestimated compared to 2008 (based on either elevated 2 hour glucose or elevated fasting glucose concentrations). Also, the absolute number of women included was relatively low and the estimated prevalence of GDM thereby reported with some statistical uncertainty. Finally, the diagnostic criteria of GDM (WHO 1999) selected in this study influence the prevalence of GDM compared to other international criteria. If instead, the International Association of Diabetes and pregnancy Groups [Citation101] cut-off values based on venous plasma glucose concentrations values were used the estimated prevalence would have been different. Thus, including fasting values at or above 5.1mmol/l would have tended to increase the prevalence compared to the 6.1mmol/l used in this study. On the other hand, including 2 hours values at or above 8.5mmol/mol would have decreased the prevalence compared to 7.8mmol/l used in this study. While 2 hours venous and CWB glucose concentrations may be assumed comparable, this is not the case for fasting blood glucose concentrations [Citation231]. Thus, fasting and random venous blood glucose concentrations have been reported higher than CWB glucose concentrations. On the other hand, the 2 hours venous blood concentrations after an OGTT have been reported slightly lower than CWB glucose concentrations [Citation231]. Thus, the WHO 1999 cut-off values for GDM based on venous plasma was higher for fasting glucose measurements (7.0mmol/l versus 6.1mmol/l) whereas no difference was reported for 2 hour values (7.8mmol/l) [Citation83]. This was in accordance with a recent study from India where it was concluded that in settings where venous plasma glucose estimations are not possible (like in Greenland), CWB glucose can be used to test for GDM, using lower 2 hours cut-off value CWB glucose concentrations (7.8mmol/l versus 8.5mmol/l) to maximise the sensitivity [Citation232].
In conclusion, it was considered best to use the WHO 1999 diagnostic criteria since they included cut-off values for CBW glucose. Despite some differences in the two samples, the effect on the estimated prevalence of GDM and testing effectiveness was considered of minor significance, making the prevalence and the testing effectiveness comparable in the two samples.
Microvascular complications among Greenlanders and non-Greenlanders with T2D
Microvascular complications among Greenlanders and non-Greenlanders with T2D were studied in Nuuk in 2016 [Citation223]. All patients with a permanent address in Nuuk diagnosed with T90 (T2D) according to the International Classification of Primary Care (second Edition) system [Citation233] were identified electronically. Patients born in Greenland were considered to be Greenlanders while patients born outside Greenland were considered to be non-Greenlanders.
The information available from the lifestyle table in the EMR was extracted electronically. The information included year of diabetes diagnosis, smoking habits, physical activity, height, weight, blood pressure, home blood pressure, HbA1c and UACR. In addition information about gender and age, medical treatment, and results of screening for microvascular complications was recorded. Only the most recent test result and only tests performed within a 2 year period were included. The duration of the diabetes was calculated as 2016 minus the year of the diagnosis. Patients were categorised as smokers if daily smoking was recorded in the lifestyle table, and physically inactive if less than 5000 steps per day were recorded in the lifestyle table.
The standard guidelines for measuring height and weight were defined as measurements with the patient wearing light clothing but no outerwear nor shoes. However, self-reported height and weight may also have been used in some settings. Home blood pressure measurements based preferably on an average of at least 12 measurements were included. If not available, most recent blood pressure measurements in the medical office performed on sitting patients after 5 min of rest were included. Blood pressure measurements were performed using the automatic blood pressure Easy Rapid device from Pic Solutions [Citation234]. Analysis of urine (UACR) was performed on Architecht® 8000T (Abbott Laboratories, Copenhagen, Denmark). Analysis of venous blood for HbA1c was performed on Tosoh G8 HPLC Analyser. The analyses were performed at The Central Laboratory at Queen Ingrid’s Hospital.
Microvascular complications
The proportion of patients treated with glucose-lowering drugs (Anatomical Therapeutic Chemical (ATC) codes A10), lipid-lowering drugs (ATC codes C10), and antihypertensive drugs (ATC codes C02-C04 and C07-09) was calculated based on electronically prescriptions in the EMR. Retinal imaging was performed using the Daytona Ultra-Widefield Optos® retinal camera. If any sign of retinopathy was described by the ophthalmologist, retinopathy was considered present. The classification was based on the worst affected eye. Thus, according to the International Diabetes Federation, the preferred test to screen for retinopathy was the non-mydriatic retinal photography using a fundus camera [Citation230]. If UACR was 2.5 to 25mg/mmol, patients were categorised with microalbuminuria while patients with UACR at or above 25mg/mmol were categorised with nephropathy in accordance with international guidelines [Citation183,Citation229,Citation230]. If the result of the examination of vibration perception of the feet performed by a chiropodist using a biothesiometer from Rova Company® were 25 mV or above, the patients were categorised with neuropathy. Vibration perception threshold was considered the gold standard for the diagnosis of diabetic peripheral neuropathy [Citation235].
Strengths and limitations
The major strength of this analysis was that all patients diagnosed with diabetes were included in the study. Furthermore, microvascular complications were defined and categorised based on internationally accepted methods. However, categorisation with microalbuminuria and nephropathy was only based on the most recent result and not on confirmed values in sterile urine as recommended in clinical practice. This may tend to overestimate the prevalence of microalbuminuria and nephropathy reported in this study. However, most likely the comparison between Greenlanders and non-Greenlanders was not affected since any misclassifications probably were equally distributed in the two groups. Also, not all patients were screened for microvascular complications. It cannot be excluded that the rates of complications differ among patients following the screening program and those that do not. Higher rates may be observed among patients not attending the screening program. However, this less likely affected the comparison since equal screenings rates were observed for Greenlanders and non-Greenlanders. Another limitation in this study was the low number of non-Greenlandic females in the study reflecting the gender imbalance among non-Greenlanders in Nuuk. Two-thirds of the immigrants were males [Citation223]. Non-Greenlandic males that have migrated to Greenland may also differ from Greenlandic males in other parameters than could be captured in this study. Thus, some residual confounding could not be excluded.
In conclusion, the prevalence of microvascular complications could be estimated among all patients in Nuuk with T2D using internationally accepted methods. Some residual confounding could not be excluded. Furthermore, not all patients were actually screened for microvascular complication, which may tend to underestimate the prevalence of microvascular complications, but this was not considered to affect the comparison between Greenlanders and non-Greenlanders.
Main results and discussion
Monitoring of an ongoing national diabetes care programme was feasible in the geographically dispersed population of Greenland. During the last 10 years, the prevalence of diagnosed diabetes and quality of diabetes care in Greenland has been studied and monitored continuously [Citation179,Citation217–Citation223]. In repetitive cross-sectional studies, it was demonstrated that a model based on initial analysis, innovation, and implementation of new strategies could be used to increase the awareness and detection of diabetes and gestational diabetes. This process was realised by the diabetes group. Furthermore, it was possible to integrate the EMR in the diabetes project from the very beginning. It allowed statistical extractions of data and made the model feasible.
Contact with health care system in Greenland 2010–2011
A total of 46,802 persons had at least one contact with the primary health care system in the period 1 June 2010 to 31 May 2011 [Citation217]. Therefore, 83% (46,802/56,419) of the whole population has been in contact with the primary health care system within a 1 year period. The proportion of females was 90% and higher compared to 76% of males (p < 0.001). The age and gender-specific proportion of the population that has been in contact with the primary health care system in Greenland is illustrated in .
Figure 3. Percentage of population in Greenland with at least one contact with the health care system in Greenland between 1 June 2010 and 31 May 2011. The figure was based on published data [Citation217].
![Figure 3. Percentage of population in Greenland with at least one contact with the health care system in Greenland between 1 June 2010 and 31 May 2011. The figure was based on published data [Citation217].](/cms/asset/6b09585e-d622-437a-a202-f02c57330071/zich_a_1709257_f0003_b.gif)
Males between 20 and 50 years old represented the group with the lowest use of primary health care (71–73%). The high proportion of persons in Greenland with annual contact to the health care system in Greenland indicates that public demand of health care services is common, and also that the majority of people in Greenland have access to primary care.
The frequent use of health care services was comparable to levels reported in other Nordic countries with annual contact rates at 85–90% of the whole population [Citation51–Citation53]. Also, the more frequent use of health care among females compared to males was a phenomenon observed in the other Nordic countries. It may partly be explained by routine perinatal care, but may also reflect gender-specific health behaviour. Also, the higher prevalence of use among children and older people was as expected. In addition to a high prevalence of airway and middle ear infections among Greenlandic children [Citation236–Citation238], children were offered routine health care checks, and they were offered participation in an immunisations programme in Greenland. The higher prevalence of contact among older people most likely reflected the higher prevalence of disease and disabilities in this group [Citation21,Citation215].
In general, the majority of the population in Greenland had been in contact with the primary health care system. Thereby, a possibility existed to detect new cases of diabetes and other chronic conditions among patients in the primary health setting. Opportunistic case-finding of diabetes in contrast to a population screening programme represented several advantages. Patients contacting the health care voluntarily were assumed to be interested in their own health. Timing is based on the patients’ initiative. Furthermore, no evidence of reduced cardiovascular mortality existed using a population-based screening for diabetes [Citation239]. Screening programmes were expensive, and effective screening was complicated in the dispersed population throughout all of Greenland. Since 1997, screening for cervical cancer has been offered to adult women in Greenland. Yet, screening rates of around 40–54% have been reported in Greenland and remained much lower than the target rate around 85% [Citation240]. On the other hand, middle-aged males representing the group with the lowest use of primary health may be among those with the highest risk of undetected diabetes. Alternative access opportunities to health for this group could be considered. Lifestyle Café, a concept based on a drop-in offering and a patient-centred lifestyle assessment available outside normal clinic opening hours. This concept may represent an effective alternative.
In conclusion, despite the challenge of delivering health care in the geographically widespread population of Greenland, more than 80% of the total population have been in contact with the primary health care within a 1-year period. Thus, opportunistic case-finding among the majority of the population was a possible strategy for reducing the number of undetected cases of diabetes in Greenland, and was chosen for use in the new Greenlandic diabetes initiative.
Diagnostic activity in 2010–12 and 2014–15
A total sample of 1008 persons was identified electronically filtering for all adults in Greenland aged 35 years or above born on the first day of a month. Of those, 157 (23 diagnosed with diabetes and 134 without diabetes) were tested with measurement of HbA1 at least once in the 27 month observation period starting from 1 June 2010. The overall estimate of diagnostic activity regarding diabetes 2010–2012 was 15.6% (157/1008) among adults aged 35 or above.
In the second observational period, 1 January 2014 to 31 December 2015, a total of 10,127 persons (6,346 females and 3,781 males) were identified, in whom HbA1c had been measured at Queen Ingrid’s Hospital in 2014 or 2015. 18.1% of the whole population has so far been tested at least once within a 2 year period. The age and gender-specific proportion of the population tested is illustrated in .
Table 2. Age and gender-specific proportion of total population in Greenland tested with HbA1c during 2014 or 2015. The table was constructed based on formerly published data [Citation220].
Females were tested more often than males in all age groups except among the 70–79 year age group, where no difference was observed. The proportion tested increased with age in groups from 16.6% among the youngest (20–39 years old) to 50.1% among the oldest (70–79 years old) for the whole group. The proportion of patients tested (18.1% of the whole population or 24.0% of adults aged 20–79 years) in 2014–2015 was much higher than the 15.6% in 2010–2012 documenting thusly increased diagnostic activity. This may reflect increased awareness of diabetes in the health care system as well as in the general population. In particular, the proportion of females tested was high. This may partly be due to the more frequent use of health care system among females compared to males in Greenland [Citation217]. In addition, routine screening with HbA1c in the first trimester among all pregnant women was implemented in the guidelines for gestational diabetes in Greenland in 2014. This may also have contributed to the higher proportion of females tested in the younger age groups. A minor contribution to the increased number of person tested with HbA1c may be a result of more patients being diagnosed with diabetes in 2015 compared to 2010, because these patients were offered an HbA1c test more than once a year.
