ABSTRACT
Objectives: To characterise cold sensitivity using a semi-structured interview, physical examination, thermal quantitative sensory testing (QST), and laser speckle contrast analysis (LASCA). Methods: Eight women and four men, ages 22–74, with cold sensitivity were interviewed and examined by an occupational physician. Thermal perception thresholds were established using QST, on the pulp of the index and little finger of the most affected hand. Skin perfusion in the dorsum of the hand was measured using LASCA, at baseline, after two-minute 12°C water immersion, and during rewarming. Results: The physical examination yielded few findings indicative of vascular or neurosensory pathology. One subject (8%) had impaired thermal perception thresholds. LASCA at baseline showed absent proximal-distal perfusion gradients in six subjects (50%), and a dyshomogeneous perfusion pattern in five (42%). Perfusion on a group level was virtually unchanged by cold stress testing (median 52.5 PU; IQR 9.0 before versus 51.3 PU; IQR 27.2 afterwards). Conclusions: Physical examination and thermal QST offered little aid in diagnosing cold sensitivity, which challenges the neurosensory pathophysiological hypothesis. LASCA indicated disturbances in microvascular regulation and could prove a useful tool in future studies on cold sensitivity.
Introduction
Cold sensitivity
Cold sensitivity (CS), or cold intolerance, is defined by Campbell and Kay as a collection of acquired symptoms, resulting in an abnormal aversion to cold, with pain, altered sensibility, stiffness or colour changes, which may occur after a traumatic injury [Citation1]. The condition has mostly been reported in conjunction with hand injuries, such as traumatic amputations [Citation2], nerve or vascular injury [Citation3,Citation4], cold injury [Citation5], or hand-arm vibration (HAV) syndrome [Citation6]. In a population-based case–control study, the condition was associated to frostbite, nerve injury, rheumatic disease, migraines, and vascular disease, while a high body mass index (≥25 kg/m2) appeared protective [Citation7]. The pathophysiology behind CS is obscure, but hypotheses regarding vascular and neurosensory mechanisms have been presented [Citation8–Citation10]. The diagnosis of CS is based on the symptoms reported by the individual, and questionnaires such as the Cold Intolerance Symptom Severity (CISS) score [Citation11]. Subjects with CS report a major negative impact on the quality of life and work ability [Citation2], but workers’ compensation claims are often rejected, partly because of the lack of objective measures [Citation12]. Therefore, there is a need to clearly define CS from a clinical point of view, and find methods that objectively characterise the condition.
Evaluation of small nerve fibre function
Thermal quantitative sensory testing (QST), is an established psycho-physical method to assess the thermal perception thresholds of the fingers [Citation13]. It reflects the function of the small afferent sensory nerves; Aδ fibres for the sensation of cold stimuli and C fibres for warmth. Impaired thermal perception thresholds have been reported in diabetes neuropathy [Citation14], HAV syndrome [Citation15], and after cold injury [Citation16]. In the present study, it was included to evaluate signs of small fibre neuropathy, which would support the neurosensory pathophysiological hypothesis for CS [Citation17].
Evaluation of peripheral microcirculation
Laser speckle contrast analysis (LASCA) can be used to continuously evaluate cutaneous blood perfusion in large areas [Citation18]. LASCA has been shown to be reproducible [Citation19], and correlate well with other laser Doppler techniques [Citation20], which in turn have demonstrated a linear relationship with microvascular blood flow in the range of the skin perfusion capacity [Citation21]. Apart from evaluating mean perfusion in larger image fields, smaller regions of interest (ROIs) can be defined, and a proximal-distal perfusion gradient in the fingers calculated [Citation22]. Also, the colour-coded, high-resolution Doppler images can be assessed for small hypo- or hyper-perfused areas, indicating microvascular damage [Citation23]. In previous studies, LASCA has been used on subjects with burn wounds [Citation24], primary and secondary Raynaud’s phenomenon [Citation25], and systemic sclerosis [Citation26]. To the authors’ knowledge, only one previous study has evaluated subjects with CS, reporting markedly decreased perfusion in the affected fingers [Citation27]. However, this small study (N = 6) only investigated subjects with known peripheral nerve injury, used an older laser Doppler perfusion imager with lower resolution and no ROIs, and cold stress testing (CST) that was not ISO-standardised.
Objectives
The objective of this case series was to characterise cold sensitivity using a semi-structured interview, physical examination, thermal quantitative sensory testing, and laser speckle contrast analysis. The study was also intended to test the feasibility of standardised cold stress testing in cold-sensitive subjects.
Material and methods
Study design and setting
The present study was a case series on cold-sensitive subjects, conducted at the Occupational and Environmental Medicine Clinic at the University Hospital of Umeå, during the winter months (October and November) of 2019.
