ABSTRACT
The aim of this work was to compare the prevalence of opisthorchiasis, diphyllobothriasis, and ascariasis among the rural indigenous and long-term resident people of Khanty-Mansi Autonomous Okrug (KMAO) in the years 1988–89 and 2018–19. Helminth infections were identified by faecal microscopic examinations conducted during health check-ups. We analysed 399 medical records for years 1988–89 and 549 records for 2018–19. There were found a decrease in the prevalence of ascariasis among the indigenous people, but the region remains a hotbed of fish-transmitted helminthiases. The spread of D. latus infestation has remained close to 5% in the indigenous adults. The number of opisthorchiasis-infected children, both indigenous and non-indigenous, has increased significantly (p < 0.05). Among the indigenous adults, opisthorchiasis in 2018–19 was at as high level as in 1988–89 (57.5% vs 54.4%). The non-indigenous adults had O. felineus infestations in 2018–19 frequently than in 1988–89 (p = 0.06). The results of our study on the prevalence of helminth infection in the population of the northern Ob River basin agree with the many years average annual incidence of helminthiases in KMAO.
Introduction
In the Arctic, the diversity of mammal parasites is lower than in other geographic areas, however the vulnerability of humans to helminthiases there remains high [Citation1,Citation2]. Peroral infestations are especially common in high-latitude regions. Experts believe that in pre-European era the helminthiases were the most common epidemic diseases of indigenous northerners. Various species of parasitic helminths or their eggs were found in the mummies of ancient Aleuts, Canadian and Greenland Inuit [Citation3–5]. Palaeopathological studies have showed the presence of helminth-induced lesions in the indigenous population of the Arctic zone of Western Siberia in the 17 th–20 th centuries [Citation6,Citation7].
In the 1930 s, along with the industrial development of the Russian Arctic, Siberia and the Far East, the state administration started to implement there a system of sanitary and preventive measures against parasitic diseases [Citation8]. Some progress was made, but the prevalence of foodborne helminthiases in the high-latitude regions remained high and significantly exceeded the all-national average [Citation9,Citation10]. Western Siberia stood out, in this concern, as one of the most impaired regions of Russia. The prevalence of diphyllobothriasis in the North of Western Siberia in 2000–11 was 4–8 times higher than the Russian Federation average, and the prevalence of endemic for the region opisthorchiasis – 15-30 times higher [Citation11].
Notice that the regional figures pertain to the region as a whole, while the situation in the groups of indigenous people living in remote northern areas is different. Indeed, our previous study has shown that in the 1980 s, the level of infestation among the indigenous people leading close to traditional lifestyle was even higher than that in the region’s numerically predominant, westernised population. As recent economic and social transformations in Russia affected the quality and accessibility of public health services, it is of a particular interest to assess the current picture of helminth infestations in the indigenous population of the North of Western Siberia.
The aim of this work was to compare the prevalence of opisthorchiasis, diphyllobothriasis and ascariasis among the rural indigenous and long-term resident people of Khanty-Mansi Autonomous Okrug – Ugra in the years 1988–89 and 2018–19.
Materials and methods
In this study, we consider the parasitological situation in the rural population of one of the northern regions of Western Siberia – the Khanty-Mansi Autonomous Okrug – Ugra (herein – KMAO).
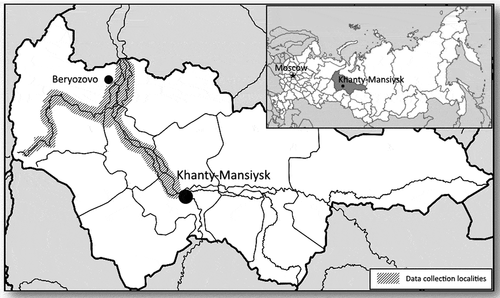
Data were obtained during health check-ups in 1988–89 and 2018–19 in eight rural settlements of Beryozovsky district and several rural settlements of Beloyarsky and Oktiabrsky districts of KMAO. The sites are situated above the 60 th parallel north, in high-latitude areas; see its localisation on the .
Our focal points are three diseases caused by endemic helminths. Two of the diseases, opisthorchiasis (ICD-10 code B66.0) and diphyllobothriasis (ICD-10 code B70.0), are fish-transmitted helminthiases. The infestations with Opisthorchis felineus (Rivolta, 1884) Blanchard, 1895 (Trematoda: Opisthorchiidae) and Diphyllobothrium latum (Linnaeus, 1758) Lühe, 1910 (Cestoda: Diphyllobothriidae) occur when humans consume insufficiently processed freshwater fish, which are intermediate hosts of the parasites. The third disease is soil-transmitted ascariasis (ICD-10 code B77). It develops by the infestation with Ascaris lumbricoides (Linnaeus, 1758) Rudolphi, 1808 (Nematoda: Ascarididae). Despite the northern location of KMAO, the seasonal temperature and humidity variations there do not preclude a possibility for A. lumbricoides eggs to mature in the open grounds [Citation12].
