ABSTRACT
In a remote region of western Alaska where tuberculosis (TB) incidence remains relatively high, a rapid molecular detection assay (Xpert MTB/RIF) was introduced four years ago with goal of improving the ability to diagnose active pulmonary tuberculosis (TB). Our aggressive testing programme was intended for all patients acutely evaluated for pulmonary TB at our regional hospital and multiple clinics over a large area. All 223 consecutive patients evaluated for active pulmonary TB were tested with Xpert MTB/RIF (Xpert) per our protocol of which 192 (86.1%) had at least one additional (paired) sputum sample collected for standard acid-fast bacilli (smear) microscopy and culture. Fourteen patients eventually became culture-positive for Mycobacterium tuberculosis (MTB), all but one having initially tested positive (MTB detected) by Xpert (sensitivity 92.9%). All remaining culture-negative individuals had tested negative (not detected) by Xpert (specificity 100%). By contrast, smear microscopy sensitivity and specificity was 64.3% and 98.9% respectively. This represents the addition of four active TB patients detected by Xpert over smear. In remote regions, the ability of Xpert to quickly and reliably detect TB while determine which patients are not contagious represents a huge healthcare savings as in most cases these patients will not require hospitalized isolation.
Introduction
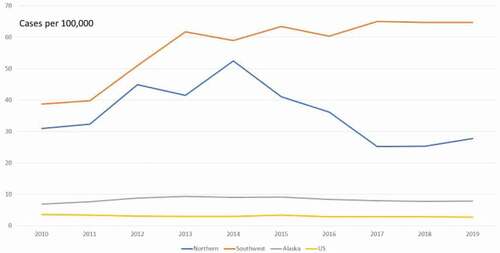
It is not debated that “ … TB remains a serious public health issue in the circumpolar region … ” [Citation1] and Alaska is no exception [Citation2] with its tuberculosis (TB) incidence (2019 provisional rate 8.1 per 100,000) [Citation3] among the highest in the USA (U.S). In particular, western Alaska (sub-divided into Northern and Southwest regions) [], bordering the Bering Sea with primarily indigenous populations, has been most affected with rates up to eight times that of the above reported Alaska rate. Western Alaska is where US Public Health Service isoniazid field trials first demonstrated preventive efficacy during the last century [Citation4], which not only helped bring a devastating TB epidemic to a close but also helped to guide future US treatment strategy.
Figure 1. Western Alaska (sub-divided into Northern and Southwest regions) tuberculosis incidence, 2010–2019. All Alaska rates are 3-year moving averages, U.S. rate is year-to-year. Data points for 2010 and 2011 contain rates for years prior to 2010 in their 3-year average. Data for year 2019 is provisional.
As demonstrated in the developing world [Citation5], the Xpert MTB/RIF assay (Xpert), based on polymerase chain reaction methodology, is a quick and reliable on-site diagnostic TB assay (sensitivity 90.4%, specificity 98.4% [Citation6]). Even prior to approval by the US Food and Drug Agency (FDA) [Citation7,Citation8], its use in remote Alaska was considered a promising new strategy to more efficiently diagnose active pulmonary TB when combined with the standard acid-fast bacilli (smear) microscopy and culture [Citation9]. This new efficiency of adding a highly sensitive test with timely results in a remote setting added clarity to suspected TB patient management. Prior to the advent of Xpert, the first lab confirmation (smear microscopy) of TB could take up to a week to process at our one centralised State Lab hundreds of miles away in Anchorage due to transportation distance and weekend lab closure. With the advent of on-site Xpert results, the diagnostic picture becomes much clearer. If TB is detected, appropriate treatment and isolation is started often at the “point-of-care”. If not initially detected but later found to have TB by culture, at least the patient is presumably not a contagious threat while awaiting culture results. Also, the patient is not unnecessarily respiratory isolated, often at a great expense with loss of liberty. In a non-remote area, this would not be such an issue.
Prior to use of Xpert, it would not be unusual for suspected TB patients to be unnecessarily confined-a valid human rights issue and at times a great expense-for up to a week while awaiting central lab results that might determine treatment and community intervention.
