ABSTRACT
Exposure to mercury (Hg) is a global concern, particularly among Arctic populations that rely on the consumption of marine mammals and fish which are the main route of Hg exposure for Arctic populations.The MercuNorth project was created to establish baseline Hg levels across several Arctic regions during the period preceding the Minamata Convention. Blood samples were collected from 669 pregnant women, aged 18–44 years, between 2010 and 2016 from sites across the circumpolar Arctic including Alaska (USA), Nunavik (Canada), Greenland, Iceland, Norway, Sweden, Northern Lapland (Finland) and Murmansk Oblast (Russia). Descriptive statistics were calculated, multiple pairwise comparisons were made between regions, and unadjusted linear trend analyses were performed.Geometric mean concentrations of total Hg were highest in Nunavik (5.20 µg/L) and Greenland (3.79 µg/L), followed by Alaska (2.13 µg/L), with much lower concentrations observed in the other regions (ranged between 0.48 and 1.29 µg/L). In Nunavik, Alaska and Greenland, blood Hg concentrations have decreased significantly since 1992, 2000 and 2010 respectively with % annual decreases of 4.7%, 7.5% and 2.7%, respectively.These circumpolar data combined with fish and marine mammal consumption data can be used for assessing long-term Hg trends and the effectiveness of the Minamata Convention.
Background
Mercury is a global health issue and it is of particular concern among Arctic populations that rely on the consumption of some marine species, which have elevated concentrations of methylmercury (MeHg). MeHg bioaccumulates in the tissues of living organisms and is biomagnified in top-predators of aquatic food webs, such as predatory fish and marine mammals. This is particularly an issue among Inuit populations in the Arctic, who regularly consume traditional foods (also sometimes referred to as country of subsistence foods) such as fish and marine mammals and are therefore exposed to elevated levels of MeHg, even though these regions are far from sources of emissions [Citation1,Citation2]. Due to mercury’s long-range transport capability and bioaccumulation in the Arctic food chain, elevated concentrations of total Hg in blood have been measured in Inuit populations in Greenland and Canada as well as in the Faroe Islands [Citation3–5]. In the populations where the main source of Hg exposure is from dietary sources, total Hg in blood is a good approximation of MeHg exposure.
Hg compounds are known to be toxic to humans and other organisms, particularly organic Hg compounds such as MeHg which have adverse effects on neurobehavioural and cardiovascular and health [Citation6–8]. Public health crisis drew attention to the health impacts of mercury in the 1950s, due to mercury poisoning and associated Minamata disease and Niigata disease in Japan [Citation9], and mercury poisoning outbreaks in Iraq [Citation10]. The negative health consequences of chronic low-dose MeHg exposure, especially in utero, are well documented and include neurological and cardiac health effects among populations who rely on the consumption of fish and marine mammals [Citation11–15]. Prenatal exposure to Hg is of particular concern because the neurobehavioural impacts of Hg exposure can be permanent [Citation16,Citation17]. Exposure to Hg during foetal life can affect the central nervous system even at levels with no clinical consequences for the mother [Citation18] due to the ability of MeHg to readily cross the placental and brain barriers, which is not the case for inorganic Hg [Citation19]. Some institutions have developed blood Hg guidance values to identify “at-risk” populations. A provisional blood MeHg guidance value of 8 µg/L for pregnant women, women of childbearing age and children (<18 years of age) was developed by Health Canada [Citation20]. The US EPA derived a recommended reference dose of 5.8 µg/L (based on a benchmark dose level of 58 µg/L mercury recommended by the National Research Council [Citation21]. The Commission on Human Biological Monitoring in Germany has also established a human monitoring value (HBM I) of 5 µg/L for adults and children [Citation22].
The Minamata Convention on Mercury was adopted in 2013 and ratified in August 2017, becoming legally binding for all parties [Citation23]. Among the circumpolar Arctic countries, Canada, the USA, Denmark, Norway, Sweden, Finland and the Russian Federation have signed the Convention, and Iceland has accessioned [Citation23]. The formal objective of the Convention under Article 1 is “to protect human health and the environment from anthropogenic emissions and releases of mercury and mercury compounds” [Citation23]. Major highlights of the Minamata Convention include a ban on new mercury mines, the phase-out of existing ones, the phase-out and phase-down of mercury use in several products and processes, control measures on emissions to air and on releases to land and water, and the regulation of the informal sector of artisanal and small-scale gold mining. The Convention also addresses interim storage of mercury and its disposal once it becomes waste, sites contaminated by mercury and health issues [Citation24]. The Parties agreed to control and (where feasible) to reduce emissions of Hg and Hg compounds. The Convention also calls for additional research on issues related to Hg.
