ABSTRACT
The aim of this study was to evaluate whether patients with type 2 diabetes (T2D) who had stopped attending their diabetes treatment system (referred to as “lost to follow-up”, LTF) but who succeeded in improving their glycaemic control after returning to the diabetes treatment system had changes in their diabetes medication when compared with similar patients who did not show improvement. “LTFs” who had baseline haemoglobin A1 c (HbA1 c) ≥53 mmol/mol and succeeded in reducing HbA1 c ≥ 6 mmol/mol during a 12–30 month follow-up period after adhering again to their diabetes treatment system were compared with “LTFs” who had an unsatisfactory change in HbA1 c or with “LTFs” who maintained good glycaemic control throughout the 12–30 month follow-up period. Unsatisfactory change in HbA1 c was determined as HbA1 c ≥ 53 mmol/mol and change <6 mmol/mol after the 12–30 month follow-up period in their diabetes treatment system or HbA1 c < 53 mmol/mol when returning to the diabetes treatment system but ≥53 mmol/mol at the end of the 12–30 month follow-up period. “LTFs” with improvement in glycaemic control used a higher number of different anti-hyperglycaemic agents (P < 0.001) and their dosages of metformin increased (P < 0.05) when compared with “LTFs” without improvement or “LTFs” with satisfactory glycaemic control. Cholesterol-, LDL-cholesterol- and triglyceride-concentrations decreased during the 12–30 month follow-up period (P < 0.05) in “LTFs” with improved glycaemic control, but not in the other groups. “LTFs” with T2D who had poor glycaemic control seemed to require an increase in their anti-diabetic medication when attempting to improve their glycaemic control.
Introduction
A proportion of patients with type 2 diabetes (T2D) do not attend diabetes clinics as prescribed by the health-care personnel for a variety of reasons. Poor adherence may be linked to the type of care provided, demographic factors, patients beliefs to medication, and perceived patient burden regarding the treatment procedures [Citation1]. Especially in young patients’ inadequate glycaemic control can be explained with poor adherence to medication [Citation2]. These “LTFs” may be exposed to a notable risk of diabetic complications thus reducing their quality of life and raising the costs of diabetes treatment [Citation3–Citation5]. Very little is known about what happens when “LTFs” are recruited back into the T2D treatment system in primary care.
Previous study findings have shown that in the public primary health-care system, 1 in 10 patients with T2D is an “LTF” [Citation4]. Although “LTFs” with T2D are generally difficult to bring back into the system, those with poor glycaemic control and who are successfully re-attached to the diabetes treatment system seem to benefit from being recalled [Citation5]. Further, this subgroup of “LTFs” with T2D seems to benefit, at least glycaemic control improves, from intensive communication and counselling with the health-care professionals [Citation6]. However, it is unknown whether there are any differences in the changes in diabetes medication between “LTFs” who improved their glycaemic control and those who did not.
Parameters such as blood pressure (BP) and lipid concentrations may also affect, even independently, the wellbeing and cardiovascular outcomes of patients with T2D [Citation7–Citation10]. How these parameters are affected when glycaemic control is improved is unknown.
The aim of the study was to compare the changes made to the diabetes medication in “LTFs” with T2D after re-attaching to the diabetes treatment system. As a secondary outcome, we examined whether there were differences in other studied parameters than glycosylated haemoglobin A1 c (HbA1 c) between “LTFs” with or without an improvement in glycaemic control.
Materials and methods
Study design and setting
This study was a “real-life” longitudinal observational register-based cohort study using data retrospectively obtained from an electronic patient record system. In 2009, a project was initiated for improving the glycaemic control of those who did not attend to T2D care system in the public primary healthcare in the city of Vantaa, Finland. We recorded retrospectively the influence of the clinical work performed by the community primary health-care nurses and general practitioners [Citation4].
The ethics committee of the Hospital District of Helsinki and Uusimaa (Nr 91/13/03/01/2011, 9.1.2013) and the health authority of the city of Vantaa (Dno SOSTER 3124/2011/092) approved this study. According to the ethics committee of the Hospital District of Helsinki and Uusimaa, and health authority of Vantaa, the study participants did not need to sign a Statement of Informed Consent because the study was retrospective, based on patient charts and the investigators did not contact the “LTFs”.