Study subjects
Through a large, questionnaire-based epidemiological research project called Cold and Health in Northern Sweden (CHINS), subjects experiencing CS (N = 504) were identified [Citation7,Citation28]. First, an information letter was sent to possible study subjects, living in the municipalities closest to the clinic. They were then asked for participation by telephone and written informed consent was obtained during the clinic visit. The study protocol was approved by the Swedish Ethical Review Authority (DNR 2019-02082).
Study protocol
Study subjects were instructed to avoid strenuous physical activity, vibration exposure, nicotine, and caffeine for 3 hours before the examination. Exclusion criteria were a history of severe hypertension, congestive heart failure, obstructive lung disease, liver or kidney failure, diabetes mellitus, vasoactive medications, obstructive sleep apnoea, alcohol or substance abuse, or pregnancy. Firstly, the subjects were interviewed about their experiences of CS, based on a semi-structured protocol with questions on perceived symptoms, onset, course, and consequences for work and leisure time. Then, the subjects underwent physical examination performed by an occupational physician (AS). The symptomatic hand was examined; if both were equally affected the dominant hand was selected. It was inspected for signs of ulcers, calluses or nail changes. Two-point discrimination (Touch-Test® Two-Point Discriminator) and monofilament sensibility (Touch-Test® Monofilament five-piece set) in the volar aspects of the fingertips was tested. Wrist pulses (including Allen’s test) and capillary refill time were evaluated. Blood pressure was measured in a seated position, on the right upper arm, using an automatic gauge (Omron i-C10, Hoofddorp, The Netherlands), and peripheral saturation with a pulse oximeter (OxyMax N-65, Nellcor Puritan Bennet LLC, Pleasanton, CA, USA). Screening for length-dependent polyneuropathy was performed using a 128 Hz tuning fork applied to the medial malleoli, as well as 25°C and 40°C temperature rollers on the dorsum of the feet (Rolltemp, Somedic SenseLab AB, Sösdala, Sweden).
Thereafter, thermal QST was carried out according to the current standard at the hospital, using a SenseLab Modular Sensory Analyser (MSA), coupled to a 25 × 50 mm thermode (Somedic SenseLab AB, Sösdala, Sweden) [Citation29]. Finger skin temperature (FST) ≥28.0°C was required before testing, and achieved by active warming, if necessary. Measurements were conducted on the pulp of the index and little finger of the symptomatic hand. When perceiving thermal stimuli, subjects were instructed to press a button, which triggered digital read-out of thermode temperature and prompted return to starting temperature. Thermal stimuli started at 32.0 ± 0.1°C and ranged from 5.0°C to 52.0°C. The method-of-limits was used [Citation30], with five consecutive cold stimuli followed by five warm stimuli, random inter-stimuli intervals of 4–6 s, temperature change rate of 1.0°C/s during stimuli, and 3.0°C/s during return. With this procedure, warm detection threshold (WDT), heat pain threshold (HPT), cold detection threshold (CDT), and cold pain threshold (CPT) were established.
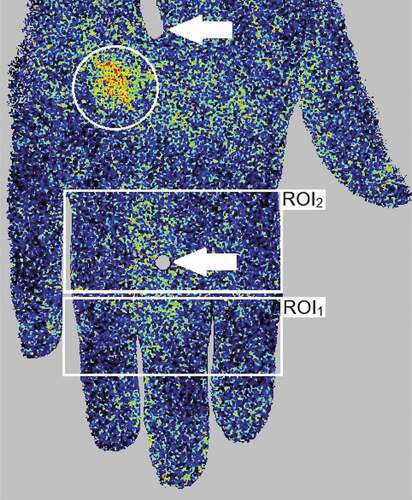
Finally, evaluation of cutaneous circulation was performed using a high-resolution laser Doppler perfusion imager (PeriCam PSI, Perimed AB, Järfälla, Sweden). It utilises a laser beam with a wavelength of 785 nm, reaching the dermis of the skin at a depth of approximately 0.3 mm, regardless of skin pigmentation. When the laser light hits moving erythrocytes, a Doppler shift occurs, meaning that the wavelength of the reflected laser light is altered. The magnitude and frequency distribution of wavelength changes are related to the number and velocity of erythrocytes, and by analysing the pattern of back-scattered laser light an index of perfusion is obtained [Citation31]. This indirect measurement of microcirculation is often referred to as “flux”, and expressed in arbitrary “perfusion units” (PU). The scanner head was positioned 20 ± 1 cm from the skin surface, perpendicular to a non-reflective pad provided by the manufacturer. The scanner captured the dorsum of the hand, from the metacarpal bones to the distal phalanges of all digits. The image acquisition rate was set to 1 Hz. A laser crosshair corresponding to felt pen marks on the hand ensured consequent positioning. FST of the pulp of the index finger was repeatedly measured using an infrared thermometer (Testo 845, Alton Hampshire, UK). After 30 minutes of acclimatisation, sitting comfortably with adequate clothing (clo 0.7–0.8) in a room with controlled temperature (23 ± 1°C) and humidity (29 ± 10%), a one-minute baseline LASCA measurement was performed. Thereafter, CST was conducted, using a refrigerated water bath (Lauda Alpha RA 12, Lauda-Königshofen, Germany). The subjects were instructed to submerge the hand, down to the ulnar styloid process, into circulating water of 12.0 ± 0.1°C for 2 minutes, according to the ISO 14835-1:2016 [Citation32]. No plastic gloves were used, to avoid pockets of insulating