The introduction of HbA1c as a diagnostic tool in 2010 seems to have affected the diagnostic activity importantly as a supplement to fasting CWB glucose performed with or without OGTT. Thus, HbA1c measurement was much more convenient for the patient, since it can be obtained at any time and requires no overnight fasting period prior to the test [Citation46,Citation225].
Still, the number of males aged below 60 years old tested with HbA1c was much lower than among females. Consequently, undiagnosed diabetes in this group may be a larger problem than among females. Therefore, new testing strategies need to be considered, targeting this group. Also, testing strategies must be considered, targeting people living in the settlements and people less privileged socio-economically. Observations from the populations surveys indicated that the prevalence of diabetes decreased with urbanisation in Greenland [Citation44].
To sum up, the diagnostic activity regarding diabetes has increased within a few years. The introducing of HbA1c as a diagnostic tool together with increased awareness of diabetes were the most obvious reasons for the increased activity observed. However, undiagnosed diabetes remains a problem that needs to be addressed more in depth.
Prevalence of diagnosed diabetes from 2008 to 2017
The prevalence of diagnosed diabetes was estimated in 2008, 2010, 2012, 2014 and 2017 using the diabetes profile in EMR, except for the brief period during and after replacement of the former EMR with a new EMR in 2015 [Citation218,Citation219,Citation221]. The time trend of diagnosed adult diabetes is shown in . Since the beginning of the diabetes project in 2008, a threefold increase in the prevalence has been measured to be from 1.14% to 3.45% among adults aged 20 or above. In 2017, the prevalence of diabetes was 3.3% among adults aged 20–79.
Table 3. Prevalence of diagnosed diabetes among adults in Greenland 2008–2017. The table was constructed based on previously published data [Citation218,Citation219,Citation221], and new unpublished data from 2017.
shows the age and gender-specific prevalence of diabetes among Greenlanders and non-Greenlanders in 2014. The prevalence among adults aged 20–79 was significantly lower among Greenlanders compared to non-Greenlanders, 2.36% versus 3.69% (p < 0.001). The higher prevalence among non-Greenlanders was a result of higher (p < 0.001) prevalence among non-Greenlandic males (4.70%) compared to Greenlandic males (2.15%). In contrast, the prevalence among non-Greenlandic females (1.53%) was lower (<0.001) than among Greenlandic females (2.57%). The prevalence among Greenlandic males (2.15%) was lower than among Greenlandic females 2.57% (p = 0.008). For both males and females the prevalence increased with age group. The highest prevalence was observed in the age group 70–79, 10.62% among Greenlanders and 17.37% among non-Greenlanders. However, the prevalence was based on few patients with an age lower than 40 in all groups. Additionally, the group of female non-Greenlanders only comprised few patients, and the prevalence must be evaluated in this perspective.
Table 4. Age and gender-specific prevalence of diabetes among Greenlanders and non-Greenlanders in 2014. The table was constructed based on previously published data [Citation221].
The most obvious explanation of the increased prevalence was increased diagnostic activity as mentioned in the previous section. However, a real increase in background incidence may also have contributed, driven by the increasing prevalence of overweight. And, obesity may also have contributed [Citation21]. Furthermore, reduced mortality with proper treatment of detected diabetes cases may have influenced the prevalence positively. On the other hand, the recent trend of moving from Greenland to Denmark among older retired persons may affect the prevalence negatively, since the prevalence of diabetes was higher among the elderly [Citation224].
The most recent prevalence of diabetes among adults aged 20 or above of 3.45% was in line with the most recent population survey (2014) estimated the prevalence of diabetes [Citation21]. Among 537 persons examined with HbA1c in 2014, 6.7% were categorised as having diabetes [Citation21]. Of those, 60% were already diagnosed with diabetes corresponding to a prevalence of diagnosed diabetes around 4.0%. However, the prevalence of diagnosed diabetes was much lower than the 9–10% reported in the population surveys where the diagnosis was based on OGTT.
The newly described mutation among Inuit associated with elevated postprandial hyperglycaemia, but not elevated HbA1c may contribute to the proportion of undiagnosed patients. A recent study indicated that up to 30% of patients with this mutation had an increased postprandial hyperglycaemia that may persist undiagnosed if only tested with HbA1c and not OGTT [Citation241]. Future research projects have to address this issue including evaluation of complications to this Inuit specific subtype of diabetes. The prevalence of diagnosed diabetes was close to the global average of diagnosed diabetes in 2017, around 4.4% (50% undiagnosed among 8.8% estimated to have diabetes) [Citation28], and lower than in countries like USA (6.2%), Canada (5.2%), UK (4.7%), New Zealand (4.6%), and Australia (4.5%) [Citation161].
The higher prevalence of diagnosed diabetes among Greenlandic males compared to Greenlandic females may reflect the lower diagnostic activity and the less frequent use of primary health care among males compared to females [Citation220,Citation242]. Also, a higher prevalence of obesity among Greenlandic females compared to males may contribute to the higher female prevalence [Citation21,Citation243].
The higher prevalence among non-Greenlanders compared to Greenlanders was in contrast to most observations among Ingenious people around the globe [Citation8,Citation25,Citation27,Citation150,Citation161]. In general, the prevalence of diabetes among Indigenous people was two to four times higher than in the general population in many countries.
However, Greenlanders differed in some ways from many other indigenous populations in ex-colonial countries. Greenlanders represented the majority of the population in Greenland, self-government existed, and Greenlandic was the official language spoken. This was in contrast to many Indigenous populations that have been marginalised as a minority, and have lost their language and territories [Citation23,Citation24]. Also, non-Greenlanders in Greenland may differ from other general populations. The majority of the non-Greenlanders were males. Furthermore, they have emigrated, lived as a minority in Greenland, and may have different lifestyles compared to general populations in their homeland. Thus, the interpretation of the higher prevalence observed among non-Greenlanders compared to Greenlanders should be approached with caution.
In conclusion, the prevalence of diagnosed diabetes has been monitored continuously within the last decade. A threefold increase in prevalence has been observed. The prevalence was increasing with age and was higher among Greenlandic females than Greenlandic males. The highest prevalence was observed among non-Greenlandic males. This was in contrast to observations among most Indigenous populations in ex-colonial countries. The comparison should, however, been made with some caution because the non-Greenlanders in Greenland may differ from other general populations.
Quality of diabetes care from 2008 to 2017
Since 2008, when the diabetes profile was implemented in the EMR, the quality of care has been estimated and monitored on a regular basis. Only temporarily was national monitoring impossible in the period during and after replacement of the former EMR with a new EMR in 2015.
As explained in the method section the baseline quality of care in Greenland was clearly overestimated and not an optimal baseline. However, despite the overestimation of the baseline in 2008, all processing of care indicators increased significantly from 2008 to 2010, during the diabetes project. Data from documents improved care. Also, distal outcome indicators for HbA1c improved from 2008 to 2010, and the proportion of patients with systolic blood pressure below 130 mmHg increased from 33% (125/465) to 39% (253/695) (p < 0.001). On the other hand, the number of patients with LDL cholesterol below 3.5mmol/l decreased from 79% to 60% (p < 0.008) during this period.
Table 5. Quality of diabetes care 2008–2017. The table was constructed based on previously published data [Citation218,Citation221], and new unpublished data from 2017.
From 2010 to 2014, a decline in all quality of care indicators was observed with LDL control as the only exception (). However, the quality of diabetes care including testing for HbAc1, retinopathy and microalbuminuria, and poor regulation of HbA1c was still improved compared to the baseline in 2008. The indicator, LDL control, was the only indicator that increased between 2010 and 2014, 60% versus 71% (p = 0.001).
When measured in 2017, the quality of diabetes care was also improved for most process of care indicators including testing for HbA1c (85% within 1 year or 95% within 2 years), LDL cholesterol (83%), retinopathy (79%), microalbuminuria (60%) compared to 81%, 72%, 45%, and 44% in 2008 (). Furthermore, annual testing of LDL cholesterol increased form 49% to 63% (p < 0.001) from 2014 to 2017.
A non-significant increase in HbA1c control from 44% to 48% (p = .0251) was also observed, whereas the number of patients with poor control (HbA1c at or above 75mmol/mol) decreased significantly from 20% in 2008 to 17% in 2017. Blood pressure control (BP<140 mmHg) increased significantly from 55% to 65% between 2014 and 2017 (p < 0.001). On the other hand, the proportion of patients tested with LDL control worsened from 71% to 64% between 2014 and 2017, indicating a need for increased focus on this risk factor.
Despite a threefold increase in the absolute number of patients with diabetes in Greenland within a 10-year observational period, the quality of diabetes in Greenland was improved for most indicators indicating a positive effect of the diabetes initiative taken. The peak performance was observed in 2010, 2 years after the initiatives were initiated. The lower, long-term outcome of quality observed eight and 10 years after the initiative compared to short-term outcome in 2010, is a phenomenon well known from other studies around the world [Citation155]. Thus, initial gains in quality of care tended to fade out over time [Citation244,Citation245]. Long-term monitoring of the effects of quality care initiatives has been suggested to maintain the quality achieved [Citation245,248]. However, the major organisational changes including extending the diabetes programme to a lifestyle initiative in 2011, reorganising the whole primary health care sector in Greenland from 16 districts into five health care regions in 2013, and changing the national EMR and temporarily making national monitoring of quality impossible have also challenged the aim and possibility of maintaining high quality diabetes care. Thus, room for improvements in the actual quality of diabetes care persists.
The increased percentage of patients achieving HbA1c control (HbA1c < 53 mmol/l) may partly be explained by optimised treatment. However, increased diagnostic activity and detection of cases with only slightly elevated HbA1c level may have influenced the percentage too.
The increased number of patients tested for LDL cholesterol between 2014 and 2017, may be a result of including blood lipid measurements in the standard lifestyle blood profile requisition.
The increase in blood pressure control (BP below 140/90 mmHg) observed between 2014 and 2017, may be a result of the increased focus on hypertension, simplification of guidelines, home blood pressure measurement, and increased availability of automatic blood pressure measurement devices in Greenland provided by the lifestyle initiative. Thus, home blood pressure measurements were generally 5 mmHg lower than office measurements [Citation210].
The 10-year follow-up on quality of care in 2017, in the presented study was quite similar to the long term follow-up (1994–2004) of the Special Diabetes Programme in Alaska [Citation73,Citation74]. The number of patients diagnosed with diabetes increased from 1,259 to 3,264 (less than 4% with T1D). A threefold increase in the number of patients with diabetes was observed as in Greenland from 2008 to 2017. Quality of diabetes care was evaluated in a subsample among those patients. Annual testing of LDL cholesterol and retinopathy was observed in 85% and 56% in Alaska compared to 63% and 55% in Greenland 2017. In the subsample in Alaska, Hba1c below 53mmol/mol was achieved among 52%, LDL cholesterol below 2.6mmol/l among 51%, and blood pressure below 130/80 mmHg among 37% of patients compared to 48% of patients tested with HbA1c below 53mmol/mol, 64% of patients tested with LDL cholesterol below 3.5mmol/l, and 65% of patients tested with blood pressure below 140/90 mmHg in Greenland in 2017 [Citation74]. These are quite similar levels of observed diabetes care quality despite the limitation in comparing different indicators used in the two studies.
The quality of diabetes care observed in the present study could be compared with the quality of care described by OECD indicators in five countries including information about the quality of care among Indigenous people in some of the countries [Citation161]. Annual testing of HbA1c at 85% in Greenland can be compared to 65% in Australia, 74% in Canada, 64% in New Zealand (39% among Maori and 99% of Pacific Islanders), 79% in Alaska, USA, and 83% among primary care patients in the UK [Citation161]. Annual lipid testing rates in Greenland at 49% (2014) and 63% (2017) were quite low and may reflect that more focus had been placed on establishing statin treatment among patients aged 40 or above rather than testing LDL cholesterol among patients already treated in accordance with the ADA [Citation202]. The proportion of T2D patients in Nuuk treated with statins, 57.8%, was higher than the number of patients tested annually in 2014 [Citation223]. Annual lipid testing was reported among 50% of primary health care patients in Australia, 64% in New Zealand, 60% among Native Americans and Alaskan Natives, and 81% among primary health care patients in the UK [Citation161]. The 2 year screening rate of retinopathy in Greenland, 79%, was quite high compared to 77% in Australia, 70% among Medicare patients in USA, 71% in New Zealand (68% Maori and 66% Pacific Islanders), 68% among patients aged 40 or above in the USA, 49% (annual) among Native Americans and Alaskan Natives, and 61% (annual) in the UK [Citation161]. On the other hand, annual screening for nephropathy in Greenland, 46%, was suboptimal and in the lower end of the comparison with 27% in primary health care settings in Australia, 64% in New Zealand (39% among Maori and 99% among Pacific Islanders), 75% of Medicare patients in the USA, 55% among Native Americans and Alaskan Natives, and 83% in the UK. Therefore, an increased focus on screening for nephropathy has to be re-initiated. The decrease in some indicators within this 10 year observational period also illustrated that continued focus – including monitoring of the quality of diabetes care – was crucial to maintaining the quality level achieved.