Study participants
Data collected for the study were based on clinical patient records obtained from the public primary healthcare of the eastern districts of Vantaa, Finland. At the time of the study, Vantaa had a population of 195 397 inhabitants and the eastern districts had 118 802 inhabitants. We identified all patients aged 18–80 years. Patients were determined to have T2D if they had an International Classification of Disease (ICD-10) code E11 in the patient charts or they were prescribed specific anti-hyperglycaemic agents for T2D between 1 January 2005 and 31 December 2009. In Finland, the public primary healthcare used ICD-10 codes to classify diseases, health related disorders, injuries, infections, and symptoms. A computer-based search was made from Finstar (Logica, Helsinki, Finland) patient chart system with a specific report generator.
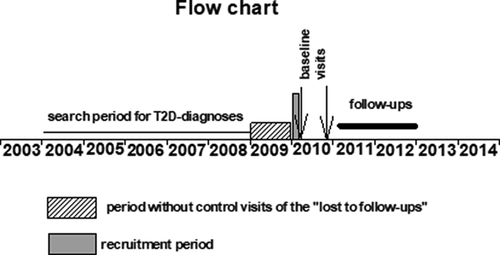
In the eastern districts of Vantaa, 3459 people fulfilled the criteria of having T2D. Patients who fulfilled T2D criteria but had not contacted the public primary health-care system during the previous 12 months (during 2009) were considered “LTFs” [Citation4]. To establish whether these “LTFs” were true “LTFs” or whether they were receiving treatment elsewhere (e.g. whether they were receiving treatment arranged in another system, private or secondary care), trained diabetes nurses from the public primary health-care system contacted them by phone. If an “LTF” was a true “LTF”, trained diabetes nurses booked an appointment including laboratory tests (baseline visit, n = 356 [Citation4]) in order to improve the diabetes treatment and to bring the “LTF” back to the public primary health-care system. In Finland in that time point, systemic call-back attempts by nurses or diabetes nurses have not been part of the usual public primary healthcare The follow-up visit was the nearest visit taking place at least one year after the initial visit (12 to 30 months after the baseline visit). Of the contacted true “LTFs”, 32% (n = 115) participated both in the baseline visit and the follow-up visit [Citation5] and composed the study population of the study. shows the flowchart of the study.
Study procedures
At the baseline visit the participant’s status was assessed, blood was drawn and diabetes counselling was provided, and treatment was enhanced when needed [Citation4]. The follow-up visit included the same assessments as the baseline visit. Based on the HbA1 c measured at the follow-up visit, the success of treatment was evaluated.
“LTFs” were divided into three groups according to their glycaemic control at the baseline visit and at the follow-up visit. One group was defined as “LTFs” with an improvement in glycaemic control, e.g. those who had a HbA1 c level ≥53 mmol/mol (7%) at the baseline visit and a reduction in HbA1 c ≥ 6 mmol/mol (0.5%) at the follow-up visit 12–30 months later [Citation6]. The second group was defined as “LTFs” without improvement, e.g. those who had a HbA1 c level ≥53 mmol/mol (7%) at the baseline visit and the decrease of HbA1 c level was <6 mmol/l (0.5%) or ”LTFs” who had a HbA1 c level <53 mmol/mol (7%) at the baseline visit but more than that at the follow-up visit 12–30 months later [Citation6]. The third group was defined as “LTFs” with good glycaemic control, e.g. those who had a HbA1 c level <53 mmol/mol (7%) and the HbA1 c level remained under this value during the 12–30 months follow-up time [Citation4]. According to international guidelines and epidemiological studies [Citation11–Citation14] diabetic complications increase significantly if the HbA1 c level is <53 mmol/mol (7%). Therefore, this level was chosen as an indicator of good glycaemic control. The level of change in HbA1 c (6 mmol/mol, 0.5%) was chosen because previous studies have shown that reducing HbA1 c by this amount reduces the incidence of complications in T2D [Citation11,Citation12]. In those patients who returned to the public primary health-care system diabetes treatment was provided according to national and international guidelines, including yearly laboratory, nurse and doctor controls and, if needed, optimising medications [Citation13,Citation14].
Primary and secondary outcomes
Trained diabetes nurses collected data from the patient records on diabetes medication before and after the 12–30 month follow-up. Based on the medication data, the recruited ”LTFs” were divided into two groups: those who were prescribed higher doses of diabetes medication or an increased number of diabetes drugs at the follow-up visit (increased medication), and those who had no changes or who were moved from one oral diabetes medication to another (no change in medication).