air inside the glove from increasing the variability of the cold exposure. The water immersion time and pain rating were continuously assessed using a photocell assembly detecting hand position, and an electronic 100 mm visual analogue scale (VAS) transducer ranging from “no pain” to “worst possible pain” (Perimed AB, Järfälla, Sweden). The subjects were instructed to remove the hand if the stimulus was too unpleasant to cope with. After CST, the hand was dried with a towel, and a 30-second LASCA measurement was immediately conducted, and subsequently repeated every third minute until the hand had rewarmed (defined as FST within 2.0°C of onset), or for a maximum of 29 minutes. The subjects were asked to hold their hand in front of them in a comfortable manner between measurements but were instructed not to actively reheat the limb. Mean perfusion was calculated during a representative 25-second time of interest (TOI) for each measurement. Two regions of interest (ROI) were specified, in accordance with previous studies [Citation22,Citation33]. Both TOI and ROI definitions have previously been tested to reduce variability [Citation34]. ROI1 captured the area between the proximal and distal interphalangeal joints of the index, middle and ring finger, and ROI2 the area between the metacarpophalangeal and proximal interphalangeal joints of the same fingers (). An absent proximal-distal perfusion gradient was defined as <10% increase in mean perfusion in ROI1, compared to ROI2. The distribution pattern was independently evaluated by two investigators (AS and BB). A homogenous pattern was defined as uniform perfusion of all examined skin, while a dyshomogeneous pattern was characterised by local hypo- or hyper-perfusion, as previously suggested [Citation22]. LASCA analyses were performed in PIMsoft (version 1.5, Perimed AB, Järfälla, Sweden).
Figure 1. An example of laser speckle contrast analysis (LASCA) imaging of the dorsal surface of the right hand, depicting regions of interest (ROI) (white rectangles), a perfusion hot spot (white circle) indicating dyshomogeneous perfusion, as well as felt pen marks (white arrows) on the middle finger and the back of the hand, for positioning
Statistical methods
Data were described as median values and interquartile ranges (IQR) for continuous variables (unless otherwise stated), and as numbers and percentages for categorical variables. Changes in perfusion were evaluated using the Wilcoxon signed-rank test and Mann–Whitney U test. Spearman’s rank correlation coefficient (rs) was calculated for FST and LASCA. Statistical analyses were performed using IBM SPSS Statistics for Windows (version 26.0, IBM Corporation, Armonk, NY, USA).
Results
Recruitment
A total of 29 subjects were eligible for participation, of which 11 (38%) accepted. Those who did not participate either declined after initial information (N = 11), could not be reached (N = 5), or had moved from the study region (N = 2). In addition, one extra CS case was recruited as a first-degree relative to one of the CHINS subjects and was asked to complete the original CHINS questionnaires [Citation7,Citation28] during the clinic visit, in which the case definition was fulfilled. The final study population consisted of eight women and four men, with a median age of 53 years (IQR 9; range 22–74).
Comparing those who participated to those who did not, there were more women (67 versus 56%), with a higher median age (53 versus 45 years), and CISS score (48 versus 42 points). However, the most important factor for participation was living in the same municipality as the clinic (50% versus 17%).
Interview and physical examination
Symmetrical distribution of symptoms in both hands was reported by 9 subjects (75%). Seven (58%) stated that only fingers were involved, while the rest described also having symptoms in the palm or on the back of the hands. Pain was reported by 10 (83%), sensory alterations by 9 (75%), stiffness by 7 (58%), and colour changes by 6 (50%). In addition, all subjects described having cold hands, or a sensation of cold even though the hand temperature was normal. None had ever experienced Raynaud’s phenomenon. Pain was reported as the most bothersome symptom by 6 subjects (50%), stiffness by 3 (25%), sensation of cold hands by 2 (17%), and sensory alterations by 1 (8%). The condition was perceived as being acquired rather than constitutional by 8 subjects (67%), and 7 (58%) reported previous injury or disease affecting the hands, such as frostbite, HAV syndrome, traumatic hand injury, carpal tunnel syndrome or rheumatic disease. First-degree heredity for CS was described by 5 (42%). The condition was perceived to have negative consequences for work and leisure time by 2 (17%) and 3 subjects (25%), respectively. No job change was reported. The median self-graded work capacity on a numerical rating scale, ranging from 0 to 10, was 8 (IQR 4; range 2–10). Unusually frequent use of gloves (single or double layer) was reported by 11 subjects (92%), and 6 (50%) had used artificial heating devices (i.e. electric gloves or heat packs). Eight subjects (67%) also reported CS in the feet.