In conclusion, the quality of diabetes care improved from 2008 to 2017, and was comparable to the level reported internationally. The actual quality of care in 2017, was lower than observed in 2010, shortly after the diabetes project was started, underlining the need for continued focus as crucial in maintaining the level of quality achieved. Major organisational changes, including expanding the diabetes project with a new lifestyle initiative, reorganising primary care, and using the new EMR system, were challenges to the diabetes initiative. Although improvements have been achieved, room for improvement still exists for enhancing the present quality of diabetes care. These must be addressed in the future.
Prevalence of gestational diabetes and testing effectiveness between 2008 and 2014
In 2008, a total of 233 Greenlandic women were included in the study sample although excluding mothers of twins, and pregnant women without available information or medical record. Sixty-eight per cent (233/342) of all deliveries in Nuuk were included corresponding to 28% (233/829) of all singleton deliveries in Greenland during 2008. In 2014, 727 Greenlandic women were included in the sample corresponding to 92% (727/794) of all singleton deliveries this year. No women were excluded due to pre-pregnant diabetes or use of oral steroids neither in 2008, nor in 2014. No women delivered more than once in each sample.
Basic characteristics of women included in 2008 and 2014, are shown in .
Table 6. Basic characteristics of women included in the 2008 and 2014 study of GDM. The table was constructed based on already published data including recalculated data from the 2014 [Citation179,Citation222].
Smoking was more frequent (55% vs. 51%) among women in the 2014 sample compared to the 2008 sample (p < 0.001). Otherwise, no differences were seen between the two samples.
Risk factors, testing effectiveness, and prevalence of GDM are shown in . The proportion of women with at least one risk factor for GDM (among women with information available for all risk factors) was higher, 50.1% in the 2014 sample, compared to 36.9% in the 2008 sample. The most frequent risk factor present, in around one-third of the cases, was pre-pregnancy BMI at or above 27 kg/m2. A significant increase in testing effectiveness was measured from 53% in 2008, to 67% in 2014 (p = 0.028). The testing effectiveness among Nuuk residents in the 2014 sample was much higher, 84% (60/74), than among residents outside of Nuuk 61% (125/204) (p = 0.002). Also, the proportion of women tested was higher in 2014 compared to 2008, 29.6% versus 19.7% (p < 0.003). In 2008, two cases of GDM were identified compared to 15 in 2014. This corresponded to a prevalence of GDM among all women of 0.9% and 2.1% in 2008 and 2014, respectively, and of 4.3% and 7.0% among tested women in 2008 and 2014, respectively. The increased prevalence was not statistically significant due to the small numbers. However, an increasing trend seems to emerge. No women met the present criteria used in Greenland (2 hour CWB glucose at or above 9.0 mmol/l) in 2008 compared to five women in 2014.
Table 7. Risk factors, testing effectiveness and prevalence of GDM among women in 2008 and 2014 study. The table was constructed based on already published data including recalculated data from the 2014 [Citation179,Citation222].
As seen, the testing effectiveness has improved significantly from 53% to 67% between 2008 and 2014. The actions taken to increase awareness of GDM among health care professionals were most likely the main reasons for the better outcome, including workshops, GDM guidelines, and education. However, outside Nuuk, the testing effectiveness was only 61% and unacceptably low compared to 84% in Nuuk. Low testing effectiveness and disparity in testing effectiveness have also been observed among other Indigenous populations. As mentioned before, a study from New Zealand demonstrated a relatively low screening rate at 63% of pregnant mothers [Citation116]. In addition, much lower (56%) among Maori mothers than (76%) among mothers of European origin [Citation116].
A total prevalence of GDM at 0–2% among pregnant women was relatively low and comparable with prevalence in north Europe at around 2–4% and lower than reported in south Europe and among many Indigenous populations and minorities like Native Americans, Asians, Hispanics and African-Americans living in USA and aboriginals in Australia and Maori and Torres Strait Islanders in New Zealand with prevalence 6–8% [Citation99,Citation109]. However, the prevalence of GDM among tested women was 7.0% and thus moderate to high in a global perspective. Even this prevalence may be underestimated, since no fasting glucose measurements were included in 2014. Surely, the population prevalence was underestimated due to the relatively low screening rates observed outside Nuuk. Furthermore, GDM may also be present among mothers without risk factors. A study from Australia reported a 40% increase in the prevalence of GDM among aboriginals after changing strategy from selective risk factor-based testing to universal testing of all pregnant women [Citation115]. The lower testing effectiveness outside Nuuk was understandable, because the only obstetrics department in Greenland was located in Nuuk. Furthermore, the smaller, geographically isolated primary health care centres were challenged in delivering all manner of health care. Lack of specialist and midwives, and a high number of short-term healthcare workers also represented a real hindrance to providing continuity in the prenatal care. Routine, universal testing of all pregnant women in gestational week 24 may represent a more simplistic approach that may be executed more effectively than the present high risk testing model using different testing intervals. Also, the very high cut-off values used in Greenland has to be reconsidered in order to detect cases of GDM that could benefit from lifestyle interventions. However, this decision has to be balanced with the difficulty in using the cumbersome OGTT, the risk of labelling pregnant women as sick, and then taking into consideration both economic and organisational challenges.
The very high prevalence of smoking observed in both samples was alarming and indicated a need to focus more on that issue. The higher prevalence of smoking in 2014 reflects both a higher proportion of smokers among of residents outside Nuuk and a higher proportion of outside Nuuk residents in the 2014 sample compared to the 2008 sample. The prevalence of smokers has consequently been reported higher among inhabitants outside Nuuk compared to Nuuk citizens [Citation21].
In conclusion, the prevalence of GDM in Greenland seemed relatively low, but with a tendency to increasing frequency. Testing effectiveness has improved significantly between 2008 and 2014. However, around a third of the Greenlandic women, that should have been tested, were not tested. The testing effectiveness was poorer outside Nuuk and an alternative testing strategy has to be considered to improve testing effectiveness further.
Microvascular complications among Greenlanders and non-Greenlanders with T2D
A total of 393 patients living in Nuuk (295 Greenlanders and 98 non-Greenlanders) had been diagnosed with T2D, and the prevalence of microvascular complications (neuropathy, microalbuminuria/nephropathy and retinopathy) was measured [Citation223]. The basic characteristics and prevalence of microvascular complications are shown in and .
Table 8. Basic characteristics and proportions of patients with microvascular complications. The table was constructed based on already published data [Citation223].
Table 9. Basic characteristics and proportion of patients with microvascular complications. The table was constructed based on already published data [Citation223].
The 2 year screening rates for microvascular complications were 85% for retinopathy, 66% for microalbuminuria and 65% for neuropathy.
No difference in screening rates was observed among Greenlanders and non-Greenlanders. No difference in basic variables was observed among Greenlanders and non-Greenlanders. However, the proportion of females was lower, 9% (9/98), among non-Greenlanders than 57% (169/295) among Greenlanders (p < 0.001). No differences between Greenlandic males and females were observed. However, among non-Greenlanders, only nine were females, and no certain, conclusive comparison with non-Greenlandic males could be made due to the low number of non-Greenlandic females.
Neuropathy was observed among 49.6% of all patients with no differences observed between Greenlanders and non-Greenlanders. Internationally, divergent results have been reported related to ethnicity and neuropathy [Citation129]. However, ethnicity was not an internationally accepted risk factor of neuropathy [Citation131]. This is in accordance with the results of the present study.
Microalbuminuria was observed less frequently among Greenlanders, 24.9%, compared to 37.5% among non-Greenlanders (p = 0.047). The combined prevalence of microalbuminuria and nephropathy was 35.6% (93/261) with no difference between Greenlanders and non-Greenlanders (p = 0.122). Microalbuminuria was negatively associated (logistic regression) to being Greenlander (β = -0.595, p = 0.044) [Citation223]. However, no significance was observed after adjusting for age and gender (β = -0.379, p = 0.252) [Citation223].
The prevalence of microalbuminuria at around 24% among Greenlanders in this study was relatively low in a global perspective. On average 39% of 32,208 patients from 33 different countries had microalbuminuria [Citation139]. Higher prevalence was observed among some minorities like Asians and Hispanics at around 55%. Yet, a prevalence of microalbuminuria or nephropathy affecting more than a third of all patients in Nuuk underlines the importance of screening for this condition in order to optimise blood pressure and glycaemic control.
Retinopathy was only present in 7.0% among Greenlanders compared to 21.4% among non-Greenlanders (p = 0.001). Retinopathy was negatively associated (logistic regression) to being Greenlander (β = -1.284, p < 0.001) and positively associated with HbA1c (β = 0.020, p = 0.017) [Citation223]. The association to being non-Greenlander remained significant after adjusting for age, gender, HbA1c, and duration of diabetes (β = -1.042, p = 0.026) indicating that Greenlanders may be less prone to retinopathy than non-Greenlanders [Citation223]. This conclusion was in line with the first and only prior study of complications among Greenlanders, in which the prevalence of retinopathy was estimated to be 11% compared to 21% among Danes [Citation152]. Globally, ethnicity has been established as an independent risk factor of retinopathy [Citation123,Citation124,Citation129]. Greenlandic ethnicity seems to be related to a lower risk of that diabetes-related complication. The documented prevalence of retinopathy was quite low compared to 35–40% observed in Canada, many European countries, Southeast Asia, and Australia [Citation124–Citation127]. On the other hand, the prevalence of retinopathy was higher than in countries with a low prevalence (around 5%) like Denmark, Finland and parts of France [Citation124]. The observed prevalence of retinopathy among Greenlanders, 7.0%, was also much lower than the 33% reported among the Inuit in Canada [Citation168]. However, the low prevalence of retinopathy in Greenland has to be observed carefully in the future, since the duration of diabetes, a solid risk factor of retinopathy, among both Greenlanders and non-Greenlanders was quite low in the present study, on average around 7 years.
In conclusion, despite a relatively short time with diabetes and a mean duration of 7 years, microvascular complications were observed frequently among both Greenlanders and non-Greenlanders. Greenlanders had a remarkably lower prevalence of especially retinopathy than non-Greenlanders – and may thus be less prone to this complication. No difference was observed in the prevalence of neuropathy and in the combined prevalence of either microalbuminuria or nephropathy among Greenlanders and non-Greenlanders. The common prevalence of microvascular complications in connection with diabetes underlined the need for continued and sustained focus on diabetes care and for increasing the access to screening for complications in Greenland.
Conclusion
Monitoring of an ongoing national diabetes care programme proved feasible in the geographically dispersed population of Greenland. During the last 10 years, the prevalence of diagnosed diabetes and quality of diabetes care in Greenland has been studied and monitored continuously [Citation179,Citation217–Citation223]. In repetitive cross-sectional studies, it was demonstrated that a model based on analysis, innovation and implementation of new strategies could be used to increase awareness and detection of diabetes and gestational diabetes. The process was realised by the former diabetes group and present lifestyle group. Furthermore, it proved possible to integrate the EMR in the diabetes program from the very beginning. It allowed statistical extractions of data and made the model feasible.
Despite the challenges of delivering health care in the geographically wide spread population of Greenland, more than 80% of the total population was in contact with the primary health care system within a calendar year period. Thus, opportunistic case-studying of the majority of the population was a possible strategy in reducing the number of undetected cases of diabetes in Greenland, and was chosen for the new Greenlandic diabetes initiative.