As secondary outcomes, we studied patient characteristics including sex, baseline body mass index (BMI), duration of diabetes, diagnosed retinopathy or albuminuria. Baseline BP, blood lipids (cholesterol, low density lipoprotein [LDL] -cholesterol, high density lipoprotein [HDL] -cholesterol, triglycerides), haemoglobin, alanine transaminase, creatinine and prevalence of proteinuria were recorded. When data were available BP, cholesterol, LDL-cholesterol, HDL-cholesterol, triglycerides, creatinine and prevalence of proteinuria were recorded and compared with the respective baseline status in all three groups of glycaemic control.
Statistical analysis
Data are presented as percentage (n) or mean (standard deviation, [SD]) or median (interquartile range [IQR] 25%-75%). Prevalence differences were tested using cross-tabulation and Chi-Square test. Magnitude differences were analysed with Kruskal-Wallis analysis of variance followed by Dunns’ method or ANOVA followed by Bonferroni corrected t-test. Comparisons between before-after situations were carried out with paired t-test, Wilcoxon signed rank-test or cross-tabulation and Chi-Square test when applicable. A P-value <0.05 was considered statically significant.
Results
Characteristics of the study participants at baseline
At the baseline visit, BMI was higher in “LTFs” with improved glycaemic control than in the other groups. At the baseline visit, “LTFs” with improvements had higher triglyceride and lower HDL-cholesterol concentrations than those who maintained good glycaemic control (). shows the characteristics of the study participants at the baseline visit. “LTFs” without improvement had a longer duration of T2D than those who had good glycaemic control ().
Table 1. Characteristics of the study participants (loss of follow-ups, LTFs) at baseline.a.
Table 2. Number of loss of follow-ups (LTFs) using a specific type of anti-hyperglycaemic agent at the baseline and at the follow-up visit.
Diabetes medication
An increase in dosage or in the number of different T2D drugs during the follow-up was higher in “LTFs” with improvements (32/36, e.g. 89%) than in those without improvement in glycaemic control (17/27, e.g. 63%, P < 0.05) or those with good glycaemic control (16/52, e.g. 31%, P < 0.001). “LTFs” without improvement also had more increases in their medication than those with good glycaemic control (P < 0.05). There was an increase in the use of DPP-4 (dipeptidyl peptidase 4)-inhibitors in all studied groups (). At the baseline visit, the groups did not differ in the dosage of metformin used. The dosage of metformin increased in “LTFs” with improvements from 1000 mg (median, IQR 25–75%: 0–2500 mg) to 2000 mg (1500–3000 mg, P < 0.05) but not in those without improvement (from 2050 ± 960 mg [mean±SD] to 1956 ± 878 mg) or in those whose glycaemic control remained good (from 1298 ± 1197 mg to 1432 ± 1042 mg). No other systematic changes in medication were observed during the follow-up ().
Changes in different parameters during follow-up in different glycaemic control groups
About half (23–30/52) of the “LTFs” who remained in good glycaemic control also underwent other laboratory tests and BP checks than HbA1 c. Roughly one third of those “LTFs” who were not in optimal glycaemic control at baseline but showed improvement (9–16/36) or who showed no improvement (10–16/27) participated in the T2D check-ups during the follow-up period. In “LTFs” with improvement and who participated in these check-ups, total cholesterol, LDL-cholesterol and triglyceride concentrations decreased ().
Table 3. Change in different parameters during the 12–30 month follow-up period in different glycaemic control groups of loss of follow-ups (LTFs).a.
Discussion
Improved glycaemic control in recruited “LTFs” was associated with increases in both dosage and the number of drugs used for T2D treatment. There seemed to be changes in medications, especially an increase in the use of DPP4-inhibitors, even among those “LTFs” with good glycaemic control during the follow-up. The laboratory follow-ups were generally performed unsatisfactorily and good clinical care guidelines [Citation13,Citation14] were not followed adequately. A longer duration of T2D was associated with worse glycaemic control. Non-optimal lipid status seemed to improve if a “LTF” was able to improve his/her glycaemic control during follow-up.