Helminth infections were identified by Kato-Katz stool examination technique, which is recommended by the World Health Organization for intestinal helminth epidemiological surveys [Citation13]. The tests conducted the stuff certified clinical laboratory technicians of the mobile medical and diagnostic department of the KMAO-Ugra Center for Professional Pathology. The authors received the test results in the form of anonymised records.
As far as the target population is of small number and settlements are remote and hard to cover within one year, we aggregated data for the two-years periods, 1988–89 and 2018–19. An additional restriction is that we only could have the information about the presence or absence of helminthiasis in a particular patient at the time of examination. Hence, we could only estimate the prevalence of helminthiases, i.e. the number of positive tests related to the total number of test [Citation14]. However, a standard for Russian medical statistics is the incidence rate, which is the number of first reported cases in the calendar year among the population of a particular territory. These reasons make it impossible to directly compare our material with the data from the Russian Statistical Agency.
The term Indigenous people in this work designate ethnic Mansi and Khanty. Their way of life now we can define as post-traditional. The non-indigenous people are at least second generation of the region residents. They are predominantly ethnic Russians and Komi. In both these groups, artisanal fishery provides a significant share of their food supply.
A preliminary analysis has not revealed gender differences in infestation within the population groups. Subjects were allocated into two age groups: children 5–15 years old, adults above 15. Accordingly, we further describe the situation within the population groups (indigenous and non-indigenous) with the subdivision for age groups (children and adults), without taking sex into account. The number of subjects by years of collecting data, population and age groups are shown in .
Table 1. Number of subjects in study groups
We used Statistica 8.0 and Microsoft Excel software for statistical analysis. A Chi-square test adjusted for maximal likelihood was used to compare study groups by the prevalence of helminthiasis.
Results
See the prevalence of helminthiases in study groups in . This indicator reflects the cases of each type of parasite in question. Since there are subjects infested by more than one type of helminth, the total number of infested (in the last column of the tables) is not the sum of values from the previous columns.
Table 2. Intestinal helminthiases prevalence in KMAO indigenous people in 1988–89 and 2018–19 (per cent)
Table 3. Intestinal helminthiases prevalence in KMAO non-indigenous people in 1988–89 and 2018–19 (per cent)
Among the indigenous people the opisthorchiasis prevalence increased in children from 0.7% in 1988–89 to 28.6% in 2018–19, but it did not practically changed in adults. The frequency of Diphyllobothrium infestation has not changed significantly from 1988–89 to 2018–19; among adults, it has been and remained high (5.3% in 1988–89, 4.8% in 2018–19).
Ascariasis, which accounted for a significant number of cases in 1988–89, was not detected in 2018–19.
The cumulative level of infestations among the children of indigenous people increased from 8.2 to 28.6% (p < 0.01). The prevalence of helminthiases in the indigenous adults was and continued to be high: 54% in 1988–89, and 58% in 2018–19; the difference is statistically insignificant.
The parasitological situation in the non-indigenous rural population has aggravated (). The overall infestation rate has increased among both children (p < 0.03) and adults (p < 0.05). The growth was at the account of cases of opisthorchiasis (p = 0.004 in children, p = 0.06 in adults); in both age groups, the prevalence of ascariasis and diphyllobothriasis has not changed significantly.
Discussion
As stated earlier, our materials characterise the prevalence of invasion, but not the incidence, a traditional index of medical statistics. In addition, the sizes of our study groups do not reflect the real ratio of the indigenous and non-indigenous populations. In particular, indigenous people accounted in 1988–89 for 44.4 and in 2018–19 for 56.9% of the rural population of Beryozovsky district of KMAO, while in our study groups they constituted 81.7 and 69.4% respectively to periods. Besides, ethnic Khanty and Mansi make up only 1.9% of the population in KMAO. As the official medical statistics [Citation15,Citation16] provide data on the disease incidence for the region as a whole, disregarding ethnic groups, we cannot directly compare our materials with theirs. Therefore, we can only consider the general consistency of our results with the figures on helminthiasis incidence shown in available publications.