In 2016, this new Xpert rapid testing technology was added to the TB programme administered by our healthcare system based in Bethel, Alaska involving our regional hospital plus 46 clinics of various size in greater than 50 surrounding village communities over an area larger than the state of Washington. What follows is our experience over the programme’s first 3 years.
Methods
Sputum samples were collected for testing with Xpert from all patients who presented at risk for active pulmonary TB during the study period from 2016 to 2019 as part of our standard best practices TB control programme modelled after the Centres for Disease Control and Prevention (CDC) guidelines [Citation10]. Any Xpert testing done as non-acute was excluded from the study.
Prior to Xpert testing, this newly FDA-approved rapid TB assay, with its in-house hospital turn-around time of 2 h, was integrated into our Adult TB Guideline (Guideline) [Citation9]. Concisely, this new Guideline recommended collecting paired sputum samples (one for Xpert, one for smear/culture) initially for all acutely suspected TB patients (determined by standard risk factors in our Guideline) in the hospital or village clinic, regardless of chest x-ray (CXR) capability, followed by two more consecutive sputum smear/culture samples. Based on the Xpert result, any continuation of respiratory isolation, need of travel or treatment was considered with this new clarity of the patient’s diagnosis using Xpert technology. All other standard testing and patient management were carried out as before (including eventual CXR if not initially done when able to travel safely, usually by small plane) per CDC recommendations.
A minimum of one paired sputum for both Xpert (test processed only at lab of local hospital in Bethel) and smear microscopy with culture (tests only done at State Lab in Anchorage), with the goal of at least two more smear/culture samples over the next 48-h period, was a programme requirement for patients presenting to our service unit chronologically per our Guideline. For the purpose of the study, an active pulmonary TB diagnosis was based solely on the sputum culture results regardless what Xpert or other testing technology available at the State Lab. No other body fluids per FDA mandate were tested with Xpert and no TB clinical diagnoses without positive culture was included in the study. Also, any incidental TB diagnosis or diagnosis of a resident elsewhere traced back to our area was not included in the study.
All sputum samples tested for Xpert were done in the local hospital by trained laboratory technicians. Those samples collected in the hospital were processed on the same day while those collected in the village were usually delayed until the next day due to required air transportation (in our roadless area) to the lab in Bethel. Since smear/culture sputum samples collected were only processed at the centralised State Lab, this could take at least 3 days but up to a week due to reasons stated in the Introduction.
The Xpert test sensitivity and specificity and the percentage of Xpert-tested patients participating with a paired sputum collection were used as metrics for TB programme efficacy.
Not originally begun as a research project, laboratory data collected from this effort over a three-year period was afterwards evaluated for diagnostic efficiency using the above metrics. This formed the basis of an operational research study which was approved by the Yukon-Kuskokwim Health Corporation (YKHC) Executive Board following review by the YKHC Human Studies Committee.
Results
Over a 3-year period, 223 consecutive patients were evaluated for active pulmonary TB and all were tested with Xpert per our protocol. From these patients, we were able to collect at least one other (paired) sputum sample for smear microscopy and culture from 192 patients (86.1%). From this latter group, fourteen patients (11 male/3 female, ages 21–87 years) [] eventually cultured positive for Mycobacterium tuberculosis (MTB). All but one of these had initially tested positive (MTB detected) by Xpert (sensitivity 92.9%). All the remaining 178 individuals with paired sputum samples who were culture-negative for MTB initially tested negative (not detected) by Xpert (specificity 100%). By contrast, smear microscopy detected nine of the culture-positive patients for a sensitivity and specificity 64.3% and 98.9%, respectively. This represents the addition of four TB patients detected by Xpert over smear [] (or 28.6 additional cases detected per 100 culture-confirmed TB cases) for this small patient population. No rifampin resistance was detected. The active TB testing positivity was 7.3% (i.e., percentage of those cultured for TB symptoms {192} who eventually grew out culture-positive MTB {14}).