Among participants with improvement in glycaemic control, the main change was an increase in the number of anti-hyperglycaemic agents prescribed as well as an increase in the dosage of metformin which was not observed in the other two groups. Yet there was no difference in the dosage of metformin between the groups at the beginning of the follow-up and therefore we cannot claim that there would have been a systematic underuse of this drug at the beginning of the follow-up in any of the groups studied. According to international and national diabetes treatment guidelines, which Finnish public primary care is supposed to follow [Citation13,Citation14] metformin is the first-line medication in the treatment of T2D if contraindications do not inhibit its use [Citation15]. Metformin has been shown to improve the prognosis of T2D patients [Citation16]. The increasing communication, and thereby increasing exchange of information, between the T2D treatment system and the recruited “LTFs” with improved glycaemic control which we observed [Citation6] did not solely explain the improved glycaemic status. Recommendation based [Citation13–Citation15] increases in medication also seemed to be required for favourable results in T2D treatment of the “LTFs”.
Interestingly, use of DPP-4-inhibitors increased in all of the groups during the follow-up. Therefore, we cannot conclude that taking these preparations into the treatment repertoire would have hadst a general positive effect on the glycaemic status of the studied “LTFs”. The DPP-4-inhibitors also belong to the recommended second-line T2D-medications [Citation13–Citation15]. Yet nothing has been shown to be superior to metformin [Citation15,Citation16]. We were not able to show an association between improvement in glycaemic control and an increase in the use of DPP-4-inhibitors so the main reason for the observed increased use of this type of medication may have been aggressive marketing. Just at the time of this study, DPP-4-inhibitors were novel preparations and they were strongly marketed. This may have been the reason for initiating DPP-4-inhibitors as additive medication or replacing parts of previous medication with DPP-4-inhibitors, even in patients with good glycaemic control.
Actions which improved glycaemic control in those recruited “LTFs” who were able to improve their glycaemic control may also have induced modest improvements in some other parameters which are related to better vascular outcomes in T2D patients [Citation8–Citation10]. However, it is difficult to draw very strong conclusions about this because although the general practitioners followed good clinical care guidelines and monitored HbA1 c-concentrations as recommended [Citation13–Citation15] there were other vital parameters which were not as well monitored during the follow-up. Marking T2D diagnosis on patient charts does not guarantee that T2D is properly treated [Citation17] and neglecting actions which belong to proper T2D treatment is not uncommon in primary care [Citation18–Citation20]. We had difficulties in getting follow-up data about physical measurements such as weight and BP although these parameters may have prognostic value for T2D patients [Citation21]. This holds true also with certain laboratory tests which also may have similar value in the treatment of these patients [Citation21]. Furthermore, the number of eligible patients was small. The present data are 10 years old. According to recent Finnish epidemiological studies [Citation22], the prevalence of T2D has not, however, decreased since the times of these data were collected [Citation23]. Slight changes in recommendations for treatment have been made since that time [Citation13,Citation14]. This could mean that a bigger proportion of putative “LTFs” who finally attend to treatment could nowadays be helped to yield better glycaemic control than was possible in 2010–2012. Yet, this does not rule out the fact that the present data call for better adherence to T2D treatment guidelines in primary care.
Conclusions
Improved glycaemic control in recruited “LTFs” was associated with increases in metformin dosage and use of other diabetes medications. Improved medication combined with previously described increase in contacting the public primary care that improved glycaemic control may also have induced improvements in some other parameters related to better cardiovascular outcomes in T2D patients.
Data statement
The data are available with a reasonable request to the authors.
Acknowledgments
We thank Michael Horwood, PhD, for reviewing the language.
Disclosure statement
The authors report no conflict of interest
Additional information
Funding
References
- Polonsky WH, Henry RR. Poor medication adherence in type 2 diabetes: recognizing the scope of the problem and its key contributors. Patient Prefer Adherence. 2016 Jul 22;10:1299–7.
- Feldman BS, Cohen-Stavi CJ, Leibowitz M, et al. Defining the role of medication adherence in poor glycemic control among a general adult population with diabetes. PLoS One. 2014 Sep 26;9(9):e108145. . eCollection 2014.
- Fullerton B, Erler A, Pohlmann B, et al. Predictors of dropout in the German disease management program for type 2 diabetes. BMC Health Ser Res. 2012;12(1):8. .
- Kauppila T, Laine MK, Honkasalo M, et al. Contacting dropouts from type 2 diabetes care in public primary health care: description of the patient population. Scand J Prim Health Care. 2016;34(3):267–273.
- Laine MK, Kauppila T, Honkasalo M, et al. Impact of intervention on metabolic outcomes among dropouts with type 2 diabetes. Adv Med Sci. 2017;63(1):5–8.
