ABSTRACT
Living at high latitudes is associated with vitamin D (VD) deficiency. An ideal setting to study this is the Antarctic continent, which has temporary inhabitants, but the magnitude of the effect of living in Antarctica and the effects of VD supplementation on this population remain unclear. We performed a systematic review and meta-analysis to assess the effect of temporary residence in Antarctica and impact of VD supplementation on VD status of this population. Random‐effects meta‐analyses were performed to assess serum 25-hydroxyvitamin D (25(OH)D) concentration changes after Antarctic residence (13 studies, 294 subjects) and after VD supplementation (5 studies, 213 subjects). Serum 25(OH)D mean difference after temporary residence in Antarctica was -15.0 nmol/L (95%CI: -25.9, -4.2; I²=92%). Subgroup meta-analyses of studies evaluating Antarctic summer and winter stays showed 25(OH)D only decreases when overwintering (winter 25(OH)D change -17.0 nmol/L [95%CI: -24.1, -9.8; I²=83%] vs. summer 25(OH)D change 1.3 nmol/L [95%CI: -14.6, 17.1; I²=86%]). The meta-analysis of VD supplementation studies in Antarctica showed a mean 25(OH)D increase after supplementation of 10.8 nmol/L (95%CI: 3.3, 18.3; I²=88%). In conclusion, VD status significantly worsens after inhabiting Antarctica, particularly when over-wintering. VD supplementation can prevent worsening of VD status and should be considered in this population.
Introduction
Vitamin D (VD) deficiency is a health condition that affects an important proportion of the worldwide population [Citation1]. VD deficiency can lead to bone mineralisation defects such as rickets in children and osteomalacia in adults [Citation2]. Also, studies in the general population have shown an association of VD deficiency with increased fracture risk and other health problems, including cancer, cardiovascular problems, autoimmune diseases, respiratory infections, and allergies [Citation3–8]. VD status can be easily evaluated by measuring serum or plasma 25-hydroxyvitamin D (25(OH)D) by different methods, although these are not always comparable [Citation9]. Establishing cut-off values of what constitutes normal VD status and VD deficiency has been difficult and controversial among experts and scientific societies, and it is possible that these thresholds differ at different ages and populations [Citation10]. Although variable definitions have been suggested, the Endocrine Society has proposed a serum 25(OH)D concentration below 50 nmol/L (20 ng/mL) to define VD deficiency [Citation11], cut-off value that will be used in this work.
VD is a liposoluble pleiotropic hormone that in humans is mainly synthesised in the skin from cholesterol precursors upon exposure to solar UVB radiation (wavelength 290 to 315 nm), and in a smaller proportion acquired from dietary sources such as oily fish [Citation3]. Low sun exposure is associated with VD deficiency, with progressively more severe deficiency at higher latitudes. These high latitudes significantly affect VD skin synthesis in the winter months in locations above 40º in both hemispheres. At extreme circumpolar latitudes, sunlight may be insufficient for VD synthesis year-round [Citation12,Citation13]. In Antarctica, despite the stratospheric ozone layer hole every austral spring, solar UV radiation reaching the surface is shallow at high latitudes and low solar elevation angles [Citation14,Citation15]. Frequent clouds accentuate this phenomenon of low UV radiation in some Antarctic regions, such as in the South Shetland Islands, where many Antarctic bases and a large portion of the population are located [Citation15].
Antarctica has unique extreme environmental conditions that pose an existential challenge to humans who live and work there, particularly over long periods. In addition to high latitudes, several biological and environmental factors of life on Antarctica are associated with a higher risk of vitamin D deficiency. Reduced sun exposure due to long winter season with little daytime, harsh cold temperatures, severe winds requiring full cover clothing, and sunscreen use in spring and summer seasons also increase the risk of VD deficiency [Citation16]. Moreover, the cold temperatures and extreme polar climate oblige its residents to spend most of their time indoors. The seasonality and duration of stays in Antarctica are highly variable, but can probably be classified well into two groups: those staying year-long or overwintering, as they inhabit Antarctica from one spring or summer to the next, and on the other hand, those with shorter stays that usually include only some spring and summer weeks or months.