Before the physical examination, the median FST at baseline was 30.4°C (IQR 5.4), with four subjects (33%) having FST below the clinical cut-off for sensory testing (28.0°C). Examination of the skin revealed no ulcers, calluses, or nail changes. Other tests showed reduced 2-point discrimination (N = 1 subject; 7 mm), reduced monofilament sensibility (N = 2; 2 g target force required, indicating diminished light touch), abnormal radial (N = 1) or ulnar (N = 1) Allen´s test, and presence of distal interphalangeal joint arthritis (N = 1). The blood pressure was elevated (>140/90 mmHg) in three subjects (25%), all of whom had previous diagnoses of hypertension. Peripheral saturation was normal (≥95%) in all subjects. No sensory deficits in the feet were found. Findings are summarised in .
Table 1. Subject characteristics and findings
Thermal quantitative sensory testing (QST)
For the index finger, the median WDT was 35.5 (IQR 1.5); HPT 47.7 (IQR 4.7); CDT 29.0 (IQR 1.3); and CPT 9.8 (IQR 7.2). For the little finger, median WDT was 37.1 (IQR 4.0); HPT 49.0 (IQR 3.3); CDT 28.4 (IQR 4.0); and CPT 6.7 (IQR 7.2). However, the lowest temperature of the thermode (5.0°C) only elicited cold pain responses in six subjects (50%) for the index finger and four (33%) for the little finger. One subject (8%) had impaired thermal perception thresholds, according to the currently used cut-offs (WDT >42.0°C, CDT <23.0°C).
Cold stress testing (CST) and laser speckle contrast analysis (LASCA)
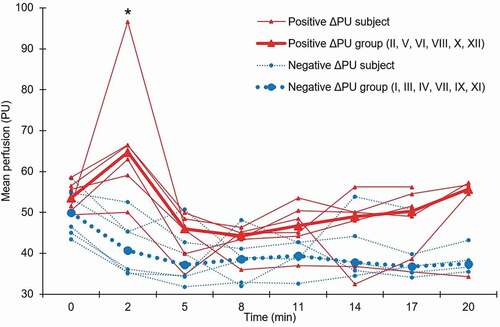
All subjects completed CST. The median VAS maximum was 55 mm (IQR 53; range 18–100), and several reported that pain was replaced by numbness as the dominant feature during the second part of CST (). At baseline, the proximal-distal perfusion gradient was absent in six subjects (50%), and the perfusion pattern dyshomogeneous in five (42%). CST lowered the median FST from 30.4°C (IQR 5.4) to 16.0°C (IQR 1.2) (). The perfusion before and after CST was unchanged (52.5 PU; IQR 9.0 versus 51.3 PU; IQR 27.2; Wilcoxon signed-rank test p = 0.94). Subjects were categorised into having either a positive (N = 6; median 8.9; IQR 18.0) or a negative (N = 6; median −8.5; IQR 5.2) perfusion shift (ΔPU) directly after CST, and the difference between groups was statistically significant (Mann–Whitney U test p = 0.006), see . The correlation between FST and LASCA measurements varied over time (Spearman’s rank correlation coefficient 0.32≤rs≤0.77) and was statistically significant (p < 0.05) during the late rewarming phase (11–17 min).
Figure 2. Visual analogue scale (VAS) pain recordings from two-minute, 12°C water cold stress testing. Thin solid lines depict individual subjects, thick dotted line the median
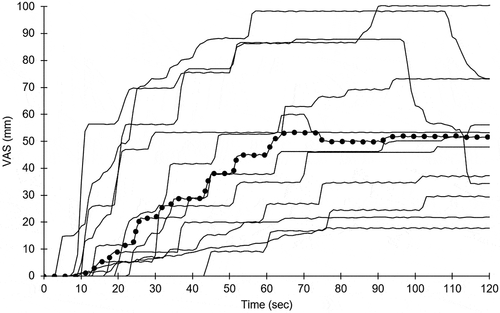
Figure 3. Finger skin temperature (FST) for the pulp of the index finger, measured by an infrared thermometer, before (0 min), directly after cold stress testing (2 min) and during the rewarming period (5–20 min). Subjects have been categorised into two groups according to fast (FST ≥28.0°C within 15 minutes after cold stress testing) or slow rewarming, and the median for each group is presented. Roman numerals represent the subject number
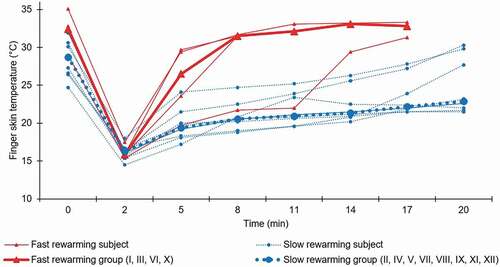
Figure 4. Mean perfusion for the dorsum of the hand, measured by laser speckle contrast analysis (LASCA), before (0 min), directly after cold stress testing (2 min) and during the rewarming period (5–20 min). Subjects have been categorised into two groups according to a positive or negative change in perfusion (ΔPU) between baseline (0 min) and directly after cold stress testing (2 min), and the median for each group is presented. Roman numerals represent the subject number. PU: Perfusion units. *Significant difference between groups (Mann–Whitney U test p = 0.006; with positive outlier excluded p = 0.010)
Discussion
Key results
The study subjects described uniform symptoms of cold sensitivity, but with varying origin and consequences. The physical examination yielded few findings indicative of vascular or neurosensory pathology. Impaired thermal perception thresholds were only found in one subject (8%). Objective evaluation of microcirculation, using laser speckle contrast analysis, showed an absent proximal-distal perfusion gradient in six subjects (50%) and a dyshomogeneous perfusion pattern in five (42%) at baseline. Also, six (50%) had a paradoxical increase in perfusion directly after cold stress testing.