Table 1. Chronological listing of active tuberculosis cases (defined by culture growth on at least one sputum sample) with demographic information from study population tested with Xpert MTB/RIF over a 3-year period, 2016–2019.
Discussion
It is our intent to share the results of our adding improved technology to our TB control programme with similar healthcare systems, especially in the circumpolar region, who may benefit from our experience.
The Xpert performance in our limited population favourably compared to published data [Citation5]. This included the improvement noted of Xpert over standard smear microscopy [Citation11] for rapid detection of MTB (with the added benefit of the test result in hours instead of days). Reasonably, faster, and more accurate detection might result in less TB transmission and as a result, fewer new cases in the long term (but cases may increase short term due to improved detection). The ability of Xpert to quickly and reliably determine which patients are not contagious represents a huge healthcare savings as in most cases these patients will not require hospitalised isolation.
It is noted that Case #11 [] is the only patient with eventual culture-positive MTB that was “not detected” by Xpert. In addition, this patient’s 4+ smear microscopy result highlighted Xpert’s shortcoming in this case. Upon review, it was noted anecdotally that in the initial paired sputum collection an apparent low quality “watery” sample was tested with Xpert and a much higher quality sample was sent to the State Lab for smear microscopy and culture. Since our lab and Xpert itself had no quality requirement for Xpert, it was understandable that a “not detected” result followed from this sample that was likely saliva only. In retrospect, a repeat Xpert assay should have occurred, especially with an abnormal CXR and clinical suspicion for TB. If that had been done, detection likely would have occurred, and the study sensitivity would have been 100% instead of the reported 92.9% for this small culture-positive group. Our lab policy for Xpert changed as a result requiring a respiratory therapy (RT)-collected sputum (which assumed an RT would more reliably collect a proper sputum sample) which included visual confirmation of the specimen. This underscores the importance of clinical judgement in determining whether to repeat a questionable result or when to appropriately end respiratory isolation.
Our smear microscopy sensitivity of 63.4% was in line with a published 3-sputum study [Citation12] result of 67.5% although it is noted that a more recent study [Citation11] resulted in a lower 38.8% figure. It is possible the higher smear sensitivity figures reflect a more advanced TB disease population including ours. This point is supported by the fact that in the latter study with low smear sensitivity, the Xpert sensitivity was also much lower at 74.1% than comparative studies reporting greater than 90% values.
The reason why a second (paired) sputum was not collected from more than 86.1% of the initial patients evaluated for active pulmonary TB is not entirely known as this data was not recorded. However, such a level of compliance is still acceptable. Likely reasons for why a second sputum sample was not collected on these 31 individuals includes; patient refusal, inadequate or insufficient specimen as determined in the lab, inability to produce a second specimen at presentation and unable or unwilling to retry on a subsequent day, and medical personnel forgetting to collect a separate second sputum specimen.
A valid human rights concern has been raised [Citation9] that an unnecessary delay in TB testing pre-advent of Xpert and in particular in remote areas errs on patient isolation-confinement for the sake of community safety. This concern has been lessened anecdotally by Xpert as clinically stable-suspected TB patients, with MTB “not detected” by Xpert, can now be immediately released to the community as “not currently contagious” with public health follow-up as immediate diagnostic clarity has been added to their status. Previously a test result from a far-away lab would be required for this to occur. In the same manner, patients otherwise qualifying for hospital admission might forego “airborne precautions” isolation which had been practiced before. The above clarity in a patient’s TB status could have potential huge cost savings. Metrics related to this issue were not collected.
Our study results confirm our TB programme efficacy by virtue of the high number of participants getting Guideline-recommended paired sputum samples as well as the Xpert test sensitivity and specificity favourably comparing to published data. Although no treatment improvement metric, including time to treatment, treatment completion time, time of non-treatment isolation, or cost savings were studied, all can reasonably be presumed improved with the addition of Xpert rapid testing. This is in line multiple studies [Citation8,Citation13,Citation14] which have confirmed such results pertaining to efficient TB medical care, improved health facility and community safety and cost savings [Citation15]. As a reminder, even with cost savings, concerns for sustainability of this new technology has been raised for the developing world [Citation16].