The Minamata Convention also calls for conducting an effectiveness evaluation to ensure the convention is successfully protecting human health and the environment from Hg. Evers et al. [Citation25] proposed metrics for evaluating effectiveness of the convention, which included the collection of human biomonitoring data to track changes in Hg exposure. Arctic studies such as the Circumpolar Blood Study (1994–1997), that were developed to measure persistent organic pollutants in maternal blood [Citation26,Citation27], have been used as an evaluation tool for the effectiveness of the Stockholm Convention. Similarly, blood Hg data from current and past studies of Hg exposure in pregnant women can be used to evaluate the effectiveness of the Minamata Convention.
People living in the Arctic have some of the highest blood MeHg concentrations on Earth [Citation4]. This MercuNorth study included participation from the USA, Canada, Denmark/Greenland, Iceland, Norway, Sweden, Finland and Russia, and was initiated to assess human exposure to mercury in pregnant women before the ratification of the Minamata Convention. The objective of this paper is to describe levels of total mercury in the blood of pregnant women from across the circumpolar Arctic before 2017, which can serve as a baseline to evaluate the effectiveness of the Minamata Convention.
Methods
Population and recruitment
Participants of the MercuNorth study were originally recruited from separate studies conducted in the USA, Canada, Greenland, Iceland, Norway, Sweden, Finland and Russia. Each participating country recruited pregnant women on a voluntary basis (convenience sample) following their own recruitment procedure, between 2010 and 2016. Many of these women were recruited as part of a subset of other ongoing surveys. Between 25 and 196 pregnant women were recruited in each country, resulting in 669 participants in this study. All participants were pregnant women between the ages of 18 and 44 years with an average age of approximately 28 years. Ethical approval and consent was obtained for each study location, respectively, by the following: Alaska Area Indian Health Service IRB (Institutional Review Board); Research Ethics Committee at the CHU de Québec Research Centre – Université Laval; Ethical Committee for Scientific Investigations in Greenland (KVUG); National bioethics committee of Iceland (VSNb2014070006/03.01); Regional Committees for Medical and Health Research Ethics; Ethical Review Board in Umeå (Dnr 2014/400-31); Ethics Committee of the Northern Ostrobothnia Hospital District; and Ethics Committee at the Northwest Public Health Research Center (IRB0006281). Study details for each study location are listed in .
Table 1. MercuNorth study details and participant characteristics
Blood samples
A trained nurse or laboratory technician performed collection. A total of 16 ml of whole blood was collected in two EDTA tubes: a 6-ml tube (BD 367,863) and 10-ml tube (BD 366,643). A 2-ml aliquot of whole blood was transferred in a Sarstedt vial for Hg analysis. Vials were stored frozen at −20°C until shipment to the Toxicology laboratory at “Institut National de Santé Publique du Québec” (INSPQ) or to another calibrated laboratory (Greenland: Institute for Bioscience – Arctic Research Centre, Aarhus University, Denmark; Alaska: CDC’s NCEH laboratory, Russia: North-Western Branch of “Typhoon” lab). In Inari (Finland), whole blood was collected using Li-heparin tubes as part of the KolArctic study, although this difference in tubes used is not expected to impact any comparison of study results.
Total mercury in blood was quantitatively determined by inductively coupled plasma mass spectrometry (ICP-MS, ELAN DRC II system, PerkinElmer). Samples were diluted 20-fold with a solution containing 0.5% (v/v) ammonium hydroxide and 0.1% (v/v) octylphenol ethoxylate. An external calibration curve was prepared by diluting the corresponding volume of human blood (from healthy volunteers) 20-fold with the same diluent and then spiking with different volumes of 1 mg L−1 multi-elements standard solution (SCP Science, PlasmaCal ICP-MS Verification Standard 1, #141-110-011). The internal standard for both calibration curve and samples analysis was 195Pt.
The internal quality control (QC) of the analyses was ensured by analysing non-certified reference after calibration, after every 10th sample as well as at the end of each analytical sequence. The internal QC was within 1 standard deviation of the expected values. QMEQAS – Quebec Multielement External Quality Assessment Scheme (QM-B-Q1108, QM-B-Q1201, QM-B-Q1302, QM-B-Q1505 and QM-B-Q1512) blood reference materials were used for the total mercury analysis.