The comparison of the rates of infestation in indigenous and non-indigenous groups (see ) shows that in children they do not differ significantly in either 1988–89, or 2018–19. The total prevalence of helminthiases among the adults was and remained higher in the indigenous than in non-indigenous district residents (p < 0.05).
The infestation with A. lumbricoides decreased significantly in both the indigenous children and adults (p < 0.01 in both age groups); the differences between the non-indigenous age subgroups by the periods are statistically insignificant. Presumably, the high prevalence of ascariasis among indigenous northerners in 1988–89 could partly be conditioned by the necessity to supplement their diet with vegetables from local vegetable gardens due to the lack of traditional foods. Indeed, in the last quarter of the 20 th century, indigenous people increased the consumption of vegetables. Particularly due to the fact that practically all the rural children of the high-latitude regions of Western Siberia all the wintertime stayed in the boarding-schools where they were fed with the “Soviet nationwide” type of a diet, which included vegetables [Citation10,Citation17]. However, it was not customary food for indigenous people, and the boarding-school students were not taught the manual skills to prepare vegetables. The circumstance increased the risk to contract soil-transmitted helminthiases.
In the last 20–25 years, the cooking and household practices of indigenous people noticeably westernised. That resulted in a reduction of the prevalence of ascariasis, even though little or no changes have occurred in sanitation in the majority of settlements where we collected data (in the second decade of the 21 st century there is still no in-house water and sewage piping).
The spread of D. latus infestation has mostly remained the same. The differences between the time periods are insignificant inside the age and population subgroups. The population subgroups are also indiscernibly differ at the both time periods. Our data on the D. latus infestations among the residents of remote settlements are in concert with the reported incidence of diphyllobothriasis in the population of KMAO as a whole: in 1992–2010 the reduction in the number of newly identified cases per year averaged only 2% [Citation15]. The prevalence of diphyllobothriasis in our study groups is close to 5% in both 1988–89, and 2018–19. That agrees with the data on extremely high incidence (41.6 per 100,000 population, four times higher than the Russian Federation average) registered in the middle Ob River Region and KMAO as a part of it [Citation15,Citation18].
Opisthorchiasis is the most common human helminthiasis in the northern Ob River rural areas. While the many years average incidence of opisthorchiasis in Russia was estimated at 15.2 per 100 thousand population, in KMAO as a whole it was 374.0 per 100 thousand, and in Beryozovsky district in some years it reached 620 per 100 thousand [Citation15,Citation16]. The results of our study support the opinion that the Beryozovsky district and adjacent areas is a territory with extremely high prevalence of opisthorchiasis.
Let us consider the differences in the level of infestation with O. felineus in our study groups. Between the indigenous and non-indigenous children it did not differ in either 1988–89 or in 2018–19, whereas in the adults, the proportion of infested Khanty and Mansi is consistently and significantly higher than that among non-indigenous long-term residents. In the indigenous adult group, however, unlike the non-indigenous, the difference between time periods is insignificant (). That means, among the children, indigenous as well as non-indigenous, the prevalence of opisthorchiasis has markedly increased, while the level of infestation in adult non-indigenous population went up, heading towards rather consistently high that of the indigenous northerners.
Table 4. Opisthorchiasis prevalence in KMAO rural population in 1988–89 and 2018–19 (per cent)
We suppose, the elements of the traditional way of life of indigenous people of Western Siberia, including food processing techniques, which they partly preserved, serve as a protecting factor restraining further growth of the opisthorchiasis prevalence.
A staple of the traditional diet of the indigenous people was parasitologically less dangerous fishes of the salmon (Salmonidae), and sturgeon (Acipenseridae) families. Nowadays, cyprinid fish, the main carrier of O. felineus, account for more than 60% of the total catch in the Ob-Irtysh basin [Citation19]. Traditionally, Khanty and Mansi people rarely used them for food. Small fishes of Cyprinidae species were considered weed and relatively large members of the family (carp, ide) were non-prestigious or used as a famine-time food. According to the old custom of the Khanty, the ide fish of spring and summer catch was only suitable to feed a dog, while humans consumed the fish of pre-winter catch, dry-cured and freezed-out for about two months [Citation10]. This traditional technique well corresponds with the modern technology of food industry. It instructs to keep the large fish at a temperature not above −28°C for at least 32 hours or in saturated saline brine at least 40 days in order to disinfect it from the metacercaria of O. felineus.