Interpretation and comparison
In our study, the description of symptoms was consistent with the definition of CS by Campbell and Kay [Citation1]. One additional symptom that was not included in their definition, but reported by all subjects in the present study, was the experience of habitually cold hands. As early as in 1985, CS was defined as “an icy cold feeling that can progress to pain” [Citation35], and “an unpleasant sense of coldness” was highlighted as an important symptom in a later thesis on the topic [Citation36]. In a future amendment of the CS definition, this symptom should be considered for inclusion. Importantly, all subjects negated observable signs of Raynaud’s phenomenon, which underlines the notion that CS is indeed a separate condition. First-degree heredity for CS was reported by almost half the subjects, and as far as the authors are aware, this has not previously been described. Few (17%) had experienced a negative impact on work ability and none had changed job as a consequence. This is contrasted by previous studies on CS, where a more profound impact on work ability was reported, with job change in 4–38% [Citation37,Citation38]. The difference might be due to a lesser intensity of symptoms in our study, the positive effect of behavioural changes (e.g. warming metal hand tools before use, increasing clothing, and acquiring heated gloves), or the high ambition regarding work participation facilitation in Sweden.
The routine physical investigation yielded few findings and did not appear to be sufficient for evaluating CS. The two subjects that had decreased sensitivity to monofilaments both reported tangible exposure to HAV, which could explain this mild sensory deficit. The abnormal Allen’s tests in two cases were not associated with any clear vascular symptoms and could represent an anatomical variant. Finally, findings of finger joint arthritis were explained by a previously established diagnosis of rheumatic disease, which has previously been associated to CS [Citation7]. Thermal QST only showed impaired thermal perception thresholds in one subject, with previous relevant HAV exposure, which is a likely explanation. If CS is primarily of neurosensory origin, more signs of nerve dysfunction would have been expected in the present study. In comparison, Jørum and Opstad investigated naval soldiers with CS after cold injury and found impaired WDT, CDT, and CPT thresholds in the feet, indicative of small fibre neuropathy [Citation16]. There were less findings in the hands, but the median CPT thresholds in the hands appeared to be higher than (16.6°C) than in our study (6.7–9.8°C), suggestive of cold allodynia. In a thesis by Smits, thermal QST was performed on 32 hand surgery patients with CS, and compared to 15 healthy controls [Citation17]. Mean CISS was 48, compared to a median of 48 in the present study, which corroborates a similar intensity of CS. In this work, significantly impaired WDT and CDT were found in patients with CS, compared to healthy controls. However, 81% of tested patients had either nerve lesions or full amputations, meaning that it was evident beforehand that neurosensory injury would be present in the majority of cases, regardless of CS symptoms. Also, QST testing was performed in different locations depending on where the patient experienced the most symptoms, rather than standardising the anatomical test site, which limits comparison. Finally, a study by Giertmühlen et al. showed a low correlation between self-reported intensity of CS and CPT [Citation39], which is in line with our findings. One methodological consideration is that the lower limit of thermode temperature (5.0°C) in the present study appeared insufficient to provoke cold pain in all subjects, indicating that the protocol needs refining. To summarise, results of thermal QST in cold-sensitive subjects are ambiguous and require further study.
Immersion in 12°C water for 2 minutes was tolerated by all subjects. There was a large variation in VAS ratings (), and several experienced that the stepwise increase in pain during the first minute was subsequently attenuated, and changed to numbness during the latter part of the provocation, which has also previously been described [Citation40]. The baseline FST varied between subjects but was homogenously lowered directly after water immersion, indicating sufficient and consequent CST. The rewarming period showed different patterns of FST normalisation, with some subjects having a slow, passive rewarming in a linear fashion, while others seemed to actively reheat much faster (). One previous study using CST (16°C water for 5 minutes) reported temperature recovery for 92% of healthy subjects after 12 minutes of rewarming [Citation41]. Another study on mixed volunteers (with and without HAV exposure) subjected to CST (15°C water for 1 minute) showed 64% recovery within 15 minutes [Citation42]. Finally, one study with a more intensive CST (10°C water for 10 minutes) showed 56% recovery within 15 minutes in healthy military conscripts [Citation43]. In comparison, the present study only had four subjects (33%) showing recovery within 15 minutes. Although differences in CST protocols, FST evaluation technique, and ambient temperature limit comparison, there seems to be a tendency towards longer rewarming time in the present study, indicating an attenuated cold response.