- Kauppila T, Eriksson JG, Honkasalo M, et al. Relationship between number of contacts between previous dropouts with type 2 diabetes and health care professionals on glycaemic control: A cohort study in public primary health care. Prim Care Diabetes. 2019;13(5):468–473.
- Duckworth W, Abraira C, Moritz T, et al. VADT Investigators. Glucose control and vascular complications in veterans with type 2 diabetes. N Engl J Med. 2009;360(2):129–139.
- Nichols GA, Joshua-Gotlib S, Parasuraman S. Independent contribution of A1C, systolic blood pressure, and LDL cholesterol control to risk of cardiovascular disease hospitalizations in type 2 diabetes: an observational cohort study. J Gen Intern Med. 2013 May;28(5):691–697.
- Feingold KR, Grunfeld G Diabetes and dyslipidemia, endotext. https://www.ncbi.nlm.nih.gov/books/NBK305900/
- de Havenon A, Majersik JJ, Tirschwell DL, et al. Blood pressure, glycemic control, and white matter hyperintensity progression in type 2 diabetics. Neurology. 2019;92(11):e1168–e1175.
- Patel A, MacMahon S, Chalmers J, et al. ADVANCE Collaborative Group. Intensive blood glucose control and vascular outcomes in patients with type 2 diabetes. N Engl J Med. 2008;358(24):2560–2572.
- Zoungas S, Arima H, Gerstein HC, et al. Collaborators on Trials of Lowering Glucose (CONTROL) group. Effects of intensive glucose control on microvascular outcomes in patients with type 2 diabetes: a meta-analysis of individual participant data from randomised controlled trials. Lancet Diabetes Endocrinol. 2017;5(6):431–437.
- Inzucchi SE, Bergenstal RM, Buse JB, et al. Management of hyperglycaemia in type 2 diabetes, 2015: a patient-centred approach. Update to a position statement of the American Diabetes Association and the European Association for the Study of Diabetes. Diabetologia. 2015;58(3):429–442.
- American Diabetes Association. 15. Diabetes Advocacy: standards of Medical Care in Diabetes-2018. Diabetes Care. 2018;41(Suppl 1): S152–S153. .
- Standl E, Schnell O, McGuire DK, et al. Integration of recent evidence into management of patients with atherosclerotic cardiovascular disease and type 2 diabetes. Lancet Diabetes Endocrinol. 2017;5(5):391–402.
- Gorter KJ, van de Laar FA, Janssen PG, et al. Diabetes: glycaemic control in type 2 (drug treatments). BMJ Clin Evid. 2012;0609:ii: 0609.
- Goudswaard AN, Lam K, Stolk RP, et al. Quality of recording of data from patients with type 2 diabetes is not a valid indicator of quality of care. A cross-sectional study. Fam Pract. 2003;20(2):173–177.
- Modesti A, Bartaloni R, Bellagamba F, et al. Health care delivery in type 2 diabetes. A survey in an Italian primary care practice. Prim Care Diabetes. 2015;9(1):9–14.
- Fürthauer J, Flamm M, Sönnichsen A. Patient and physician related factors of adherence to evidence based guidelines in diabetes mellitus type 2, cardiovascular disease and prevention: a cross sectional study. BMC Fam Pract. 2013;14(1):47.
- Brugos-Larumbe A, Aldaz-Herce P, Guillen-Grima F, et al. Assessing variability in compliance with recommendations given by the International Diabetes Federation (IDF) for patients with type 2 diabetes in primary care using electronic records. The APNA study. Prim Care Diabetes. 2018;12(1):34–44.
- Staff M, Roberts C, March L. The completeness of electronic medical record data for patients with Type 2 Diabetes in primary care and its implications for computer modelling of predicted clinical outcomes. Prim Care Diabetes. 2016;10(5):352–359.
- Mehta N, Stenholm S, Männistö S, et al. Excess body weight, cigarette smoking, and type II diabetes incidence in the national FINRISK studies. Ann Epidemiol. 2020 Feb;42:12–18. Epub 2020 Jan 9.
- Ylihärsilä H, Lindström J, Eriksson JG, et al. Prevalence of diabetes and impaired glucose regulation in 45- to 64-year-old individuals in three areas of Finland. Diabet Med. 2005 Jan;22(1):88–91.