Further affecting VD status is the change in dietary habits and foods that people receive during their Antarctic stay [Citation17]. Food in Antarctica is entirely brought from other continents and none is grown naturally or cultivated due to low temperatures and Antarctic regulations. The reliance on preserved foods, and the limited connections with other continents to obtain natural foods, especially in the winter season, can generate a high negative impact on the dietary intake of VD, especially for people staying long-term [Citation17]. All of these factors produce extreme living conditions in Antarctica that might affect VD status and its inhabitants’ health.
Due to the high rates of VD deficiency in the population of countries located at high latitudes, VD supplementation has been recommended to treat this deficiency and, in the medium and long term, to prevent associated diseases [Citation18]. However, few studies have evaluated VD supplementation’s impact on people during their stay in Antarctic bases and expeditions. Besides, it is not clear if VD supplementation at usual dosing recommended by clinical guidelines is enough to avoid low VD levels during residence in Antarctica [Citation19–21]. To understand how residence in Antarctica affects VD status and the impact of VD supplementation in this particular population, we performed a systematic review and meta-analysis of studies that measured VD concentrations in healthy humans during their residence in different Antarctic bases. We believe that these data will provide fundamental insights into VD status at the high latitudes and extreme climatic conditions of Antarctica, which can contribute to policy development on preventing VD deficiency at very high latitudes, including the Antarctic continent.
Methods
Identification of studies and study eligibility criteria
We performed a systematic review and meta-analysis according to PRISMA (Preferred Reporting Items for Systematic reviews and Meta-Analyses) [Citation22], on studies that evaluated vitamin D status and studies about VD supplementation in residents of the Antarctic continent. We searched in PubMed/Medline, Web of Science, and Scopus databases. The algorithm keywords included: “vitamin D” “cholecalciferol”, and “ergocalciferol” combined with “Antarctic” or “Antarctica”, from start to 1 December 2020 (last update). We limited the search to humans.
We also manually reviewed references of studies found through search algorithms to avoid missing any relevant research on the topic. Then, two authors (CC and AB) independently reviewed the selected abstracts to define inclusion. The eligibility criterion was that the study included serum levels of 25(OH)D measured before and after a residency in any Antarctic bases. The only exclusion criteria were the unavailability of numerical data of serum 25(OH)D concentrations of the groups of subjects reported in each study.
Data extraction
All data required for the systematic review and meta-analysis were extracted independently by two authors (CC and AB) from studies considered according to the eligibility criteria as follows: first author, publication year, country and Antarctic base/research station of study performance, intervention, enrolment year, demographic characteristics of the study population (n, age, sex, body weight, height, and body mass index), length of follow-up, methods used for measurement of serum 25(OH)D level, and outcomes about change in serum 25(OH)D level. In some cases, body mass index was calculated by the researchers of this review using the formula weight/height2. In studies that evaluated the effect of VD supplementation, we extracted data on VD dosing and duration of supplementation. Follow-up duration was extracted as days, months, and years, but the data were transformed into approximate days. Mean ± range date age was transformed into a mean ± standard deviation (SD). Data of 25(OH)D concentrations were obtained as nmol/L or ng/ml. To include them in this systematic review and subsequent meta-analysis, all data were converted to nmol/L (1 ng/ml * 2.496 = nmol/L). If statistics were presented with Standard Error of the Mean (SEM), to include them in this review these data were converted to SD.
Data analysis
We extracted numerical data of the mean 25(OH)D blood concentrations in groups of subjects at each specific timepoint that was measured. The first analysis was performed, including the 25(OH)D at baseline and after an entire stay. Then, subgroup analysis was also performed, dividing the 25(OH)D change data according to Antarctic stays in summer months (January to February) and the stay in Antarctic winter (any months between March and September). Finally, a VD supplementation meta-analysis was done comparing the VD level before and after vitamin D supplementation. The statistical analysis was carried out using the Review Manager 5.3 software (the Cochrane Collaboration, 2014, Nordic Cochrane Center, Copenhagen, Denmark). The 25(OH)D level was used as the summary measurement for the meta-analysis, and the results are presented as forest plots. Heterogeneity and inconsistency of the measures were identified through Cochran’s Q statistical test and I2 test, respectively. The random-effects model was applied with inverse variance according to the results of the individual studies. The inconsistency test (I2 > 75%) was used as an indicator for high heterogeneity. The publication bias was assessed through a funnel plot. In all analyses, a confidence interval of 95% was used, and P < 0.05 was considered statistically significant.