Regarding LASCA, Della Rossa et al. performed a large (N = 76) LASCA study on healthy subjects (HS), primary Raynaud’s syndrome patients (PRP) and systemic sclerosis patients with secondary Raynaud’s syndrome (SSc). They reported absent proximal-distal perfusion gradients in 26%, 55%, and 74%, respectively [Citation25]. Another LASCA study by Rosato et al. showed absent gradients in 6% of HS, 95% of PRP and 88% of SSc [Citation33]. This compares to 50% of the subjects in our study. In the study by Della Rossa et al., a dyshomogeneous perfusion distribution at baseline was reported in 5% of HS, 20% of PRP and 84% of SSc, in comparison with 42% in our study. Also, they included CST with hand immersion in 16°C water for 90 seconds, a milder provocation than in the present study. Still, they found a mean decrease in perfusion assessed by LASCA of −19% in HS, which is representative of the well-established normal vasoconstrictive response upon cold stimuli of the hand [Citation44]. Conversely, virtually no change (−1.2%) in the group as a whole was found in the present study, but subjects could be categorised based on a positive or negative perfusion shift (ΔPU) directly after CST. The group with a paradoxical positive shift had a trend towards higher perfusion throughout the follow-up time (). Previous research has suggested that thermal stimulation of C fibres in the skin can induce an axon flare response, characterised by local vasodilation, and perfusion increase as measured by LASCA [Citation45]. It is possible that such local vasodilatory responses could affect the mean perfusion of the entire image, although it was not readily observed in the visual representation of data. Finally, Ruch et al. studied subjects with CS after peripheral nerve injury, assessing perfusion using an older laser Doppler perfusion imager before and after 20 minutes of 5–8°C air CST [Citation27]. They found markedly decreased perfusion in the affected fingers during all stages of testing, which also contrasts our findings. However, the results are not immediately comparable to the present study since other methods were used both for CST and perfusion evaluation. Also, the study was performed in close proximity to the injury (mean 53 days), meaning that neurosensory rearrangement was likely still in process and possibly affecting the vasoregulation. To conclude, the present study shows deviations from the suggested normal perfusion patterns, albeit less pronounced than, and in some parts opposing what has been found in previous studies. Since LASCA is non-invasive, reproducible, and rapidly performed, it could in the future prove suitable for implementation in arctic healthcare settings, when assessing cold-related neurovascular hand symptoms.
Limits
One major limit with the present study was the fact that the subjects reported varying background for CS. In trying to understand the pathophysiology behind the condition, a more homogenous group (i.e. only subjects with CS after nerve injury, or cold injury) would have been preferable. On the other hand, these case series illustrate the many different backgrounds to presenting a similar clinical description of CS symptomatology. Gender differences have been reported for CS [Citation7], but such effects were not considered in the present study due to the small material. Also, in the present study, there was a large variation in age, which might have affected the neural and vascular function, as well as the response to CST, but was not adjusted for.
Performing CST for only 2 minutes might have been an insufficient time to provoke a full thermoregulatory response. Five-minute CST is described in the ISO-standard [Citation32] but was not opted on since there was a concern that CS subject might not endure the longer provocation time, leading to premature abortion at different stages. Also, longer CST has been reported to induce the Hunting response, or cold-induced vasodilation [Citation46], which could potentially disturb the interpretation of the microvascular response in the normal temperature range [Citation47]. Finally, the observed clinical reactions and high VAS ratings support that five-minute CST would indeed have been unfeasible.
Strengths
One strength of this study was that the subjects were recruited from a well-controlled database, which meant that many relevant historical parameters were available for analysis, such as previous diseases and injuries. Also, several factors known to immediately influence vasoregulation (e.g. nicotine use and HAV exposure) were controlled for. All subjects were examined by the same investigators, during the same winter period, in a controlled environment. Mixing qualitative and quantitative methods captured many different aspects of CS.
Conclusions
Cold sensitivity in the hands was mainly characterised by cold-related pain and sensory alterations. A sensation of cold hands was also a dominating symptom and should be considered for inclusion in the definition of CS. Based on the interview, the condition clearly differs from Raynaud’s phenomenon. Physical examination and thermal QST offered little aid in diagnosing cold sensitivity, which challenges the neurosensory pathophysiological hypothesis. However, these modalities might still prove valuable in a clinical setting when assessing neurovascular hand symptoms from a differential diagnostic perspective. Laser speckle contrast analysis indicated disturbances in microvascular regulation and could be a useful tool in future studies of cold sensitivity. Despite subjects having an abnormal aversion to cold, standardised two-minute, 12°C cold stress testing was feasible.