Diagnostic activity regarding diabetes increased during two periods from 2010 to 2012 and from 2014 to 2015. Introduction of HbA1c as a supplementary diagnostic tool combined with increased awareness of diabetes were the most obvious reasons for the observed activity increase. However, undiagnosed diabetes remained a problem that needed to be addressed further.
The prevalence of diagnosed diabetes was monitored continuously during the last decade. A threefold increase in the number of patients was measured. The prevalence increased with age and was higher among Greenlandic females than among Greenlandic males. The highest prevalence was observed among non-Greenlandic males. This was in contrast to observations from among most Indigenous populations in ex-colonial countries, where the prevalence of diabetes among Indigenous people in general has been reported to be 2–4 times higher than among the non-Indigenous populations. The comparison should, however, be evaluated with caution because the non-Greenlanders in Greenland may in fact differ from other general populations.
The quality of diabetes care improved from 2008 to 2017, and was comparable to the level reported internationally. The actual quality of care in 2017, was lower than observed in 2010, shortly after the diabetes project was started underlining the need for continued focus as crucial in maintaining the level of quality already achieved. Major organisational changes, including expanding the diabetes project with a new lifestyle initiative, the establishment of new regional primary care program, and the use of a new EMR system proved to be challenges for the diabetes initiative. Although improvements have been achieved, room for improvement still exists in the present quality of diabetes care. This must be addressed in the near future.
The prevalence of GDM in Greenland was relatively low (4–7% of tested women), but with a tendency towards increased levels. Testing effectiveness improved significantly from 53% to 67% between 2008 and 2014. However, around a third of the Greenlandic women that should have been tested were not tested. The testing effectiveness was poorer outside Nuuk, 61% compared to 84%, and an alternative testing strategy has to be considered to improve testing effectiveness in the future on the national level.
Despite relatively short times dealing with diabetes, with a mean duration of 7 years, microvascular complications were observed and frequently, among both Greenlanders and non-Greenlanders. Greenlanders had a remarkably lower prevalence of retinopathy than non-Greenlanders and may thus be less prone to this complication. However, the common prevalence of microvascular complications related to diabetes underlines the need for continued and sustained focus on diabetes care in Greenland.
Perspectives
In this thesis, it was demonstrated that a new model of diabetes care was feasible in its implementation also in the dispersed population of Greenland based on continuous monitoring and repeated analysis, innovation, and implementations processes. The model was used to increase awareness of diabetes, the prevalence of diagnosed diabetes, the quality of diabetes care, the management of gestational diabetes, and the feasibility of screening for microvascular complications. The integration of a diabetes profile in the former EMR and a lifestyle table in the new EMR were essential additions for improving both clinical management and regional and national monitoring of diabetes and diabetes care.
Diagnostic activity and case-finding have increased substantially after the implementation of HbA1c as a diagnostic tool in 2010. Consequently, the prevalence of diagnosed diabetes has steadily been increasing over the last 10 years. However, undiagnosed diabetes remains a problem and additional strategies need to be considered in the future.
The proportion of middle-aged men that has been tested for diabetes was lower than among females, and strategies may be considered targeting gender and age groups in a specific way. Testing opportunities could be provided in settings outside the traditional health care system including in the workplace, at leisure facilities, and in other settings. Furthermore, using Hba1c as the primary screening tool alone may not be sufficient to reduce the proportion of undiagnosed cases in Greenland. Carriers of the specific Inuit diabetes mutation may stay undiagnosed if tested with HbA1c alone and not with OGTT. This Inuit specific diabetes form must be explored more in the future including the associated risks for complications.
The quality of diabetes care has improved from 2008 to 2010. However, further improvements were still required. For example the proportion of patients with poorly regulated diabetes (Hba1c at or above 75mol/mol) was still high in 2017. More feasibly, access to physicians or nurses with a special interest in diabetes could address this issue rather than relying on visiting, consulting physicians engaged only in short time shifts, which is the present reality in many locations outside of Nuuk. Skype consultation with physicians or nurses in Nuuk offers a possibility that already has been started in a pilot test setting. The implementation of common EMR for all patients in Greenland could be used as a step towards considering all patients based on their addresses rather than dealing only with those solely affiliated to local health care clinics. In this way all patients in Greenland would benefit from high quality primary health care available in a large central primary health care clinic. Furthermore, the model of diabetes care could be extended to many other areas of primary care including both chronic and non-chronic conditions. Potentially, the quality of primary health care in Greenland could improve after such a re-organisation.
The management of gestational diabetes care improved from 2008 to 2014, through the development of organisation of care, clinical guidelines, and information provided to and gleaned from patients. Furthermore, testing effectiveness improved from 53% (2008) to 69% (2014). In Nuuk, a quite acceptable testing effectiveness of 84% was observed, whereas the national performance level was suboptimal, missing in fact approximately one-third of all women that should have been tested. An alternative and a more simple testing strategy may be considered. Testing all pregnant women once in pregnancy at gestational week 24 may be considered as proposed by The International Federation of Gynaecology and Obstetrics. Also, the cut-off values used in Greenland must be reconsidered, because the present cut-off was based on venous plasma values rather than on capillary whole blood glucose concentrations. This would result in an increased prevalence of GDM and an increased need for lifestyle interventions and treatments of new cases. Thus, the decision has to be balanced with the resources available within the health care system. An analysis is needed of the consequences of different strategies and cut-off values. Furthermore, it should be explored whether baseline Hba1c measured early in pregnancy could be used to identify women at risk of GDM in Greenland as observed in some international studies.
The lower prevalence of retinopathy observed among Greenlanders compared to non-Greenlanders in Nuuk should also be confirmed in a larger study including all Greenlanders with diabetes. Other areas that should be explored in upcoming research projects include the prevalence of diagnosed T1D and the quality of diabetes care for this group, including not only quality of care, but possible differences in ethnicity, the presence of monogenic forms of diabetes in Greenland, and the prevalence of macrovascular complications. Furthermore, a study of distal outcome indicators is wished for, i.e. the rate of lower extremity amputation, end-stage renal disease, and cardiovascular death among patients with diabetes. Finally, undetected diabetes among children need to be explored, because children were not included in the former studies of diabetes in Greenland.
In a global perspective, the model used to improve awareness and the quality of diabetes management in Greenland may also be used in other remote settings in the future along with the implementation of electronic medical record systems, which would allow continued monitoring and feasible access to the evaluation of initiatives taken.
Learning points
A health care model based on continued monitoring, analysis, and adjustment of initiatives taken, can be used to improve diabetes care even in the dispersed population of Greenland.
A universal electronic medical record system represents a possibility to facilitate diabetes care in the clinical consultation, at regional and national level.
A multifaceted initiative targeting the organization, the health care professionals, and the patients can be used to increase diagnostic activity revealing the prevalence of diagnosed diabetes, quality of diabetes care, testing for gestational diabetes and microvascular complications.
The Greenland model of diabetes care potentially can improve the health care of other chronic diseases in Greenland and may be useful in other remote settings.
Additional
A public defence of this doctoral thesis took place at Arhus University at 8 March 2019 and the author received the higher doctoral degree.
Acknowledgments
This thesis presents the result of cooperation between many people and institutions for more than a decade. I am deeply thankful for the massive support and the willingness to cooperate that I have met everywhere. This thesis could not have been completed without financial support. I am very thankful for the post-doctoral donation from the Greenland Research Council, which made this thesis possible. The initial donation in 2007 from Novo Nordisk A/S, to the health care system in Greenland, started the national diabetes program. This is very praiseworthy.
Disclosure Statement
No potential conflict of interest was reported by the author.
Additional information
Funding
References
- WHO | Diabetes [Internet]. WHO. [cited 2016 Feb 16]. Available from: http://www.who.int/mediacentre/factsheets/fs312/en/
- Guariguata L, Whiting DR, Hambleton I, et al. Global estimates of diabetes prevalence for 2013 and projections for 2035. Diabetes Res Clin Pract. 2014 Feb 1;103(2):1–49.
- Fowler MJ. Microvascular and macrovascular complications of diabetes. Clin Diabetes. 2011 Jul 1;29(3):116–122.
- Cheung N, Mitchell P, Wong TY. Diabetic retinopathy. Lancet Lond Engl. 2010 Jul 10;376(9735):124–136.
- Webster AC, Nagler EV, Morton RL, et al. Chronic kidney disease. Lancet. 2017 Mar 25;389(10075):1238–1252.
- Lancet T. Type 2 diabetes—time to change our approach. Lancet. 2010 Jun 26;375(9733):2193.
- Beagley J, Guariguata L, Weil C, et al. Global estimates of undiagnosed diabetes in adults. Diabetes Res Clin Pract. 2014 Feb;103(2):150–160.
- al NM et. Global complication rates of type 2 diabetes in Indigenous peoples: A comprehensive review - PubMed - NCBI [Internet]. [ cited 2017 Jul 13]. Available from: https://www.ncbi.nlm.nih.gov/pubmed/18768236
- Pedersen ML. Diabetes mellitus in Greenland. Dan Med J. 2012 Feb;59(2):B4386.
- Jørgensen ME, Bjeregaard P, Borch-Johnsen K. Diabetes and impaired glucose tolerance among the inuit population of greenland. Diabetes Care. 2002 Oct;25(10):1766–1771.
- Grønlands Statistik [Internet]. [cited 2017 May 23]. Available from: http://www.stat.gl/default.asp?lang=en
- Indlandsisen smelter [Internet]. 2012 [cited 2017 Oct 10]. Available from: http://videnskab.dk/gronland-en-tikkende-klimabombe/indlandsisen-smelter
- Niclasen B, Mulvad G. Health care and health care delivery in Greenland. Int J Circumpolar Health. 2010 Dec 18;69(5):437–487.
- Bjerregaard P, Young TK. The circumpolar Inuit: health of a population in transition. Copenhagen: Munksgaard; 1998.
- Moltke I, Fumagalli M, Korneliussen TS, et al. Uncovering the genetic history of the present-day greenlandic population. Am J Hum Genet. 2015 Jan 8;96(1):54–69.
- Hvalfangst, handel og kristen mission i Grønland [Internet]. [cited 2017 Oct 9]. Available from: https://europas-lande.dk/dan/Lande/Gr%C3%B8nland/Historie/Hvalfangst%2C%20handel%20og%20kristen%20mission/mellem/
- [email protected]. Grønland [Internet]. [ cited 2017 Oct 9]. Available from: http://danmarkshistorien.dk/leksikon-og-kilder/vis/materiale/groenland/
- Grønland - historie (1500–1979) | Gyldendal. Den store danske [Internet]. [ cited 2017 Oct 9]. Available from: http://denstoredanske.dk/Geografi_og_historie/Grønland/Grønlands_samfund,_kultur_og_historie/Grønland_(Historie)/Grønland_(Historie_-_1500-1721)
- Bjerregaard P, Curtis T. Greenland population study. Cultural change and mental health in Greenland: the association of childhood conditions, language, and urbanization with mental health and suicidal thoughts among the inuit of Greenland. Soc Sci Med. 1982 2002 Jan;54(1):33–48.
- Larsen CVL, Curtis T, Bjerregaard P. Harmful alcohol use and frequent use of marijuana among lifetime problem gamblers and the prevalence of cross-addictive behaviour among Greenland Inuit: evidence from the cross-sectional inuit health in transition Greenland survey 2006–2010. Int J Circumpolar Health. 2013;72:19551.
- Dahl-Petersen IK, Lytken Larsen CV, Nielsen NO, et al. Befolkningsundersøgelsen i Grønland 2014. Levevilkår, livsstil og helbred. National Institute of Public Health, University of Southern Denmark; 2016.
- Rolskov AS, Bjorn-Mortensen K, Mulvad G, et al. Rapid change in the ciprofloxacin resistance pattern among Neisseria gonorrhoeae strains in Nuuk, Greenland: time to reconsider preventive and treatment strategies. Int J Circumpolar Health. 2015;74:26916.
- Gracey M, King M. Indigenous health part 1: determinants and disease patterns. Lancet Lond Engl. 2009 Jul 4;374(9683):65–75.