The quality and accuracy of the analytical methods in these studies were assessed by participation in an interlaboratory comparison programmes. These include PCI – programme de comparaisons interlaboratoires – Interlaboratory Comparison Program for Metals in Biological Matrices (Canada), QMEQAS (Canada), PMQAS – Priority Metals Quality Assessment Scheme (Canada), LAMP – Lead and Multielement Proficiency Program (USA), State of New York Department of Health (USA) and G-EQUAS – External Quality Assessment Scheme (Germany). Some countries/regions used their national laboratory to conduct mercury analyses. These laboratories also participated in the PCI Interlaboratory Comparison Program for Metals in Biological Matrices QC programme, to demonstrate adequate performance for total mercury in blood. In Denmark, the quality and accuracy of the analytical method were ensured by participation in the Quality Assurance of Information in Marine Environmental monitoring interlaboratory comparison programme [Citation30,Citation31]. In addition, comparative analyses for a subset of blood samples (n=30) was required to verify the comparability of results obtained by INSPQ’s toxicology laboratory and the national laboratories from the USA, Denmark and Russia.
Statistical analyses
Baseline analyses of blood Hg levels, descriptive statistics (geometric means (GMs), 95% confidence intervals (95% CI) and ranges) and percentage exceeding guidance value were calculated for each study region. Overall F-test and multiple comparisons between regions with pairwise t-tests were assessed using analysis of variance (ANOVA) with Hg concentrations log transformed given the skewedness distribution.
Temporal trend analyses of blood Hg levels were performed separately on regions for which data from previous or subsequent sampling campaigns were also available (1992–2013 for Nunavik, 2000–2012 for Alaska and 2010–2015 for Greenland). ANOVA with year of sampling treated as a categorical factor was used. Changes in log transformed concentrations across the years of sampling were evaluated using contrasts. The contrast tested the linear trend using linear contrast coefficients for unequally spaced time points. The mean estimates obtained from the model for each time point were transformed back to the original scale to obtain a GM and its 95% CI. Annual percentages of change were calculated for each region. Regression slopes were compared for Alaska and Nunavik using ANOVA with an interaction term between year of sampling (treated as continuous) and region (treated as categorical) variables, starting in 2000 when data from both regions were available (Greenland was excluded due to a limited number of shared time points). Adjusted analyses based on participants’ age or gestational week were not possible, as information on participants’ age or gestational week were not available on an individual basis for all regions. Analyses were performed using SAS software, version 9.4 (SAS Institute Inc., Cary, NC, USA).
Results
Whole blood mercury concentrations in pregnant women and comparisons between circumpolar Arctic regions
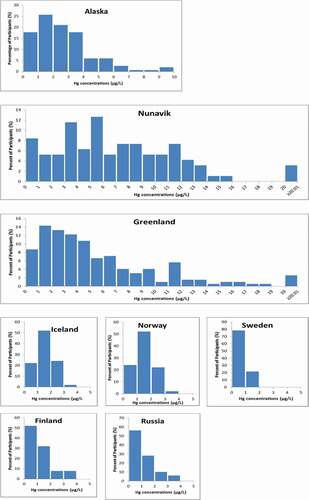
The highest GM concentrations of Hg in whole blood were found in Nunavik (5.20 µg/L), which were more than 10 times higher than the lowest GM concentrations, reported in Sweden (0.48 µg/L)(). Blood Hg concentrations in Greenland and Alaska were 3.79 and 2.13 µg/L, respectively. Blood concentrations of Hg in Iceland, Norway and Russia were similar and not significantly different from each other, but higher than in Finland and Sweden which had the lowest levels. Due to small sample sizes in some regions, 95th percentile CIs could not be calculated.
Table 2. Blood Hg concentrations in pregnant women by study regions from 2010 to 2016
The widest range of concentrations were observed in Nunavik and Greenland, and Alaska to a lesser extent (), compared to the other study regions. This is further illustrated in , which shows the distribution of Hg concentrations in each study region. All participants from Iceland, Norway, Sweden, Finland and Russia had blood Hg concentrations within a small range, all below 4 µg/L. Concentrations of Hg in Alaska, Nunavik and Greenland, however, were greatly varied with several participants having concentrations that were much higher than the majority of other participants from the same region.