Acknowledgments
The authors gratefully acknowledge the statistical guidance of Dr Per Liv at the Department of Epidemiology and Global Health at Umeå University.
Disclosure statement
The authors report no conflict of interest.
Additional information
Funding
References
- Campbell DA, Kay SP. What is cold intolerance? J Hand Surg Br. 1998;23(1):3–10.
- Lithell M, Backman C, Nyström A. Pattern recognition in post-traumatic cold intolerance. J Hand Surg Br. 1997;22(6):783–787.
- Novak CB, Anastakis DJ, Beaton DE, et al. Cold intolerance after brachial plexus nerve injury. Hand (N Y). 2012;7(1):66–71.
- Klocker J, Peter T, Pellegrini L, et al. Incidence and predisposing factors of cold intolerance after arterial repair in upper extremity injuries. J Vasc Surg. 2012;56(2):410–414. .
- Hutchison RL. Frostbite of the Hand. J Hand Surg-Am. 2014;39(9):1863–1868.
- Carlsson IK, Rosén B, Dahlin LB. Self-reported cold sensitivity in normal subjects and in patients with traumatic hand injuries or hand-arm vibration syndrome. BMC Musculoskelet Disord. 2010;11(1):89.
- Stjernbrandt A, Carlsson D, Pettersson H, et al. Cold sensitivity and associated factors: a nested case–control study performed in Northern Sweden. Int Arch Occup Environ Health. 2018;91(7):785–797.
- Ruijs AC, Jaquet JB, van Riel WG, et al. Cold intolerance following median and ulnar nerve injuries: prognosis and predictors. J Hand Surg Eur Vol. 2007;32(4):434–439.
- Carlsson IK, Nilsson JÅ, Dahlin LB. Cut-off value for self-reported abnormal cold sensitivity and predictors for abnormality and severity in hand injuries. J Hand Surg Eur Vol. 2010;35(5):409–416.
- Smits ES, Nijhuis TH, Huygen FJ, et al. Rewarming patterns in hand fracture patients with and without cold intolerance. J Hand Surg Am. 2011;36(4):670–676.
- Carlsson I, Cederlund R, Höglund P, et al. Hand injuries and cold sensitivity: reliability and validity of cold sensitivity questionnaires. Disabil Rehabil. 2008;30(25):1920–1928.
- Graham B, Schofield M. Self-reported symptoms of cold intolerance in workers with injuries of the hand. Hand (N Y). 2008;3(3):203–209.
- Chong PS, Cros DP. Technology literature review: quantitative sensory testing. Muscle Nerve. 2004;29(5):734–747.
- Løseth S, Stalberg E, Jorde R, et al. Early diabetic neuropathy: thermal thresholds and intraepidermal nerve fibre density in patients with normal nerve conduction studies. J Neurol. 2008;255(8):1197–1202.
- Lundström R, Noor Baloch A, Hagberg M, et al. Long-term effect of hand-arm vibration on thermotactile perception thresholds. J Occup Med Toxicol. 2018;13(1):19.
- Jørum E, Opstad PK. A 4-year follow-up of non-freezing cold injury with cold allodynia and neuropathy in 26 naval soldiers. Scand J Pain. 2019;19(3):441–451.
- Smits ES Cold intolerance: from thermoregulation to nerve innervation [dissertation]. Rotterdam: Erasmus University Rotterdam; 2013.
- Briers JD. Laser speckle contrast analysis (LASCA): a nonscanning, full-field technique for monitoring capillary blood flow. J Biomed Opt. 1996;1(2):174–179.
- Roustit M, Millet C, Blaise S, et al. Excellent reproducibility of laser speckle contrast imaging to assess skin microvascular reactivity. Microvasc Res. 2010;80(3):505–511.
- Millet C, Roustit M, Blaise S, et al. Comparison between laser speckle contrast imaging and laser Doppler imaging to assess skin blood flow in humans. Microvasc Res. 2011;82(2):147–151.
- Ahn H, Johansson K, Lundgren O, et al. In vivo evaluation of signal processors for laser Doppler tissue flowmeters. Med Biol Eng Comput. 1987;25(2):207–211.
- Rosato E, Borghese F, Pisarri S, et al. Laser Doppler perfusion imaging is useful in the study of Raynaud’s phenomenon and improves the capillaroscopic diagnosis. J Rheumatol. 2009;36(10):2257–2263.
- Rosato E, Molinaro I, Rossi C, et al. The combination of laser Doppler perfusion imaging and photoplethysmography is useful in the characterization of scleroderma and primary Raynaud’s phenomenon. Scand J Rheumatol. 2011;40(4):292–298.