- King M, Smith A, Gracey M. Indigenous health part 2: the underlying causes of the health gap. Lancet Lond Engl. 2009 Jul 4;374(9683):76–85.
- Indigenous and tribal peoples’ health (The Lancet–Lowitja Institute Global Collaboration). a population study - the lancet [Internet]. [cited 2016 May 9]. Available from: http://www.thelancet.com/pdfs/journals/lancet/PIIS0140-6736(16)00345-7.pdf
- WHO | Health of indigenous peoples [Internet]. WHO. [cited 2018 Jan 4]. Available from: http://www.who.int/mediacentre/factsheets/fs326/en/
- Harris SB, Tompkins JW, TeHiwi B. Call to action: A new path for improving diabetes care for indigenous peoples, a global review. Diabetes Res Clin Pract. 2017 Jan;123:120–133.
- User S. IDF diabetes atlas - across the globe [Internet]. [cited 2018 Jan 5]. Available from: http://www.diabetesatlas.org/across-the-globe.html
- Yu CHY, Zinman B. Type 2 diabetes and impaired glucose tolerance in aboriginal populations: a global perspective. Diabetes Res Clin Pract. 2007 Nov;78(2):159–170.
- Bennett P, Burch T, Miller M. DIABETES MELLITUS IN AMERICAN (PIMA) INDIANS. Lancet. 1971 Jul 17;298(7716):125–128.
- Baier LJ, Muller YL, Remedi MS, et al. ABCC8 R1420H loss-of-function variant in a Southwest American Indian community: association with increased birth weight and doubled risk of type 2 diabetes. Diabetes. 2015 Dec;64(12):4322–4332.
- Acton KJ, Ríos Burrows N, Moore K, et al. Trends in diabetes prevalence among American Indian and Alaska native children, adolescents, and young adults. Am J Public Health. 2002 Sep;92(9):1485–1490.
- Statistics c=AU. O=commonwealth of A ou=Australian B of. chapter - diabetes [Internet]. 2013 [cited 2018 Jan 9]. Available from: http://www.abs.gov.au/ausstats/[email protected]/Lookup/CD58150AC0A36286CA257C2F0014591C?opendocument
- Cameron VA, Faatoese AF, Gillies MW, et al. A cohort study comparing cardiovascular risk factors in rural Māori, urban Māori and non-Māori communities in New Zealand. BMJ Open. 2012 Jun 8;2(3). cited 2018 Jan 9. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3378934/
- Lin S, Naseri T, Linhart C, et al. Trends in diabetes and obesity in Samoa over 35 years, 1978–2013. Diabet Med. 2017 May;34(5):654–661.
- Berthelsen A. Meddelerser om Grønland. Meddelser Om Grønl. 1940;117(3):126–127.
- Sagild U, Littauer J, Jespersen CS, et al. Epidemiological studies in Greenland 1962–1964. I. Diabetes mellitus in Eskimos. Acta Med Scand. 1966 Jan;179(1):29–39.
- Jørgensen ME, Borch-Johnsen K, Witte DR, et al. Diabetes in Greenland and its relationship with urbanization. Diabet Med J Br Diabet Assoc. 2012 Jun;29(6):755–760.
- Kromann N, Green A. Epidemiological studies in the Upernavik district, Greenland. Incidence of some chronic diseases 1950–1974. Acta Med Scand. 1980;208(5):401–406.
- Kromann N, Green A. Epidemiological studies in the upernavik district, Greenland. Incidence of some chronic diseases 1950–1974. Acta Med Scand. 1980;208(5):401–406.
- Moustgaard H, Bjerregaard P, Borch-Johnsen K, et al. Diabetes among Inuit migrants in Denmark. Int J Circumpolar Health. 2005 Sep;64(4):354–364.
- IDF diabetes atlas. Previous editions [Internet]. [cited 2017 Oct 20]. Available from: http://www.diabetesatlas.org/resources/previous-editions.html
- Jørgensen ME. Diabetes in Greenland - from Alfred Bertelsen to molecular diagnostics. Ugeskr Laeger. 2014 Sep 29;176(40):2066–2068.
- Jørgensen ME, Borch-Johnsen K, Witte DR, et al. Diabetes in Greenland and its relationship with urbanization. Diabet Med. 2012 Jun 1;29(6):755–760.
- Cheema A, Adeloye D, Sidhu S, et al. Urbanization and prevalence of type 2 diabetes in Southern Asia: A systematic analysis. J Glob Health [Internet]. 2014 Jun;4(1). [cited 2017 Dec 19]. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4073245/
- John WG. on behalf of the UK department of health advisory committee on diabetes. Use of HbA1c in the diagnosis of diabetes mellitus in the UK. The implementation of world health organization guidance 2011. Diabet Med. 2012 Nov 1;29(11):1350–1357.
- Hjellestad ID, Astor MC, Nilsen RM, et al. HbA1c versus oral glucose tolerance test as a method to diagnose diabetes mellitus in vascular surgery patients. Cardiovasc Diabetol. 2013 May 25;12:79.
- Christensen DL, Witte DR, Kaduka L, et al. Moving to an A1C-based diagnosis of diabetes has a different impact on prevalence in different ethnic groups. Diabetes Care. 2010 Mar;33(3):580–582.
- Pedersen ML, Bjerregaard P, Jørgensen ME. GAD65 antibodies among Greenland inuit and its relation to glucose intolerance. Acta Diabetol. 2014 Aug;51(4):641–646.
- Dahl-Petersen IK, Jørgensen ME, Bjerregaard P. Physical activity patterns in Greenland: a country in transition. Scand J Public Health. 2011 Nov;39(7):678–686.
- Dahl-Petersen IK, Bjerregaard P, Brage S, et al. Physical activity energy expenditure is associated with 2-h insulin independently of obesity among Inuit in Greenland. Diabetes Res Clin Pract. 2013 Dec;102(3):242–249.
- Jeppesen C, Bjerregaard P, Jørgensen ME. Dietary patterns in Greenland and their relationship with type 2 diabetes mellitus and glucose intolerance. Public Health Nutr. 2014 Feb;17(2):462–470.
- Pedersen MB, Hansen JC, Mulvad G, et al. Mercury accumulations in brains from populations exposed to high and low dietary levels of methyl mercury. Concentration, chemical form and distribution of mercury in brain samples from autopsies. Int J Circumpolar Health. 1999 Apr;58(2):96–107.
- Johansen P, Mulvad G, Pedersen HS, et al. Human accumulation of mercury in Greenland. Sci Total Environ. 2007 May 15;377(2–3):173–178.
- Jeppesen C, Valera B, Nielsen NO, et al. Association between whole blood mercury and glucose intolerance among adult Inuit in Greenland. Environ Res. 2015 Nov;143(Pt A):192–197.
- Jørgensen ME, Borch-Johnsen K, Bjerregaard P. A cross-sectional study of the association between persistent organic pollutants and glucose intolerance among Greenland Inuit. Diabetologia. 2008 Aug;51(8):1416–1422.
- Rejnmark L, Jørgensen ME, Pedersen MB, et al. Vitamin D insufficiency in Greenlanders on a westernized fare: ethnic differences in calcitropic hormones between Greenlanders and Danes. Calcif Tissue Int. 2004 Mar;74(3):255–263.
- Schæbel LK, Bonefeld-Jørgensen EC, Laurberg P, et al. Vitamin D-rich marine Inuit diet and markers of inflammation - a population-based survey in Greenland. J Nutr Sci. 2015;4:e40.
- Nielsen NO, Bjerregaard P, Rønn PF, et al. Associations between vitamin D status and type 2 diabetes measures among inuit in Greenland may be affected by other factors. PloS One. 2016;11(4):e0152763.
- Moltke I, Grarup N, Jørgensen ME, et al. A common Greenlandic TBC1D4 variant confers muscle insulin resistance and type 2 diabetes. Nature. 2014 Aug 14;512(7513):190–193.
- Mouratoff GJ, Carroll NV, Scott EM. Diabetes mellitus in Eskimos. JAMA. 1967 Mar 27;199(13):107–112.
- Mouratoff GJ, Carroll NV, Scott EM. Diabetes mellitus in Athabaskan Indians in Alaska. Diabetes. 1969 Jan;18(1):29–32.
- Scott EM, Griffith IV. Diabetes mellitus in Eskimos. Metabolism. 1957 Jul;6(4):320–325.
- Schaefer O. Medical observations and problems in the Canadian Arctic. II. Can Med Assoc J. 1959 Sep;1(81):386–393.
- Schaefer O. Glycosuria and diabetes mellitus in Canadian Eskimos. Can Med Assoc J. 1968 Aug 3;99(5):201–206.
- Thouez JP, Ekoé JM, Foggin PM, et al. Obesity, hypertension, hyperuricemia and diabetes mellitus among the cree and Inuit of northern Québec. Arctic Med Res. 1990 Oct;49(4):180–188.
- Stepanova EG, Shubnikov EW. Diabetes, glucose tolerance and some risk-factors of diabetes mellitus in natives and newcomers of chukotka. Arctic Med Res. 1991;39(Suppl):413–414.
- Murphy NJ, Schraer CD, Bulkow LR, et al. Diabetes mellitus in Alaskan Yup’ik Eskimos and Athabascan Indians after 25 yr. Diabetes Care. 1992 Oct;15(10):1390–1392.
- Schraer CD, Ebbesson SO, Adler AI, et al. Glucose tolerance and insulin-resistance syndrome among St. Lawrence Island Eskimos. Int J Circumpolar Health. 1998;57(Suppl 1):348–354.
- Ebbesson SO, Schraer CD, Risica PM, et al. Diabetes and impaired glucose tolerance in three Alaskan Eskimo populations. The Alaska-Siberia project. Diabetes Care. 1998 Apr;21(4):563–569.
- Carter EA, MacCluer JW, Dyke B, et al. Diabetes mellitus and impaired fasting glucose in Alaska Eskimos: the genetics of coronary artery disease in Alaska natives (GOCADAN) study. Diabetologia. 2006 Jan 1;49(1):29–35.
- Young TK, Schraer CD, Shubnikoff EV, et al. Prevalence of diagnosed diabetes in circumpolar indigenous populations. Int J Epidemiol. 1992 Aug;21(4):730–736.
- Schraer CD, Mayer AM, Vogt AM, et al. The Alaska native diabetes program. Int J Circumpolar Health. 2001 Nov;60(4):487–494.
- Ramesh M, Schraer C, Mayer AM, et al. Effect of special diabetes program for Indians funding on system changes in diabetes care and outcomes among American Indian/Alaska native people 1994–2004. Int J Circumpolar Health. 2008 Jun;67(2–3):203–212.
- Narayanan ML, Schraer CD, Bulkow LR, et al. Diabetes prevalence, incidence, complications and mortality among Alaska Native people 1985–2006. Int J Circumpolar Health. 2010 Jun;69(3):236–252.
- Johnson S, Martin D, Sarin C. Diabetes mellitus in the first nations population of British Columbia, Canada. part 3. Prevalence of diagnosed cases. Int J Circumpolar Health. 2002 Aug;61(3):260–264.
- Young TK, Reading J, Elias B, et al. Type 2 diabetes mellitus in Canada‚s first nations: status of an epidemic in progress. CMAJ Can Med Assoc J. 2000 Sep 5;163(5):561–566.
- Chamberlain C, McNamara B, Williams ED, et al. Diabetes in pregnancy among indigenous women in Australia, Canada, New Zealand and the United States: a systematic review of the evidence for screening in early pregnancy. Diabetes Metab Res Rev. 2013 May;29(4):241–256.
- Chamberlain C, Banks E, Joshy G, et al. Prevalence of gestational diabetes mellitus among indigenous women and comparison with non-Indigenous Australian women: 1990–2009. Aust N Z J Obstet Gynaecol. 2014 Oct;54(5):433–440.