The majority of study regions did not have any participants exceed existing Hg blood guidance values (). The only exceedances were in Nunavik, Greenland and Alaska, with the most exceedances observed in Nunavik pregnant women (37.9% ≥8 µg/L), a smaller percentage of women exceeding 8 µg/L in Greenland (23%) and Alaska (3%). It should be noted that the proportion of women in circumpolar countries exceeding health-based guidance values has been decreasing over the last few decades [Citation3], as blood Hg concentrations decline.
Multiple pairwise comparisons between study regions (as shown in ) revealed significant differences between many of the study regions. The regions with the highest concentrations, Nunavik, Greenland and Alaska, were all significantly different, respectively, from all other regions. Concentrations in participants from Iceland, Norway and Russia, however, were not significantly different. The lowest GM Hg levels were observed in Sweden; however, Sweden and Finland were not significantly different.
Table 3. Pairwise comparison of geometric means between study regions (p-values)
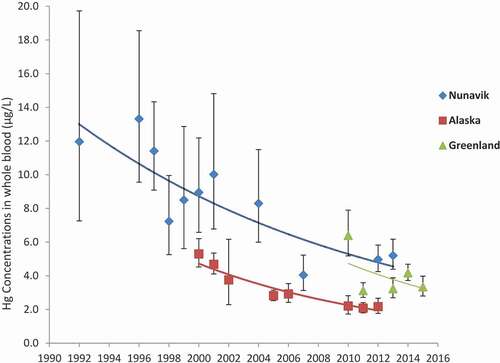
Temporal trend of blood mercury levels in pregnant women from Nunavik, Alaska and Greenland
Temporal trend analyses were conducted for the same populations in Nunavik (Canada), Alaska (USA) and Greenland, and are presented in , which presents blood Hg levels (GM and 95% CI) for these regions. Similar downward time trends were observed in these regions over the study periods (p<0.001). The annual percentage decreases in Alaska (between 2000 and 2012), in Nunavik (between 1992 and 2013) and in Greenland (between 2010 and 2015) were 7.5%, 4.7% and 2.7%, respectively. When Alaska and Nunavik were compared over similar time periods starting in 2000 when data were available for both regions, the declining trend was more pronounced in Alaska compared to Nunavik (simple slopes comparison with a p-value=0.0073). Comparisons with Greenlandic data were not made due to insufficient number of shared time points with Alaska and Nunavik. Additionally, exceedances of blood Hg guidance values decreased over the same corresponding time frame [Citation3].
Discussion
The Minamata Convention on Mercury, which became legally binding in 2017, aims to reduce Hg anthropogenic emissions and environmental concentrations over the long term [Citation24]. This, in turn, should reduce the Hg body burden of vulnerable populations such as communities in circumpolar countries whose diet heavily relies on fish and marine mammals. The strength of this study was in the coordinated international effort to provide baseline Hg data among pregnant women from across the circumpolar Arctic. Investigators from different countries recruited pregnant women using a similar methodology over a similar period. This work is complementary to other international efforts to measure baseline exposure to mercury, such as the global report “Mercury in women of child-bearing age in 25 countries” [Citation33].
It should be noted that this study presents Hg concentrations in pregnant women at several sites across the circumpolar Arctic based on a convenience sample, and should not be considered representative of all these Arctic regions, particularly as samples sizes were small in some regions. In many parts of the Arctic, population density is low and spread across several remote communities, which can make obtaining large sample sizes difficult. In addition, participants from Alaska, Nunavik and Greenland were all from Indigenous communities (Yupik and Inuit, respectively), while other study regions were not reflective of Indigenous communities. Participants from Nickel City in the Pechenga district, for example, are not reflective of Hg exposure in Indigenous populations living in the Murmansk Oblast, Russia.