Results
Study selection process
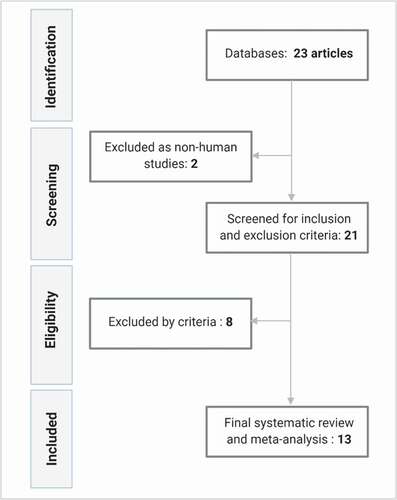
Using the search algorithm, we identified 23 abstracts published in the last 41 years (1979 – December 2020). After filtering for human studies, in total, 21 articles were reviewed by our prespecified inclusion and exclusion criteria. After this process, eight articles were excluded. One study was excluded because it did not include VD levels measured after Antarctic residence [Citation23]. The next article was excluded because the aim was a nutritional and toxicological comparison among Antarctic krill oil and dietary supplements [Citation24]. The third article was excluded because the research did not include the evaluation of VD blood concentrations in the Antarctic population but only quantified dietary vitamin D intake of the study population [Citation17]. Two articles were excluded because they incorporated data previously published in another study [Citation25,Citation26]. The last three articles that were excluded did not provide numerical data of serum or plasma 25(OH)D concentrations, and these could not be obtained by contacting the authors [Citation27–29]. Finally, thirteen studies were included in this systematic review and meta-analysis. The study selection process is summarised in .
Characteristics of the selected studies
The thirteen included studies were performed in heterogeneous populations from different countries, including England [Citation30], Australia [Citation19,Citation31,Citation32], Spain [Citation20], Argentina [Citation21,Citation33], India [Citation34], USA [Citation35,Citation36], Germany [Citation37], Uruguay [Citation38], and Japan [Citation39]. These studies were also done in several different geographical areas of the Antarctic continent according to the location of the respective nations’ bases. Antarctic bases involved in the eligible studies are shown in . The total number of subjects that participated in the selected studies were n = 265 subjects for meta-analysis regarding the seasonal variation of 25(OH)D level and n = 213 subjects for meta-analysis about the impact of VD supplementation in the Antarctic population.
Figure 2. Antarctic bases of eligible studies. Referential map about locations of Antarctic bases and stations involved in studies included in the systematic review and meta-analysis
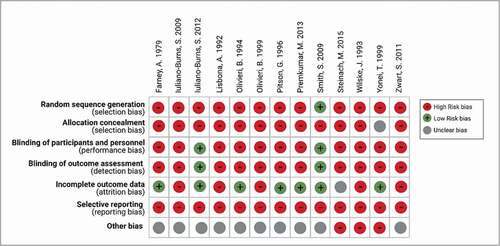
The summary of study characteristics is shown in . These include Antarctic bases, latitude, enrolment years, study type, follow-up length, doses of VD supplementation, number of study subjects, the method used to measure 25(OH)D, and main findings. The quality and risk of bias assessment for the included studies is presented in .