- Lindahl F, Tesselaar E, Sjöberg F. Assessing paediatric scald injuries using laser speckle contrast imaging. Burns. 2013;39(4):662–666.
- Della Rossa A, Cazzato M, d’Ascanio A, et al. Alteration of microcirculation is a hallmark of very early systemic sclerosis patients: a laser speckle contrast analysis. Clin Exp Rheumatol. 2013;31(2 Suppl 76):109–114.
- Ruaro B, Sulli A, Alessandri E, et al. Laser speckle contrast analysis: a new method to evaluate peripheral blood perfusion in systemic sclerosis patients. Ann Rheum Dis. 2014;73(6):1181–1185.
- Ruch DS, Vallee J, Li Z, et al. The acute effect of peripheral nerve transection on digital thermoregulatory function. J Hand Surg Am. 2003;28(3):481–488.
- Stjernbrandt A, Björ B, Andersson M, et al. Neurovascular hand symptoms in relation to cold exposure in northern Sweden: a population-based study. Int Arch Occup Environ Health. 2017;90(7):587–595. .
- Fruhstorfer H, Lindblom U, Schmidt WC. Method for quantitative estimation of thermal thresholds in patients. J Neurol Neurosurg Psychiatry. 1976;39(11):1071–1075.
- Hilz MJ, Stemper B, Axelrod FB, et al. Quantitative thermal perception testing in adults. J Clin Neurophysiol. 1999;16(5):462–471.
- Roustit M, Cracowski J-L. Non-invasive assessment of skin microvascular function in humans: an insight into methods. Microcirculation. 2012;19(1):47–64.
- International Organization for Standardization (ISO). 14835-1:2016 – mechanical vibration and shock – cold provocation tests for the assessment of peripheral vascular function – part 1: measurement and evaluation of finger skin temperature. Geneva: International Organization for Standardization; 2016.
- Rosato E, Rossi C, Molinaro I, et al. Laser Doppler perfusion imaging in systemic sclerosis impaired response to cold stimulation involves digits and hand dorsum. Rheumatology (Oxford). 2011;50(9):1654–1658.
- Rousseau P, Mahe G, Haj-Yassin F, et al. Increasing the “region of interest” and “time of interest”, both reduce the variability of blood flow measurements using laser speckle contrast imaging. Microvasc Res. 2011;82(1):88–91. .
- Engkvist O, Wahren LK, Wallin G, et al. Effects of regional intravenous guanethidine block in posttraumatic cold intolerance in hand amputees. J Hand Surg Br. 1985;10(2):145–150.
- Carlsson I Cold sensitivity in injured and normal hands. Consequences for daily life [dissertation]. Lund: Lund University; 2010.
- Collins ED, Novak CB, Mackinnon SE, et al. Long-term follow-up evaluation of cold sensitivity following nerve injury. J Hand Surg Am. 1996;21(6):1078–1085.
- Vaksvik T, Hetland K, Røkkum M, et al. Cold hypersensitivity 6 to 10 years after replantation or revascularisation of fingers: consequences for work and leisure activities. J Hand Surg Eur Vol. 2009;34(1):12–17. .
- Gierthmühlen J, Schneider U, Seemann M, et al. Can self-reported pain characteristics and bedside test be used for the assessment of pain mechanisms? An analysis of results of neuropathic pain questionnaires and quantitative sensory testing. Pain. 2019;160(9):2093–2104. .
- Traynor R, MacDermid JC. Immersion in cold-water evaluation (ICE) and self-reported cold intolerance are reliable but unrelated measures. Hand (New York, N Y). 2008;3(3):212–219.
- Cleophas TJ, Fennis JF, Van’t Laar A. Finger temperature after a finger-cooling test: influence of air temperature and smoking. J Appl Physiol Respir Environ Exerc Physiol. 1982;52(5):1167–1171.
- Dupuis H. Thermographic assessment of skin temperature during a cold provocation test. Scand J Work Environ Health. 1987;13(4):352–355.
- Brändström H, Wiklund U, Karlsson M, et al. Autonomic nerve system responses for normal and slow rewarmers after hand cold provocation: effects of long-term cold climate training. Int Arch Occup Environ Health. 2013;86(3):357–365.
- Nielsen SL, Lassen NA. Measurement of digital blood pressure after local cooling. J Appl Physiol Respir Environ Exerc Physiol. 1977;43(5):907–910.
- Unal-Cevik I. temporal and spatial quantification of pain- related small fiber functionality assessed using laser speckle contrast analysis. Pain Pract. 2018;18(7):824–838.
- Daanen HA. Finger cold-induced vasodilation: a review. Eur J Appl Physiol. 2003;89(5):411–426.
- Van der Struijs NR, Van Es EM, Raymann RJ, et al. Finger and toe temperatures on exposure to cold water and cold air. Aviat Space Environ Med. 2008;79(10):941.