While blood Hg concentrations were highest in Nunavik and Greenland, there are substantial regional variations in Hg concentrations within each Arctic region. In Greenland, for example, the samples were primarily from western Greenlandic communities (n=118) and Disko Bay communities (n=47) (which are also located on the west coast of Greenland) with GMs in these communities ranging between 1.77 and 5.68 µg/L. Northern and eastern Greenlandic communities had higher GM concentrations between 5.64 and 29.2 µg/L (albeit with much smaller sample sizes of n=14 and n=3 from northern and eastern communities). Differences in Hg concentrations among circumpolar countries may reflect differences in dietary intake among the different regions. For example in Nunavik, the local foods that contribute the most to Hg dietary intake are beluga (meat, nikku, mattaq), lake trout and seal liver [Citation34], and the contribution of each of these varies by season with the highest intake of beluga meat occurring in the late summer and fall [Citation35]. In Greenland, Inuit consume marine foods such as fish, seals and whales, and intake of Hg has been strongly associated with consumption of seals [Citation36]. In many of the other Arctic countries, marine mammals are consumed less often, and the main source of exposure to Hg is from consumption of fish, and less so marine mammals [Citation3]. Moreover, in this study, the lowest mean levels were observed in pregnant women from Inari (Finland) and Kiruna (Sweden), neither of which are located on the coast.
It is important to interpret temporal biomonitoring data in the context of dietary information. Data show that in Nunavik and Alaska, blood Hg concentrations have decreased significantly since 1992 and 2000, respectively. Blood Hg concentrations also decreased in Greenland between 2010 and 2015, although in a non-uniform manner, and declines in blood Hg are further supported by other studies in Greenland that have shown declines since the mid-1990s [Citation37]. Climate change may influence levels of Hg in wildlife among a variety of factors [Citation38]. Since no decreasing trend in Hg worldwide emissions have been reported [Citation39], this suggests that the decline in Hg exposure among pregnant women in these regions is primarily due to either a decline in fish and marine mammal consumption, or a shift towards consumption of wildlife species with lower levels of Hg. In some Arctic regions, decreases in the consumption of fish and marine mammals have been documented, due to environmental changes and a transition towards highly processed market foods as part of a more “western” diet [Citation40–42, Citation43,Citation44]. Local fish and marine mammals are an excellent source of fatty acids, nutrients and essential elements such as selenium, which may also play a key role in protecting against the adverse effects associated with exposure to Hg [Citation45–48], and a shift in diet away from these foods could result in a reduction of intake of these important nutrients. This emphasises the need for routine human biomonitoring of Hg with concomitant assessment of dietary habits and monitoring of contaminants and nutrients in foods, to better document temporal trends and balance the dietary risks and benefits of consuming these foods.
While robust time trend data indicate that blood Hg levels have decreased in pregnant women from Nunavik, Alaska and Greenland, the available historical data for our analysis were limited for our analysis of temporal trends in other circumpolar countries. In Greenland, Hg levels among pregnant women in the Disko Bay region were lower in 2010–2013 compared to levels reported for 2006, 2000, 1996 and 1994, and suggest decreased consumption of marine mammals [Citation37]. Limited available data from Norway indicate a decrease in Hg concentration between 1994 and 2014–2015, when comparing mean Hg concentration of 2.5 µg/L in the Hammerfest region and 3.4 µg/L in the Kirkenes and Bergen regions in 1994 [Citation3] with mean Hg concentrations of 1.2 µg/L in Bodo, Northern Norway in 2014–2015. In Sweden, mean Hg concentrations have been relatively stable over the last 15 years at levels <1 µg/L [Citation3]. In general, temporal trends of adult blood Hg in Arctic regions have shown a decline over time and have also been described in other Arctic regions including Yup’ik, Nunavik, coastal Chukotka and Disko Bay in maternal blood, and in whole blood of men and women from northern Sweden (Norrbotten and Vasterbotten) [Citation49].
While efforts were made to coordinate the collection of blood samples from pregnant women across the circumpolar Arctic at the same time, there was some temporal variation with samples collected over a span of 6 years (collected between 2010 and 2016). This may affect comparisons of levels of Hg between countries, particularly if Hg levels are declining at different rates across different regions. Some pregnant women were recruited during different trimesters of pregnancy; this could also further skew comparisons of Hg levels. In addition, while mean age of participants for all study regions were fairly similar (between 24.2 and 31.5 years), the difference in age between the youngest and oldest participants span 26 years. This may impact comparisons of mercury concentrations due to differences in dietary intake of Hg from fish and marine mammals as age is positively associated with consumption of marine mammals [Citation34]. Differences in the proximity of sampling periods to peak country food consumption during the year across the different sites (seasonal variation) may also impact comparisons. In addition, one limitation of this study was the analysis of only total mercury concentrations. The majority of total mercury is typically organic mercury (MeHg) in marine food eating populations; however, it would be valuable to also have data on the proportion of inorganic and organic Hg in blood for comparison with other regions around the world. Future monitoring in the circumpolar Arctic should also consider analysis of full mercury speciation in blood.