- Chamberlain C, Fredericks B, Davis B, et al. Postpartum care for aboriginal and non-aboriginal women with gestational diabetes mellitus across urban, rural and remote locations: a protocol for a cohort linkage study. Springer Plus [Internet]. 2. 2013 Oct 30 [cited 2018 Jan 9]. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4320232/
- Chamberlain CR, Oldenburg B, Wilson AN, et al. Type 2 diabetes after gestational diabetes: greater than fourfold risk among indigenous compared with non-Indigenous Australian women. Diabetes Metab Res Rev. 2016 Feb;32(2):217–227.
- Murphy NJ, Bulkow LR, Schraer CD, et al. Prevalence of diabetes mellitus in pregnancy among Yup’ik Eskimos, 1987–1988. Diabetes Care. 1993 Jan;16(1):315–317.
- Alberti KGMM, Zimmet PZ. Definition, diagnosis and classification of diabetes mellitus and its complications. Part 1: diagnosis and classification of diabetes mellitus. Provisional report of a WHO consultation. Diabet Med. 1998 Jul 1;15(7):539–553.
- CATALANO PM, HAUGUEL-DE MOUZON S. Is it time to revisit the pedersen hypothesis in the face of the obesity epidemic? Am J Obstet Gynecol. 2011 Jun;204(6):479–487.
- Lindsay RS. Many HAPO returns maternal glycemia and neonatal adiposity: new Insights from the Hyperglycemia and adverse pregnancy outcomes (HAPO) study. Diabetes. 2009 Feb 1;58(2):302–303.
- Zawiejska A, Wender-Ozegowska E, Radzicka S, et al. Maternal hyperglycemia according to IADPSG criteria as a predictor of perinatal complications in women with gestational diabetes: a retrospective observational study. J Matern Fetal Neonatal Med. 2014 Oct;27(15):1526–1530.
- Schmidt MI, Duncan BB, Reichelt AJ, et al. Gestational diabetes mellitus diagnosed with a 2-h 75-g oral glucose tolerance test and adverse pregnancy outcomes. Diabetes Care. 2001 Jul;24(7):1151–1155.
- Lauenborg J, Hansen T, Jensen DM, et al. Increasing incidence of diabetes after gestational diabetes: a long-term follow-up in a Danish population. Diabetes Care. 2004 May;27(5):1194–1199.
- Clausen TD, Mathiesen ER, Hansen T, et al. High prevalence of type 2 diabetes and pre-diabetes in adult offspring of women with gestational diabetes mellitus or type 1 diabetes: the role of intrauterine hyperglycemia. Diabetes Care. 2008 Feb;31(2):340–346.
- Mohamed N, Dooley J. Gestational diabetes and subsequent development of NIDDM in aboriginal women of northwestern Ontario. Int J Circumpolar Health. 1998;57(Suppl 1):355–358.
- Clausen TD, Mathiesen ER, Hansen T, et al. Overweight and the metabolic syndrome in adult offspring of women with diet-treated gestational diabetes mellitus or type 1 diabetes. J Clin Endocrinol Metab. 2009 Jul;94(7):2464–2470.
- Franks PW, Looker HC, Kobes S, et al. Gestational glucose tolerance and risk of type 2 diabetes in young Pima Indian offspring. Diabetes. 2006 Feb;55(2):460–465.
- Dabelea D, Knowler WC, Pettitt DJ. Effect of diabetes in pregnancy on offspring: follow-up research in the Pima Indians. J Matern Fetal Med. 2000 Feb;9(1):83–88.
- Ferrara A, Kahn HS, Quesenberry CP, et al. An increase in the incidence of gestational diabetes mellitus: Northern California, 1991–2000. Obstet Gynecol. 2004 Mar;103(3):526–533.
- Landon MB, Spong CY, Thom E, et al. A multicenter, randomized trial of treatment for mild gestational diabetes. N Engl J Med. 2009 Oct 1;361(14):1339–1348.
- Crowther CA, Hiller JE, Moss JR, et al. Effect of treatment of gestational diabetes mellitus on pregnancy outcomes. N Engl J Med. 2005 Jun 16;352(24):2477–2486.
- Brown J, Alwan NA, West J, et al. Lifestyle interventions for the treatment of women with gestational diabetes. Cochrane Database Syst Rev. 2017 May;4(5):CD011970.
- Brown J, Grzeskowiak L, Williamson K, et al. Insulin for the treatment of women with gestational diabetes. Cochrane Database Syst Rev. 2017;05(11):CD012037.
- Buckley BS, Harreiter J, Damm P, et al. Gestational diabetes mellitus in Europe: prevalence, current screening practice and barriers to screening. A review. Diabet Med J Br Diabet Assoc. 2012 Jul;29(7):844–854.
- Catalano PM, McIntyre HD, Cruickshank JK, et al. The hyperglycemia and adverse pregnancy outcome study: associations of GDM and obesity with pregnancy outcomes. Diabetes Care. 2012 Apr;35(4):780–786.
- Panel* IA of D and PSGC. International association of diabetes and pregnancy study groups recommendations on the diagnosis and classification of hyperglycemia in pregnancy. Diabetes Care. 2010 Mar;33(3):676.
- Berkowitz GS, Lapinski RH, Wein R, et al. Race/ethnicity and other risk factors for gestational diabetes. Am J Epidemiol. 1992 May 1;135(9):965–973.
- Oster RT, Toth EL. Differences in the prevalence of diabetes risk-factors among first nation, métis and non-aboriginal adults attending screening clinics in rural Alberta, Canada. Rural Remote Health. 2009 Jun;9(2):1170.
- Chamberlain C, McNamara B, Williams ED, et al. Diabetes in pregnancy among indigenous women in Australia, Canada, New Zealand and the United States: a systematic review of the evidence for screening in early pregnancy. Diabetes Metab Res Rev. 2013 May;29(4):241–256.
- Gestational diabetes. Symptoms and causes [Internet]. Mayo clinic. [cited 2018 Jan 10]. Available from: http://www.mayoclinic.org/diseases-conditions/gestational-diabetes/symptoms-causes/syc-20355339
- Sridhar SB, Darbinian J, Ehrlich SF, et al. Maternal gestational weight gain and offspring risk for childhood overweight or obesity. Am J Obstet Gynecol. 2014 Sep;211(3):259.e1–8.
- Xiang AH, Li BH, Black MH, et al. Racial and ethnic disparities in diabetes risk after gestational diabetes mellitus. Diabetologia. 2011 Dec;54(12):3016–3021.
- Hedderson MM, Darbinian JA, Ferrara A. Disparities in the risk of gestational diabetes by race-ethnicity and country of birth. Paediatr Perinat Epidemiol. 2010 Sep;24(5):441–448.
- Ferrara A. Increasing prevalence of gestational diabetes mellitus: a public health perspective. Diabetes Care. 2007 Jul;30(Suppl 2):S141–146.
- Ishak M, Petocz P. Gestational diabetes among aboriginal Australians: prevalence, time trend, and comparisons with non-aboriginal Australians. Ethn Dis. 2003;13(1):55–60.
- Yapa M, Simmons D. Screening for gestational diabetes mellitus in a multiethnic population in New Zealand. Diabetes Res Clin Pract. 2000 Jun;48(3):217–223.
- Dornhorst A, Paterson CM, Nicholls JS, et al. High prevalence of gestational diabetes in women from ethnic minority groups. Diabet Med J Br Diabet Assoc. 1992 Nov;9(9):820–825.
- Jeppesen C, Maindal HT, Kristensen JK, et al. National study of the prevalence of gestational diabetes mellitus among Danish women from 2004 to 2012. Scand J Public Health. 2017 Dec;45(8):811–817.
- Beischer NA, Oats JN, Henry OA, et al. Incidence and severity of gestational diabetes mellitus according to country of birth in women living in Australia. Diabetes. 1991 Dec;40(Suppl 2):35–38.
- Davis B, McLean A, Sinha AK, et al. A threefold increase in gestational diabetes over two years: review of screening practices and pregnancy outcomes in Indigenous women of Cape York, Australia. Aust N Z J Obstet Gynaecol. 2013 Aug;53(4):363–368.
- Daly B, Raiman I, Goodson J. Screening for diabetes in pregnancy in a regional area with a high Māori population. N Z Med J. 2017 Feb 17;130(1450):25–31.
- Porter C, Skinner T, Ellis I. The current state of indigenous and Aboriginal women with diabetes in pregnancy: a systematic review. Diabetes Res Clin Pract. 2012 Nov;98(2):209–225.
- Murphy NJ, Bulkow LR, Schraer CD, et al. Prevalence of diabetes mellitus in pregnancy among Yup’ik Eskimos, 1987–1988. Diabetes Care. 1993 Jan;16(1):315–317.
- Rao Kondapally Seshasai S, Kaptoge S, Thompson A, et al. Diabetes mellitus, fasting glucose, and risk of cause-specific death. N Engl J Med. 2011 Mar 3;364(9):829–841.
- Leasher JL, Bourne RRA, Flaxman SR, et al. Global Estimates on the number of people blind or visually impaired by diabetic retinopathy: a meta-analysis from 1990 to 2010. Diabetes Care. 2016 Sep 1;39(9):1643–1649.
- Bourne RRA, Stevens GA, White RA, et al. Causes of vision loss worldwide, 1990–2010: a systematic analysis. Lancet Glob Health. 2013 Dec;1(6):e339–349.
- Yau JWY, Rogers SL, Kawasaki R, et al. Global prevalence and major risk factors of diabetic retinopathy. Diabetes Care. 2012 Mar;35(3):556–564.
- Gupta R, Misra A. Epidemiology of microvascular complications of diabetes in South Asians and comparison with other ethnicities. J Diabetes. 2016 Jun 1;8(4):470–482.
- Sivaprasad S, Gupta B, Crosby-Nwaobi R, et al. Prevalence of diabetic retinopathy in various ethnic groups: a worldwide perspective. Surv Ophthalmol. 2012 Aug;57(4):347–370.
- Lanting LC, Joung IMA, Mackenbach JP, et al. Ethnic differences in mortality, end-stage complications, and quality of care among diabetic patients: a review. Diabetes Care. 2005 Sep;28(9):2280–2288.
- Ross SA, McKenna A, Mozejko S, et al. Diabetic retinopathy in native and nonnative Canadians. Exp Diabetes Res [Internet]. 2007;2007. [cited 2017 Jul 12]. Available from: http://www.ncbi.nlm.nih.gov/pmc/articles/PMC2248248/
- Clark A, Morgan WH, Kain S, et al. Diabetic retinopathy and the major causes of vision loss in aboriginals from remote Western Australia. Clin Experiment Ophthalmol. 2010 Jul;38(5):475–482.
- Foreman J, Xie J, Keel S, et al. The prevalence and causes of vision loss in indigenous and non-indigenous Australians: the national eye health survey. Ophthalmology. 2017 Dec;124(12):1743–1752.
- Spanakis EK, Golden SH. Race/ethnic difference in diabetes and diabetic complications. Curr Diab Rep [Internet]. 2013 Dec;13(6). [cited 2017 Jul 14]. Available from: http://www.ncbi.nlm.nih.gov/pmc/articles/PMC3830901/
- Davies M, Brophy S, Williams R, et al. The prevalence, severity, and impact of painful diabetic peripheral neuropathy in type 2 diabetes. Diabetes Care. 2006; 291518–1522.
- Edwards JL, Vincent A, Cheng T, et al. Diabetic neuropathy: mechanisms to management. Pharmacol Ther. 2008 Oct;120(1):1–34.
- Boulton AJM, Vinik AI, Arezzo JC, et al. Diabetic neuropathies: a statement by the American diabetes association. Diabetes Care. 2005 Apr;28(4):956–962.
- Davis TME, McAullay D, Davis WA, et al. Characteristics and outcome of type 2 diabetes in urban aboriginal people: the fremantle diabetes study. Intern Med J. 2007 Jan;37(1):59–63.
- Hanley AJG, Harris SB, Mamakeesick M, et al. Complications of type 2 diabetes among aboriginal Canadians: prevalence and associated risk factors. Diabetes Care. 2005 Aug 1;28(8):2054–2057.
- Hoy W, Light A, Megill D. Cardiovascular disease in Navajo Indians with type 2 diabetes. Public Health Rep Wash DC 1974. 1995 Feb;110(1):87–94.