Table 1. Summary of findings. Characteristics of studies included in the systematic review and meta-analysis involved in 25(OH)D status
Table 2. Summary of findings of studies evaluating the effect of VD supplementation on VD status
Meta-analysis of Vitamin D status during the entire Antarctic stay
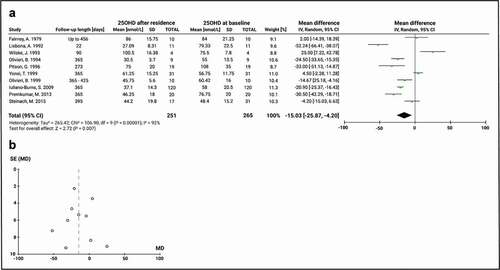
The results from the meta-analysis of VD status throughout the entire Antarctic stay of subjects can be seen in . In ten studies, the last 25(OH)D serum sample was obtained before departure from Antarctica, and results were compared to the baseline 25(OH)D serum concentration at or before arrival to the continent. The length of stay in Antarctica for these studies had a median of 365 days (range 22–456). This analysis did not include the changes in 25(OH)D serum levels in VD-supplemented subjects. The random-effects model was used to calculate the summary measurement. The result of the inconsistency test showed that there was high heterogeneity among the studies analysed (Chi2 = 106.9, df = 9, P < 0.00001; I2 = 92%). The mean baseline 25(OH)D level was 64.9 ± 19.1 nmol/L and the global effect of a full stay in Antarctica was a change of the mean 25(OH)D of −15.0 nmol/L (95% CI: −25.9, −4.2; P = 0.007), demonstrating that Antarctic residence significantly worsens VD status. No publication bias was identified according to the funnel plot ().
Figure 4. Meta-analysis of 25(OH)D serum change pre- and post- Antarctic residence in non-supplemented subjects. A) Mean difference forest plot of 25(OH)D. The mean differences were calculated based on 25(OH)D serum level at baseline and after a completed stay on the Antarctic or sub-Antarctic bases. The studies of Lisbona, A. 1992 and Iuliano-Burns, S. 2012 are included only with the non-interventional group. Values represent the mean effect sizes within a study. B) Funnel plot for publication bias. SE: Standard error of the mean difference
Meta-analysis of vitamin D status in summer and winter residence
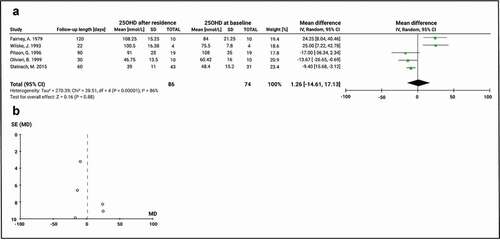
We performed subgroup meta-analyses to evaluate the impact of full clarity days in summer and higher solar UVB radiation versus full darkness days in winter with minimal or null solar UVB radiation on VD status. We analysed five studies that included 25(OH)D measurements pre- and post- Antarctic residence during January and February months, and thus, assessed variations in 25(OH)D serum concentration during summertime in Antarctica. This meta-analysis only included non-supplemented groups or periods. The mean baseline 25(OH)D level was 75.3 ± 22.9 nmol/L and the global effect of a summer stay in Antarctica had a non-significant decrease in the value of the mean 25(OH)D of 1.26 nmol/L (95% CI: −14.6, 17.1; P = 0.9). The result from the inconsistency test showed that there was high heterogeneity between the studies analysed (Chi2 = 28.5, df = 4, P < 0.00001; I2 = 86) (). Publication bias was identified according to the funnel plot (), although this could also be associated with heterogeneity among study design and effect measures.
Figure 5. Comparison of 25(OH)D before and after a summer stay in Antarctica in subjects not receiving vitamin D supplementation. A) Mean difference forest plot of 25(OH)D. The mean differences were calculated based on 25(OH)D serum level at baseline and after at least 22 days of stay among December and February. Values represent the mean effect sizes within a study. B) Funnel plot for publication bias. SE: Standard error of the mean difference
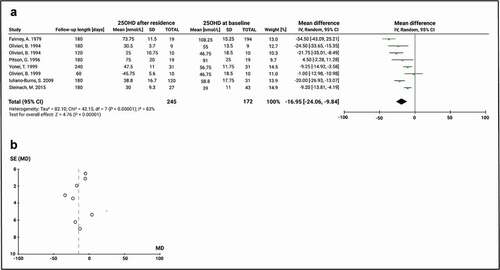
On the other hand, eight studies included Antarctic residence during winter (i.e. overwintering in Antarctica). Despite the random-effects model, the analysis showed a high heterogeneity (Chi2 = 42.2, df = 7, P < 0.00001; I2 = 83%). The mean baseline 25(OH)D level of studies evaluating the effect of overwintering was 59.7 ± 22.8 nmol/L and the global effect of a winter residence in Antarctica has a significant change of the mean 25(OH)D of −17.0 nmol/L (95% CI: −24.1, −9.8; P < 0.0001) (). The funnel plot did not identify bias ().