One limitation of this study was the lack of dietary info for interpreting blood Hg concentrations. Further biomonitoring studies are needed in all circumpolar regions in order to provide useful time trends, which should also include collection of dietary information and Hg concentrations in local foods. This will help with assessing dietary intake of mercury and whether changes in blood Hg levels over time are due to dietary changes or due to changes in Hg levels in local food items consumed.
There are significant challenges in managing Hg exposure in the Arctic [Citation41], which must be considered along with substantial food insecurity and poor nutrition. These concerns add additional complexity to recommendations and actions that doctors, nurses and public health professionals take to reduce individual and population-level exposures to MeHg. Many traditional foods are a rich source of nutrients essential for healthy pregnancies, but some also display elevated MeHg concentrations [Citation34,Citation50,Citation51]. Biomonitoring provides valuable information to inform health authorities and is needed to inform and support regulations and risk management actions at the national and international scale. Continuous biomonitoring is also important to help assess whether risk management actions aimed at reducing Hg in the environment are effectively translating into diminishing Hg exposure among human populations.
Conclusion
This paper provides baseline data of total mercury concentrations in pregnant women from circumpolar countries, just before implementation of the Minamata Convention. The highest blood Hg concentration in pregnant women from this study was found in Yupik and Inuit communities from Alaska, Nunavik and Greenland. Further biomonitoring studies are needed in all circumpolar regions in order to provide useful time trends, and allow monitoring of the efficacy of measures that will be adopted by signatory countries to limit Hg emissions worldwide. Future biomonitoring activities should be also completed in conjunction with dietary assessment to properly assess whether changes in blood Hg concentrations are due to dietary changes or due to change in Hg concentrations in traditional foods consumed.
Acknowledgments
The authors would like to thank Dr Eric Dewailly for his vision and leadership on the initiation of this MercuNorth project. The MercuNorth project was an international collaboration, and we would like to acknowledge and thank all study participants and regional partners, including all participating university personnel and hospital staff, with a special thanks to Mr Anders Ruuth from Sunderby Hospital and personnel at Kiruna Hospital, Sweden, and personnel at Landakot University Hospital, Reykjavik. We would also like to thank Elhadji A. Laouan Sidi and Caty Blanchette for statistical analyses, and thank Anna Lukina, Ron Garson and Catherine Girard for their assistance with graphics. We would also like to acknowledge funding for this project which came from multiple sources across the Arctic, including a grant from the Nordic Council of Ministers (project #14137), funding from the Swedish Environmental Protection Agency and the Danish Environmental Agency. Samples from Finland and Russia were collected and funded through the Kolarctic ENPI CBC 2007-2013 project (KO467) “Food and Health Security in the Norwegian, Russian and Finnish border regions: linking local industries, communities and socio-economic impacts”.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- AMAP. AMAP assessment 2011: mercury in the Arctic. Oslo, Norway: Arctic Monitoring and Assessment Programme (AMAP); 2011. p. xiv + 193.
- UNEP. Global mercury assessment 2013: sources, emissions, releases and environmental transport. Geneva, Swizerland: UNEP Chemical Branch; 2013.
- AMAP. AMAP assessment 2015: human Health in the Arctic. Oslo, Norway: Arctic Monitoring and Assessment Programme (AMAP); 2015. p. viii + 165.
- Basu N, Horvat M, Evers DC, et al. A state-of-the-science review of mercury biomarkers in human populations worldwide between 2000 and 2018. Environ Health Perspect. 2018;126(10):106001.
- Gibson J, Adlard B, Olafsdottir K, et al. Levels and trends of contaminants in humans of the Arctic. Int J Circumpolar Health. 2016;75(1):33804.
- Clarkson TW. The toxicology of mercury. Crit Rev Clin Lab Sci. 1997;34(4):369–11.
- Karagas MR, Choi AL, Oken E, et al. Evidence on the human health effects of low-level methylmercury exposure. Environ Health Perspect. 2012;120(6):799–806.
- Stern AH. A review of the studies of the cardiovascular health effects of methylmercury with consideration of their suitability for risk assessment. Environ Res. 2005;98(1):133–142.
- Harada M. Congenital Minamata disease: intrauterine methylmercury poisoning. Teratology. 1978;18(2):285–288.
- Bakir F, Rustam H, Rustam H, et al. Clinical and epidemiological aspects of methylmercury poisoning. Postgrad Med J. 1980;56(651):1–10.