- Schulz LO, Lalicata M, Carnes D, et al. Prevalence of diabetes and factors associated with diabetic complications in Oneida Indians. Life Sci. 1997;60(4–5):299–306.
- Chuback J, Embil JM, Sellers E, et al. Foot abnormalities in Canadian aboriginal adolescents with type 2 diabetes. Diabet Med. 2007 Jul 1;24(7):747–752.
- Brassard P, Robinson E, Dumont C. Descriptive epidemiology of non-insulin-dependent diabetes mellitus in the James Bay Cree population of Quebec, Canada. Arctic Med Res. 1993 Apr;52(2):47–54.
- Parving -H-H, Lewis JB, Ravid M, et al. Prevalence and risk factors for microalbuminuria in a referred cohort of type II diabetic patients: A global perspective. Kidney Int. 2006 Jun;69(11):2057–2063.
- Risk Factors for End-stage Renal Disease (ESRD) | Winchester Hospital [internet]. [cited 2018 Mar 9]. Available from: http://www.winchesterhospital.org/health-library/article?id=19430
- Narres M, Claessen H, Droste S, et al. The incidence of end-stage renal disease in the diabetic (compared to the non-diabetic) population: a systematic review. PLoS One. 2016 Jan 26;11(1). cited 2018 Mar 8. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4727808/
- Gregg EW, Sattar N, Ali MK. The changing face of diabetes complications. Lancet Diabetes Endocrinol. 2016 Jun 1;4(6):537–547.
- Burrows NR, Narva AS, Geiss LS, et al. End-stage renal disease due to diabetes among southwestern American Indians, 1990–2001. Diabetes Care. 2005 May;28(5):1041–1044.
- Dyck RF, Tan L. Rates and outcomes of diabetic end-stage renal disease among registered native people in Saskatchewan. CMAJ Can Med Assoc J. 1994 Jan 15;150(2):203–208.
- Joshy G, Dunn P, Fisher M, et al. Ethnic differences in the natural progression of nephropathy among diabetes patients in New Zealand: hospital admission rate for renal complications, and incidence of end-stage renal disease and renal death. Diabetologia. 2009 Aug 1;52(8):1474–1478.
- Moxey PW, Gogalniceanu P, Hinchliffe RJ, et al. Lower extremity amputations–a review of global variability in incidence. Diabet Med J Br Diabet Assoc. 2011 Oct;28(10):1144–1153.
- Dierena SV, Beulensa JWJ, Schouwa YTVD, et al. Review paper the global burden of diabetes and its complications: an emerging pandemic.
- Acs A, Ludwig C, Bereza BG, et al. Prevalence of cardiovascular disease in type 2 diabetes: a global systematic review. Value Health. 2017 Oct 1;20(9):A475.
- Young BA, Maynard C, Boyko EJ. Racial differences in diabetic nephropathy, cardiovascular disease, and mortality in a national population of veterans. Diabetes Care. 2003 Aug;26(8):2392–2399.
- Bramley D, Hebert P, Jackson R, et al. Indigenous disparities in disease-specific mortality, a cross-country comparison: New Zealand, Australia, Canada, and the United States. N Z Med J. 2004 Dec 17;117(1207):U1215.
- Hales CN, Barker DJ. The thrifty phenotype hypothesis. Br Med Bull. 2001;60:5–20.
- Pedersen ML, Jacobsen JL, Lynge AR. Micro- and macrovascular complications among Greenlanders and Danes with type 2 diabetes mellitus in Nuuk, Greenland. Int J Circumpolar Health. 2010 Apr;69(2):195–207.
- Schraer C, Adler A, Mayer A M, et al. Diabetes complications and mortality among alaska natives: 8 years of observation. Diabetes Care. Vol. 20. p. 314. 1997.
- Garratt AM, Schmidt L, Fitzpatrick R. Patient-assessed health outcome measures for diabetes: a structured review. Diabet Med J Br Diabet Assoc. 2002 Jan;19(1):1–11.
- O’Connor PJ, Bodkin NL, Fradkin J, et al. Diabetes performance measures: current status and future directions. Diabetes Care. 2011 Jul;34(7):1651–1659.
- Association AD. 1. Strategies for improving care. Diabetes Care. 2016 Jan 1;39(Supplement 1):S6–12.
- Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). UK Prospective Diabetes Study (UKPDS) group. Lancet Lond Engl. 1998 Sep 12;352(9131):837–853.
- Calsbeek H, Ketelaar NABM, Faber MJ, et al. Performance measurements in diabetes care: the complex task of selecting quality indicators. Int J Qual Health Care. 2013 Dec 1;25(6):704–709.
- Nicolucci A, Greenfield S, Mattke S. Selecting indicators for the quality of diabetes care at the health systems level in OECD countries. Int J Qual Health Care. 2006 Sep 1;18(suppl_1):26–30.
- OECD Health Care Quality Indicators Project. history and background | international journal for quality in health care | Oxford academic [Internet]. [cited 2018 Feb 6]. Available from: https://academic.oup.com/intqhc/article/18/suppl_1/1/1798452
- Si D, Bailie R, Wang Z, et al. Comparison of diabetes management in five countries for general and indigenous populations: an internet-based review. BMC Health Serv Res. 2010;10:169.
- Nicolucci A, Greenfield S, Mattke S. Selecting indicators for the quality of diabetes care at the health systems level in OECD countries. Int J Qual Health Care J Int Soc Qual Health Care ISQua. 2006 Sep;18(Suppl 1):26–30.
- Greenfield S, Nicolucci A, Mattke S. Selecting indicators for the quality of diabetes care at the health systems level in OECD countries. 2004 Oct 28. [cited 2018 Feb 6]. Available from: http://www.oecd-ilibrary.org/social-issues-migration-health/selecting-indicators-for-the-quality-of-diabetes-care-at-the-health-systems-level-in-oecd-countries_165531523300
- HealthPolicyMonitor | Surveys| University of Southern Denmark. Denmark| 12| the national indicator project [Internet]. [cited 2018 Feb 6]. Available from: http://hpm.org/en/Surveys/University_of_Southern_Denmark_-_Denmark/12/The_National_Indicator_Project.html
- Hippisley-Cox J, O’Hanlon S, Coupland C. Association of deprivation, ethnicity, and sex with quality indicators for diabetes: population based survey of 53 000 patients in primary care. BMJ. 2004 Nov 27;329(7477):1267–1269.
- Improving Diabetes Care in Rural Areas. A systematic review and meta-analysis of quality improvement interventions in OECD countries [Internet]. [cited 2018 Feb 6]. Available from: http://journals.plos.org/plosone/article?id=10.1371/journal.pone.0084464
- Diabetes Care and Outcomes. Disparities across rural America [Internet]. Medscape. [cited 2018 Feb 6]. Available from: http://www.medscape.com/viewarticle/729003
- Viswanathan V, Madhavan S, Rajasekar S, et al. Urban-rural differences in the prevalence of foot complications in South-Indian diabetic patients. Diabetes Care. 2006 Mar 1;29(3):701–703.
- Grover A, Joshi A. An overview of chronic disease models: a systematic literature review. Glob J Health Sci. 2015 Mar;7(2):210–227.
- Leung L. Diabetes mellitus and the aboriginal diabetic initiative in Canada: an update review. J Fam Med Prim Care. 2016;5(2):259–265.
- Pedersen ML. Prevalence of diagnosed type 2 diabetes mellitus in Greenland 2008: the impact of electronic database implementation on the quality of diabetes care. Int J Circumpolar Health. 2009 Feb;68(1):34–41.
- Birch E, Andersson M, Koch A, et al. Ten years of tuberculosis intervention in Greenland – has it prevented cases of childhood tuberculosis? Int J Circumpolar Health [Internet]. 2014 Jul 11;73. [cited 2017 Nov 6]. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4095760/
- Berntsen S, Karlsen APH, Pedersen ML, et al. Gonorrhoea in Greenland, incidence and previous preventive measures: a review to improve future strategies. Int J Circumpolar Health [Internet]. 2017 Jul 26;76(1). 1350092. [cited 2017 Nov 6]. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5533119/
- Rex KF, Andersen S, Krarup HB. Hepatitis B among Inuit: A review with focus on Greenland Inuit. World J Hepatol. 2015 May 28;7(9):1265–1271.
- Stensgaard T. Telemedicine in Greenland–from the early beginnings to the making of a business plan. J Telemed Telecare. 2000;6(Suppl 1):S158–9.
- Stensgaard T, Sørensen T. Telemedicine in Greenland–the creation of an evaluation plan. J Telemed Telecare. 2001;7(Suppl 1):37–38.
- Nielsen LO, Olsen S, Jarbøl DE, et al. Spirometry in Greenland: a cross-sectional study on patients treated with medication targeting obstructive pulmonary disease. Int J Circumpolar Health [Internet]. 2016 Dec 8;75. [cited 2017 Nov 6]. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5148803/
- Tvermosegaard M, Rønn PF, Pedersen ML, et al. Validation of cardiovascular diagnoses in the greenlandic hospital discharge register for epidemiological use. Int J Circumpolar Health. 2018 Dec;77(1):1422668.
- Pedersen ML, Olesen J, Jørgensen ME, et al. Gestational diabetes mellitus in Greenland: a national study of prevalence and testing effectiveness. Int J Circumpolar Health. 2016 Jan;75(1):32167.
- Pedersen ML. Management of type 2 diabetes mellitus in Greenland, 2008: examining the quality and organization of diabetes care. Int J Circumpolar Health. 2009 Apr;68(2):123–132.
- Renders CM, Valk GD, Griffin SJ, et al. Interventions to improve the management of diabetes in primary care, outpatient, and community settings: a systematic review. Diabetes Care. 2001 Oct;24(10):1821–1833.
- Shephard M, Shephard A, McAteer B, et al. Results from 15years of quality surveillance for a national indigenous point-of-care testing program for diabetes. Clin Biochem. 2017 Dec;50(18):1159–1163.
- American Diabetes Association® [Internet]. [cited 2018 Feb 14]. Available from: http://www.diabetes.org/
- European Society of Cardiology [Internet]. [cited 2018 Feb 14]. Available from: https://www.escardio.org/
- Welcome | EASD [Internet]. [cited 2018 Feb 14]. Available from: https://www.easd.org/
- English. DSAM - Dansk Selskab for Almen Medicin [Internet]. [cited 2018 Feb 14]. Available from: http://www.dsam.dk/flx/english/
- Szymezak J, Leroy N, Lavalard E, et al. Evaluation of the DCA Vantage analyzer for HbA 1c assay. Clin Chem Lab Med. 2008;46(8):1195–1198.
- Lambers Heerspink HJ, Witte EC, Bakker SJL, et al. Screening and monitoring for albuminuria: the performance of the hemocue point-of-care system. Kidney Int. 2008 Aug 1;74(3):377–383.
- Omoruyi FO, Mustafa GM, Okorodudu AO, et al. Evaluation of the performance of urine albumin, creatinine and albumin-creatinine ratio assay on two POCT analyzers relative to a central laboratory method. Clin Chim Acta Int J Clin Chem. 2012 Mar 22;413(5–6):625–629.
- Lenters-Westra E, Slingerland RJ. Three of 7 hemoglobin A1c point-of-care instruments do not meet generally accepted analytical performance criteria. Clin Chem. 2014 Aug;60(8):1062–1072.
- Shahshahani HJ, Meraat N, Mansouri F. Evaluation of the validity of a rapid method for measuring high and low haemoglobin levels in whole blood donors. Blood Transfus Trasfus Sangue. 2013 Jul;11(3):385–390.
- Nkrumah B, Nguah SB, Sarpong N, et al. Hemoglobin estimation by the HemoCue® portable hemoglobin photometer in a resource poor setting. BMC Clin Pathol. 2011 Apr;21(11):5.
- Foerster V, Severn M. Point-of-care glycated hemoglobin testing to diagnose type 2 diabetes. In: CADTH issues in emerging health technologies [Internet]. Ottawa (ON): Canadian Agency for Drugs and Technologies in Health; 2016. [cited 2017 Jun01]. Available from: http://www.ncbi.nlm.nih.gov/books/NBK476441/
- Lin DY, Blumenkranz MS, Brothers RJ, et al. The sensitivity and specificity of single-field nonmydriatic monochromatic digital fundus photography with remote image interpretation for diabetic retinopathy screening: a comparison with ophthalmoscopy and standardized mydriatic color photography11InternetAdvance publication at ajo.com. April 12, 2002. Am J Ophthalmol. 2002 Aug 1;134(2):204–213.