Acknowledgments
I wish to thank both Terri Hodges, MLT(ASCP) and Curtis Buchholz, MD, who were the Laboratory Manager and Lab Medical Director, respectively, during the study period. Their unwavering support and efforts early-on and throughout the study helped make rapid molecular detection possible in our resource limited healthcare situation. As always, Public Health Nursing in western Alaska stepped up and are to be commended for their tireless efforts in TB contact tracing and managing TB patients in our area.
Disclosure statement
No potential conflict of interest was reported by the author.
Additional information
Funding
References
- ICS-tuberculosis working group. A seven-year epidemiological review of tuberculosis in the circumpolar region 2006-2012. Available from: htpp://dhss.alaska.gov/dph/Epi/id/SiteAssets/Pages/TB/ICS-TB_report_2006-2012.pdf
- Alaska Epidemiology Recommendations and Reports. Alaska’s ongoing journey with tuberculosis in Alaska and considerations for future control. Vol. 19, No. 1, April 11, 2017. Available from: htpp://www.epi.alaska.gov/bulletin/docs/rr2017_1.pdf
- Schwartz NG, Price SF, Pratt RH, et al. Tuberculosis-USA, 2019. MMWR More Mortal Rep. 2020;69:286–4. .
- Comstock GW. Isoniazid prophylaxis in an underdeveloped area. Am Rev Respir Dis. 1962;21:9–27.
- Boehme CC, Nabeta P, Hillemann D, et al. Rapid molecular detection of tuberculosis and rifampin resistance. N Eng J Med. 2010;363:1005–1015.
- Chang K, Lu W, Wang J, et al. Rapid and effective diagnosis of tuberculosis and rifampin resistance with Xpert MTB/RIF assay: a meta-analysis. J Infect. 2012;64:580–588.
- CDC. Availability of an assay for detecting Mycobacterium tuberculosis, including rifampin-resistance strains, and considerations for its use-USA, 2013. MMWR Morg Mortal Wkly Rep. 2013;62:821–824.
- Choi HW, Miele K, Dowdy D, et al. Cost effectiveness of Xpert MTB/RIF for diagnosing pulmonary tuberculosis in the USA. Int J Tuberc Lung Dis. 2013;17:1328–1335.
- Bowerman RJ. The promise of rapid detection of active pulmonary tuberculosis in rural Alaska. Alaska Med. 2015;56:24–28.
- Nahid P, Dorman SE, Alipanah N, et al. Official American thoracic society/centers for disease control and prevention/infectious diseases society of America clinical practice guidelines: diagnosis of tuberculosis in adults and children. CID. 2017;64:1–33.
- Ampel NM Diagnosing TB with the Xpert assay vs AFB smear microscopy (reviewing Lee H-S, et al Am J Resp Crit Care Med 2019 Mar 15). Available from: htpps://www.jwatch.org/na48799/2019/03/25/diagnosing-tb-with-xpert-assay-vs-afb-snear-microscopy
- Mathew P, Yen-Hong K, Vaxirani B, et al. Are three sputum acid-fast bacillus smears necessary for discontinuing tuberculosis isolation? J Clin Microbiol. 2002;40:3482–3484.
- Park PH, Holland DP, Wade A, et al. Public health costs for tuberculosis suspects in Wake County, North Carolina, USA. Int J Tuberc Lung Dis. 2013;17:759–763.
- Chaisson LH, Roemer M, Cantu D, et al. Impact of GeneXpert MTB/RIF assay on triage of respiratory isolation rooms for inpatients with presumed tuberculosis: a hypothetical trial. CID. 2014;59:1353–1360.
- Chaisson LH, Duong D, Cattamanchi A, et al. Association of rapid molecular testing with duration of respiratory isolation for patients with possible tuberculosis in a US hospital. JAMA Intern Med. 2018;178:1380–1388.
- Adam P, Pai M. Editorial-Implementation of Xpert MTB/RIF in high-burden countries: voices from the field matter. PHA. 2019;9:78–79.