- Choi AL, Weihe P, Budtz-Jorgensen E, et al. Methylmercury exposure and adverse cardiovascular effects in Faroese whaling men. Environ Health Perspect. 2009;117(3):367–372.
- Valera B, Dewailly E, Poirier P. Environmental mercury exposure and blood pressure among Nunavik Inuit adults. Hypertension. 2009;54(5):981–986.
- Valera B, Dewailly E, Poirier P. Impact of mercury exposure on blood pressure and cardiac autonomic activity among Cree adults (James Bay Quebec, Canada). Environ Res. 2011;111(8):1265–1270.
- Valera B, Muckle G, Poirier P, et al. Cardiac autonomic activity and blood pressure among Inuit children exposed to mercury. Neurotoxicology. 2012;33(5):1067–1074.
- Weihe P, Debes F, Halling J, et al. Health effects associated with measured levels of contaminants in the Arctic. Int J Circumpolar Health. 2016;75(1):33805.
- Boucher O, Jacobson SW, Plusquellec P, et al. Prenatal methylmercury, postnatal lead exposure, and evidence of attention deficit/hyperactivity disorder among Inuit children in Arctic Quebec. Environ Health Perspect. 2012;120(10):1456–1461.
- Ethier AA, Muckle G, Bastien C, et al. Effects of environmental contaminant exposure on visual brain development: a prospective electrophysiological study in school-aged children. Neurotoxicology. 2012;33(5):1075–1085.
- Castoldi AF, Coccini T, Ceccatelli S, et al. Neurotoxicity and molecular effects of methylmercury. Brain Res Bull. 2001;55(2):197–203.
- Clarkson TW. The three modern faces of mercury. Environ Health Perspect. 2002;110(Suppl 1):11–23.
- Legrand M, Feeley M, Tikhonov C, et al. Methylmercury blood guidance values for Canada. Can J Public Health. 2010;101(1):28–31.
- National Research Council (US) Committee on the Toxicological Effects of Methylmercury. Toxicological effects of methylmercury. Washington (DC): The National Academies Press (US); 2000.
- Ewers U, Krause C, Schulz C, et al. Reference values and human biological monitoring values for environmental toxins. Int Arch Occup Environ Health. 1999;72(4):255–260.
- UNEP. Minamata Convention on Mercury. Parties and Signatories. Last accessed on Nov 27, 2018 at: [http://www.mercuryconvention.org/Countries/Parties/tabid/3428/language/en-US/Default.aspx]
- UNEP, 2013. Minamata Convention on Mercury. Text and annexes. From: (http://www.mercuryconvention.org/Convention/Text).
- Evers DC, Keane SE, Basu N, et al. Evaluating the effectiveness of the Minamata Convention on Mercury: principles and recommendations for next steps. Sci Total Environ. 2016;569-570:888–903.
- Van Oostdam JC, Dewailly E, Gilman A, et al. Circumpolar maternal blood contaminant survey, 1994-1997 organochlorine compounds. Sci Total Environ. 2004 Sep 1;330(1–3):55–70.
- Mosites E, Rodriguez E, Caudill SP, et al. A comparison of individual-level vs hypothetically pooled mercury biomonitoring data from the Maternal Organics Monitoring Study (MOMS), Alaska, 1999-2012. Int J Circumpolar Health. 2020;79(1):1726256.
- Dewailly E, Ayotte P, Muckle G, et al. 2013. Monitoring of environmental pollutants in maternal blood in Nunavik: time trend assessment and evaluation of the Arctic Char program. Synopsis of research for 2013/2014. Northern Contaminants Program (NCP), Aboriginal Affairs and Northern Development Canada, Gatineau, Canada.
- Bank-Nielsen PI, Long M, Bonefeld-Jørgensen EC. Pregnant Inuit women’s exposure to metals and association with fetal growth outcomes: ACCEPT 2010-2015. Int J Environ Res Public Health. 2019;16(7):1171.
- Dudarev AA, Dushkina EV, Sladkova Yu N, et al. Levels of exposure to metals in population of Pechenga district of Murmansk region//J. Meditsina Truda I Promyshlennaya Ekologiya (Russian Federation). 2016; (6):11–16. [Article in Russian].