- Daytona [Internet]. [cited 2018 Feb 15]. Available from: https://www.optos.com/en/products/daytona/
- Association AD. Nephropathy in diabetes. Diabetes Care. 2004 Jan 1;27(suppl 1):s79–83.
- 10. Microvascular Complications and Foot Care | Diabetes Care [Internet]. [cited 2018 Feb 14]. Available from: http://care.diabetesjournals.org/content/40/Supplement_1/S88
- ARCHITECT c8000 Clinical Chemistry | Abbott Core Laboratory [Internet]. [cited 2018 Feb 15]. Available from: https://www.corelaboratory.abbott/us/en/offerings/brands/architect/architect-c8000
- Pourhamidi K, Dahlin LB, Englund E, et al. Evaluation of clinical tools and their diagnostic use in distal symmetric polyneuropathy. Prim Care Diabetes. 2014 Apr;8(1):77–84.
- Educating people with diabetes about foot care to help reduce foot ulcers and amputations | Cochrane [Internet]. [cited 2018 Feb 19]. Available from: /CD001488/WOUNDS_educating-people-with-diabetes-about-foot-care-to-help-reduce-foot-ulcers-and-amputations
- Combined strategies to avoid foot ulcers in patients with diabetes | Cochrane [Internet]. [cited 2018 Feb 19]. Available from: /CD007610/WOUNDS_combined-strategies-avoid-foot-ulcers-patients-diabetes
- 9. Cardiovascular Disease and Risk Management | Diabetes Care [Internet]. [cited 2018 Feb 12]. Available from: http://care.diabetesjournals.org/content/40/Supplement_1/S75
- Chapelle J-P, Teixeira J, Maisin D, et al. Multicentre evaluation of the Tosoh HbA1c G8 analyser. Clin Chem Lab Med. 2010 Mar;48(3):365–371.
- WHO | Diagnostic criteria and classification of hyperglycaemia first detected in pregnancy [Internet]. WHO. [cited 2018 Feb 19]. Available from: http://www.who.int/diabetes/publications/Hyperglycaemia_In_Pregnancy/en/
- New thresholds for diagnosis of diabetes in pregnancy | News and features | News [Internet]. NICE. [cited 2018 Feb 19]. Available from: https://www.nice.org.uk/news/article/new-thresholds-for-diagnosis-of-diabetes-in-pregnancy
- Neely RD, Kiwanuka JB, Hadden DR. Influence of sample type on the interpretation of the oral glucose tolerance test for gestational diabetes mellitus. Diabet Med J Br Diabet Assoc. 1991 Mar;8(2):129–134.
- FIGO GDM and Maternal Nutrition Guidelines. FinalFIGO GDM and maternal nutrition guidelines news release VANCOUVER final Oct. 6 (1) (1).pdf [Internet]. [cited 2017 Jun 6]. Available from: http://www.figo.org/sites/default/files/uploads/News/FinalFIGO%20GDM%20and%20Maternal%20Nutrition%20Guidelines%20news%20release%20VANCOUVER%20Final%20Oct.%206%20%281%29%20%281%29.pdf
- Guidelines [Internet]. [cited 2018 Feb 15]. Available from: https://www.idf.org/e-library/guidelines/96-management-of-gestational-diabetes-in-the-community.html
- Crowther CA, Hiller JE, Moss JR, et al. Effect of treatment of gestational diabetes mellitus on pregnancy outcomes. N Engl J Med. 2005 Jun 16;352(24):2477–2486.
- 2013 ESH/ESC Guidelines for the management of arterial hypertension | European Heart Journal | Oxford Academic [Internet]. [cited 2018 Feb 19]. Available from: https://academic.oup.com/eurheartj/article/34/28/2159/451304
- 2017 Guideline for High Blood Pressure in Adults. American college of cardiology [Internet]. [cited 2018 Feb 19]. Available from: http://www.acc.org/latest-in-cardiology/ten-points-to-remember/2017/11/09/11/41/2017-guideline-for-high-blood-pressure-in-adults
- Global Initiative for Chronic Obstructive Lung Disease [Internet]. Global initiative for chronic obstructive lung disease - GOLD. [cited 2018 Feb 19]. Available from: http://goldcopd.org/
- Classes of Heart Failure [Internet]. [cited 2018 Feb 19]. Available from: http://www.heart.org/HEARTORG/Conditions/HeartFailure/AboutHeartFailure/Classes-of-Heart-Failure_UCM_306328_Article.jsp#.WosRn3yQzIU
- Reiss AE, Pedersen ML. Smoking among patients in the primary health care system in Nuuk, Greenland. Clin Nurs Stud. 2014 Sep 10;2(4):74.
- Bundgaard M, Jarbøl DE, Paulsen MS, et al. Prevalence of the use of antihypertensive medications in Greenland: a study of quality of care amongst patients treated with antihypertensive drugs. Int J Circumpolar Health [Internet]. 2012 Jun 13;71. [cited 2017 Nov 3]. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3417658/
- Olsen S, Jarbøl DE, Kofoed M, et al. Prevalence and management of patients using medication targeting obstructive lung disease: A cross-sectional study in primary healthcare in Greenland. Int J Circumpolar Health [Internet]. 2013 Feb 28;72. [cited 2015 Oct 19]. Available from: http://www.ncbi.nlm.nih.gov/pmc/articles/PMC3585771/
- Pedersen ML, Rolskov A, Jacobsen JL, et al. Frequent use of primary health care service in Greenland: an opportunity for undiagnosed disease case-finding. Int J Circumpolar Health [Internet]. 2012 Jul 24;71. [cited 2017 Nov 3]. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3417523/
- Pedersen ML, Jacobsen JL. Improvement of diabetes care in a small but geographically widely spread population in Greenland. Effects of a national diabetes care programme. Diabet Med J Br Diabet Assoc. 2011 Nov;28(11):1425–1432.
- Damsgaard L, Pedersen ML. Use of glycosylated haemoglobin as diagnostic tool in Greenland: prevalence of diagnosed diabetes mellitus. Diabetol Metab Syndr. 2013;5(1):59.
- Pedersen ML. High awareness of diabetes in the health care system in Greenland measured as a proportion of population tested with glycated haemoglobin within 2 years. Diabetol Metab Syndr [Internet]. 2017 May 3;9. [cited 2017 Nov 3]. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5415826/
- Viskum ES, Pedersen ML. Prevalence of diagnosed diabetes and quality of care among Greenlanders and non-Greenlanders in Greenland. Diabetes Res Clin Pract. 2016 Nov;121:91–98.
- Pedersen ML, Jacobsen JL, Jørgensen ME. Prevalence of gestational diabetes mellitus among women born in Greenland: measuring the effectiveness of the current screening procedure. Int J Circumpolar Health. 2010 Sep;69(4):352–360.
- Pedersen ML. Microvascular complications in Nuuk, Greenland, among Greenlanders and non-Greenlanders diagnosed with type 2 diabetes. Diabetes Res Clin Pract. 2018 Feb;136:1–6.
- Greenlands Statistics [Internet]. [cited 2016 Feb 9]. Available from: http://www.stat.gl/default.asp?lang=en
- Kowall B, Rathmann W, Landgraf R. Is HbA1c a valid and feasible tool for the diagnosis of diabetes? Diabetes Res Clin Pract. 2011 Sep 1;93(3):314–316.
- WHO | International Classification of Primary Care, Second edition (ICPC-2) [Internet]. WHO. [cited 2016 Feb 16]. Available from: http://www.who.int/classifications/icd/adaptations/icpc2/en/
- Setting: National health and nutrition examination survey (1988– 1994 and 1999–2002) and the behavioral risk factor surveillance. 2006.
- Solomon SD, Chew E, Duh EJ, et al. Diabetic retinopathy: a position statement by the American Diabetes Association. Diabetes Care. 2017 Mar 1;40(3):412–418.
- Statements | EASD [Internet]. [cited 2018 Feb 20]. Available from: https://www.easd.org/statements.html
- Guidelines [Internet]. [cited 2018 Feb 15]. Available from: https://www.idf.org/e-library/guidelines.html
- Colagiuri S, Sandbaek A, Carstensen B, et al. Comparability of venous and capillary glucose measurements in blood. Diabet Med J Br Diabet Assoc. 2003 Nov;20(11):953–956.
- Bhavadharini B, Mahalakshmi MM, Maheswari K, et al. Use of capillary blood glucose for screening for gestational diabetes mellitus in resource-constrained settings. Acta Diabetol. 2016 Feb;53(1):91–97.
- Global Family Doctor. WONCA online [Internet]. [cited 2017 May 23]. Available from: http://www.globalfamilydoctor.com/groups/WorkingParties/wicc.aspx
- Easy Rapid [Internet]. Pic solution - en. [cited 2017 May 23]. Available from: http://www.picsolution.com/content/pic/en/products/for-blood-pressure-measurement/quick-measurement-for-all/for-people-who-need-simplicity.html
- Jayaprakash P, Bhansali A, Bhansali S, et al. Validation of bedside methods in evaluation of diabetic peripheral neuropathy. Indian J Med Res. 2011 Jun;133(6):645–649.
- Koch A, Sørensen P, Homøe P, et al. Population-based study of acute respiratory infections in children, Greenland. Emerg Infect Dis. 2002 Jun;8(6):586–593.
- Koch A, Mølbak K, Homøe P, et al. Risk factors for acute respiratory tract infections in young Greenlandic children. Am J Epidemiol. 2003 Aug 15;158(4):374–384.
- Otitis Media in Greenland. studies on historical, epidemiological, microbiological, and immunological aspects. Int J Circumpolar Health [Internet]. 60;(sup2). [cited 2018 Feb 11]. Available from. http://www.tandfonline.com/doi/abs/10.3402/ijch.v60i0.18306
- Simmons RK, Griffin SJ, Witte DR, et al. Effect of population screening for type 2 diabetes and cardiovascular risk factors on mortality rate and cardiovascular events: a controlled trial among 1,912,392 Danish adults. Diabetologia. 2017 Nov 1;60(11):2183–2191.
- Holst S, Wohlfahrt J, Kjær SK, et al. Cervical cancer screening in Greenland, 1997–2011: screening coverage and trends in the incidence of high-grade cervical lesions. Gynecol Oncol. 2016 Nov;143(2):307–312.
- Manousaki D, Kent JW, Haack K, et al. Toward precision medicine: TBC1D4 disruption is common among the inuit and leads to underdiagnosis of type 2 diabetes. Diabetes Care. 2016 Nov;39(11):1889–1895.
- Pedersen ML, Rolskov A, Jacobsen JL, et al. Frequent use of primary health care service in Greenland: an opportunity for undiagnosed disease case-finding. Int J Circumpolar Health. 2012;71:18431.
- Jørgensen ME, Glümer C, Bjerregaard P, et al. Obesity and central fat pattern among Greenland Inuit and a general population of Denmark (Inter99): relationship to metabolic risk factors. Int J Obes. 2003;27(12):1507–1515.
- The Sustainability and Spread of Organizational Change (Routledge Studies in Organizational Change & Development). Amazon.co.uk: David A. Buchanan: 9780415370950: books [Internet]. [cited 2018 Feb 13]. Available from: https://www.amazon.co.uk/Sustainability-Organizational-Routledge-Studies-Development/dp/0415370957
- Dixon-Woods M, McNicol S, Martin G. Overcoming challenges to improving quality. The Health Foundation. Social Science Applied to Healthcare Improvement Research Group, Department of Health Science, University of Leicester, UK; 2012. p. 48.
- Hughes RCE, Moore MP, Gullam JE, et al. An early pregnancy HbA1c ≥5.9% (41 mmol/mol) is optimal for detecting diabetes and identifies women at increased risk of adverse pregnancy outcomes. Diabetes Care. 2014 Nov;37(11):2953–2959.