Since the 1950 s, fishermen, together indigenous and non-indigenous, have been teamed up into “fisher brigades” [collectives] and were meant to hand over all their catch to fish processing warehouses. After the catch was recorded and sorted, the families of fishermen got the most low-grade fish from it for personal needs. The practice has not only disrupted the dietary traditions of northerners, but also increased the risk of parasitic infections [Citation10,Citation17]
Although in the post-Soviet period the expropriation has discontinued, the individual fishery for the whitefish has remained limited, now due to environmental protective measures. Nowadays, catch quotas for salmons and sturgeons are allocated to those families and communities having the formal status of indigenous to secure the viability of the traditional way of life and economic activities. Our calculation shows, in 2018–20 these quotas allowed 12–18 kg of valuable species fish per year per capita in Beryozovsky district of KMAO. This is a significant volume compared to the average consumption of fish and fish products in the region, which amounted to 24.7 kg/year in 2019 [Citation20]. The availability of the fish that is relatively clear of the O. felineus infection in the indigenous families and communities could have helped to contain the spread of helminthiases.
Unlike the indigenous people, those living in the same settlements ethnic Russians, Komi and others have no fish catching allowance, as they do not formally belong to “the indigenous, numerically small, peoples”. Possibly, the growth in the prevalence of opisthorchiasis in the non-indigenous groups reflects this ethnic discrimination.
The grim helminth-related picture can partly be due to the lack of awareness on the aetiology and health consequences of infestation.
Recent social and cultural transformations turned out to be detrimental for the traditional elements of protective behaviours. Similar negative tendencies in parasitological situation have been reported in the communities of the Indians of South America [Citation21], in various indigenous groups in Malaysia [Citation22,Citation23], among the Canadian Inuit [Citation24,Citation25].
Overall, our results are consistent with the opinions of other specialists, which deem the northern Ob River region as the locality of high prevalence of fish-transmitted helminthiases [Citation11]. Our data also support the idea that the Russian North as a whole shows very weak positive dynamics concerning helminth infestations, and in many regions it is negative [Citation15,Citation16,Citation26,Citation27].
Conclusion
The results of our study on the prevalence of helminth infection in the population of the northern Ob River basin agree with the many years average annual incidence of helminthiases in KMAO.
There were found a decrease in the prevalence of ascariasis among the indigenous people, but the region remains a hotbed of fish-transmitted opisthorchiasis and diphyllobothriasis.
The number of opisthorchiasis-infected children, both indigenous and non-indigenous, has increased significantly, while among the indigenous adults the frequency of opisthorchiasis did not changed from 1988–89 to 2018–19, being consistently high.
The level of O. felineus infestations in the group of non-indigenous adults in 2018–19 appeared to be marginally higher than in 1988–89 (p = 0.06). Fishery restrictions may have contributed to the negative dynamics. Individual fishery is a basic subsistence economy for the major part of the economically deprived population in the region. Ethnic-based fishing quotas limit access to natural resources for a large part of the region’s population thus increasing the consumption of more parasite-infested fish.
Limitations
The children sample sizes substantially differ due to the organisational reasons. In 1988–89, boarding-school and kindergarten administrations procured the health check-ups, while in 2018–19 it was at the personal discretion (of parents) and the number of examinations decreased. We analysed all the primary data available during the time of collection and were not allowed to change the strategy. It cannot be ruled out that the difference in sampling method could distort the picture of changes in the prevalence of infestations.
Geolocation information
Coordinates for Beryozovo town: 63°56′04″N 65°02′40″E.
Acknowledgments
The authors are grateful to the team of the Medical and Diagnostic Department of KMAO-Ugra Center for Professional Pathology for their help in collecting data.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Jenkins EJ, Castrodale LJ, de Rosemond SJ, et al. Tradition and transition: parasitic zoonoses of people and animals in Alaska, northern Canada, and Greenland. Adv Parasitol. 2013;82:33–6.
- Dupouy-Camet J. Parasites of cold climates: a danger or in danger? Food Water Parasitol. 2016;4:1–3.
- Rausch RL. Tropical problems in the Arctic. Infectious and parasitic diseases: a common denominator. In: R. Pelizzon (ed.). Industry and tropical health. Vol. VIII. Boston: Harvard School of Public Health; 1974. p. 63–70.
- Bresciani J, Dansgaard W, Fredskild B, et al. Living conditions. In: Hansen JPH, Meldgaard J, Nordquist Jeditors. The Greenland mummies. Nuuk, Copenhagen: The Greenland Museum, Christian Eljers’ Forlag; 1991. p. 150–167.
- Waldram JB, Herring DA, Young TK. Aboriginal health in Canada: Historical, cultural, and epidemiological perspectives. 2nd ed. Univ. of Toronto Press; 2006. p. 367.