- Asmund, G., Vorkamp, K., Backus, S., Comba, M. An update on analytical methods, quality assurance and quality control used in the Greenland AMAP programme: 1999-2002. Sci Total Environ. 2004, 331(1–3): 233–45
- US NRC, 2000. Toxicological Effects of Methylmercury. Committee on the Toxicological Effects of Methylmercury, Board on Environmental Studies and Toxicology, US National Research Council (NRC). National Academy Press. http://www.nap.edu/catalog/9899.html
- Bell L, Evers D, Johnson S, et al. Mercury in women of child-bearing age in 25 countries. IPEN. 2017. p. 70. https://ipen.org/sites/default/files/documents/updateNov14_mercury-women-report-v1_6.pdf
- Lemire M, Kwan M, Laouan-Sidi AE, et al. Local country food sources of methylmercury, selenium and omega-3 fatty acids in Nunavik, Northern Quebec. Sci Total Environ. 2015;509-510:248–259.
- Pontual MM, Ayotte P, Little M, et al. Seasonal variations in exposure to methylmercury and its dietary sources among pregnant Inuit women in Nunavik, Canada. Sci Total Environ. 2020, 755(Pt 2):143196.
- Jeppesen C, Jørgensen ME, Bjerregaard P. Assessment of consumption of marine food in Greenland by a food frequency questionnaire and biomarkers. Int J Circumpolar Health. 2012;71(1):18361.
- Long M, Knudsen AS, Pedersen HS, et al. Food intake and serum persistent organic pollutants in the Greenlandic pregnant women: the ACCEPT sub-study. Sci Total Environ. 2015;529:198–212.
- Brown TM, Macdonald RW, Muir DCG, et al. The distribution and trends of persistent organic pollutants and mercury in marine mammals from Canada’s Eastern Arctic. Sci Total Environ. 2017;618:500–517.
- UNEP. Global mercury assessment 2018. Switzerland: UN Environmet Programme, Chemicals and Health Branch Geneva; 2019. ISBN: 978-92-07-3744-8.
- Knudsen A-KS, Long M, Pedersen HS, et al. Lifestyle, reproductive factors and food intake in Greenlandic pregnant women: the ACCEPT – sub-study. Int J Circumpolar Health. 2015;74(1):29469.
- Pirkle CM, Muckle G, Lemire M. Managing mercury exposure in Northern Canadian communities. CMAJ. 2016 Jul 19;pii(cmaj):151138.
- Terkelsen AS, Long M, Hounsgaard L, et al. Reproductive factors, lifestyle and dietary habits among pregnant women in Greenland: the ACCEPT sub-study 2013-2015. Scand J Public Health. 2017; 1–10.
- Bersamin A, Zidenberg-Cherr S, Stern JS, Luick BR. Nutrient intakes are associated with adherence to a traditional diet among Yup'ik Eskimos living in remote Alaska native communities: the CANHR study. Int J Circumpolar Health. 2007;66:62–70
- Wesche, S.D., Chan, H.M. Adapting to the impacts of climate change on food security among Inuit in the Western Canadian Arctic. Ecohealth. 2010, 7(3):361–73
- Achouba A, Dumas P, Ouellet N, et al. Plasma levels of selenium-containing proteins in Inuit adults from Nunavik. Environ Int. 2016;96:8–15.
- Alkazemi D, Egeland GM, Roberts LJ II, et al. New insights regarding tissue Se and Hg interactions on oxidative stress from plasma IsoP and IsoF measures in the Canadian Inuit population. J Lipid Res. 2013;54(7):1972–1979.
- Kim BM, Choi AL, Ha EH, et al. Effect of hemoglobin adjustment on the precision of mercury concentrations in maternal and cord blood. Environ Res. 2014;132:407–412.
- Lemire M, Fillion M, Frenette B, et al. Selenium and mercury in the Brazilian Amazon: opposing influences on age-related cataracts. Environ Health Perspect. 2010;118(11):1584–1589. .
- Abass K, Emelyanova A, Rautio A. Temporal trends of contaminants in Arctic human populations. Environ Sci Pollut Res Int. 2018;25(29):28834–28850.
- Harrison PA, Sidebottom AC. Alcohol and drug use before and during pregnancy: an examination of use patterns and predictors of cessation. Matern Child Health J. 2009;13(3):386–394.
- Laird BD, Goncharov AB, Egeland GM, et al. Dietary advice on Inuit traditional food use needs to balance benefits and risks of mercury, selenium, and n3 fatty acids. J Nutr. 2013;143(6):923–930.