- Slepchenko SM, Ivanov SN. Paleoparasitological analysis of soil samples from the Kikki-Akki burial ground of the 17th–19th centuries in West Siberia, Russia. J Archaeol Sci Rep. 2015;2:467–472.
- Slepchenko SM, Bugmyrin SV, Kozlov AI, et al. Comparison of helminth infection among the Native populations of the Arctic and Subarctic areas in Western Siberia throughout history: parasitological researches on contemporary and the archaeological resources. Korean J Parasitol. 2019;57(6):607–612.
- Kozlov A, Lisitsyn D. Arctic Russia. In: Kue Young T, Bjerregaard P, editors. Health transitions in Arctic populations. Toronto, Buffalo, London: University of Toronto Press; 2008. p. 71–102.
- Feshbach M, ed. Environmental and Health Atlas of Russia. Moscow: PAIMS Publ; 1995. p. 448.
- Kozlov AI, Vershubskaya GG. Medical anthropology of the indigenous people of the Russian North. Moscow: MNEPU Publ. 1999. p. 288.
- Dudarev AA, Dorofeyev VM, Dushkina EV, et al. Food and water security issues in Russia III: food- and waterborne diseases in the Russian Arctic, Siberia and the Far East, 2000–2011. Intern J Circumpolar Health. 2013;72(1):21856.
- WHO. Framework for control and prevention of soil-transmitted helminthiases in the WHO European Region 2016–2020. Copenhagen: WHO Regional office for Europe; 2016. p. 24.
- WHO. Bench aids for the diagnosis of intestinal parasites. Geneva: World Health Organization; 1994 (repr. 2012). p. 23.
- Bush AO, Lafferty KD, Lotz JM, et al. Parasitology meets ecology on its own terms: margolis et al. revisited. J Parasitol. 1997;83(3):575–583.
- Ostapenko NA, Guzeeva TM. Comparative characteristics of epidemic processes at diphyllobothriasis and opisthorchiasis in Khanty-Mansiysk Autonomous Region – ugra. Epidemiol Vaccinoprofil. 2012;2(63):52–57.
- Kozlova II, Ostapenko NA, Sisin EI, et al. On the problem of opisthorchiasis in a hyperendemic focus. Med Parasitol Parasit Dis. 2017;3:14–19.
- Kozlov AI, Kozlova MA, Vershubskaya GG, et al. Health of the indigenous people of the RF North: on the verge between centuries and cultures. Perm: RIO PGGPU; 2012. p. 159.
- Jastrebov VК. Epidemiology of diphyllobothriasis in Siberia and the Far East. Epidemiol Vaccinoprofil. 2013;5(72):25–30.
- Promotorova E. Ecology of cyprinid fishes in the lower Irtysh River Basin. Tambov: Konsalt. Co. Yukom; 2019. p. 80.
- Russian Federal State Statistics Service (Rosstat). Household food consumption in 2019. [Internet]. Rosstat. [ cited 2020 Dec 20]. Available from: https://rosstat.gov.ru/bgd/regl/b20_101/Main.htm
- Fitton LJ. Helminthiasis and culture change among the Cofan of Ecuador. Amer J Hum Biol. 2000;12:465–477.
- Hartini Y, Geishamimi G, Mariam AZ, et al. Distribution of intestinal parasitic infections amongst aborigine children at Post Sungai Rual, Kelantan, Malaysia. Trop Biomed. 2013;30(4):596–601.
- Sinniah B, Hassan AKR, Sabaridah I, et al. Prevalence of intestinal parasitic infections among communities living in different habitats and its comparison with one hundred and one studies conducted over the past 42 years (1970 to 2013) in Malaysia. Trop Biomed. 2014;31(2):190–206.
- Freeman RS, Jamieson J. Parasites of Eskimos at Igloolik and Hall Beach. In: Shephard RJ, Itoh S, editors. Circumpolar health. Toronto: Toronto University Press; 1976. p. 306–315.
- Shephard RJ, Rode A. The health consequences of “modernization”: evidence from circumpolar peoples. Cambridge: Cambridge University Press; 1996. p. 306.
- Tarasova OV, Muratova AP, Degteva GN. Morbidity as a health indicator for the children living in the Far North conditions on the territory of Nenets Autonomous Okrug. Acad J West Sib. 2011;2:43–46.
- Kokolova LM, Platonov TA, Verhovtseva LA, et al. The role of parasitic diseases in people pathology. Rus Parasitol J. 2013;2:43–48.