Figure 6. Comparison of 25(OH)D before and after overwintering in Antarctica in subjects not receiving vitamin D supplementation. A) Mean difference forest plot of 25(OH)D. The mean differences were calculated based on 25(OH)D serum level at baseline and after a residence between March and September. Values represent the mean effect sizes within a study. B) Funnel plot for publication bias. SE: Standard error of the mean difference
Meta-analysis of the effect of VD supplementation on VD status
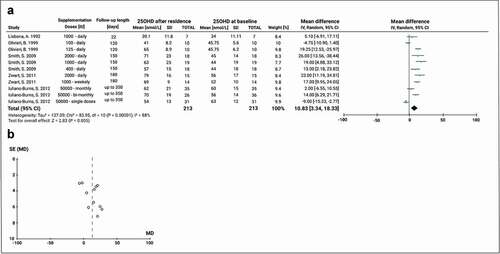
To explore the effect of VD supplementation on 25(OH)D levels during the subjects’ full stay in Antarctica, we analysed data from five experimental studies that included 11 different supplementation groups with varying dosing regimens. Daily VD doses were used in 4 studies, weekly dosing in one arm of one study, and monthly or bi-monthly supplementation in another study (). Groups were supplemented with different doses of oral VD treatment with a median daily dose of 1000 IU (range 100–2000 IU) and a median duration of supplementation of 180 days (range 22 – up to 365). Four studies included daily VD doses including 100, 125, 400, 1000, and 2000 IU. Also, one study had a VD dose of 10,000 IU weekly and one study supplemented their groups with 50,000 IU of VD monthly, every two months, or a single-dose pre-departure. Before supplementation, the VD level was 49.0 ± 8.7 nmol/L. Thus, VD supplementation increased 25(OH)D level with a significant increase of the mean 25(OH)D of 10.83 nmol/L (95% CI: 3.3, 18.3; P < 0.0001). The result from the inconsistency test showed that there was high heterogeneity between the studies analysed (Chi2 = 84.0, df = 10, P < 0.0001; I2 = 88%) (). The Funnel plot shape suggests that the meta-analysis did not have any publication bias ().
Figure 7. Meta-analysis of the effect of VD supplementation on serum 25(OH)D concentrations during Antarctic residence. A) Mean difference forest plot of the effect of VD supplementation. The mean differences were calculated based on 25(OH)D serum level at baseline and after Antarctic residence in the supplemented groups. Values represent the mean effect sizes within a study. B) Funnel plot for publication bias. SE: Standard error of the mean difference
Discussion
We conducted a systematic review and meta-analysis to evaluate all available research evidence regarding the impact of residence in Antarctica on VD status and the effect of oral VD supplementation on this population. Our results demonstrate Antarctic residence’s adverse effect on VD status, particularly among groups of subjects with an extended and overwintering residence. Nevertheless, serum 25(OH)D analyses also showed that change in 25(OH)D during Antarctic summers produced a mild non-significant reduction in VD levels, which is probably not clinically significant. Finally, VD supplementation improved VD status and is a likely effective intervention to prevent VD deficiency and worsening of VD status in people travelling to or residing in Antarctica.
Multiple factors affect the cutaneous synthesis of vitamin D, of which the most prominent is bare skin exposure to sunlight. The amount of sun exposure required to produce sufficient vitamin D levels varies by season, time of the day, and latitude [Citation40]. Previous reports evaluate the association among VD deficiency in populations living in high latitudes due to high solar angles that reduce UVB radiation and climate conditions that the residents oblige to spend more time indoors. Several studies about seasonal variation analyses demonstrated that serum 25(OH)D levels were lower in winter than in summer in high latitudes [Citation41,Citation42]. Besides, cloudy days and cold climates are associated with higher photoprotection by body clothing coverage, which is related to a lack of sun exposure [Citation43]. Moreover, the concern about the effects of stratospheric ozone depletion – that is higher in the south pole- has contributed to increase overuse of solar protection factor (SPF), which alters the incidence of UV radiation on the skin and might decrease VD production [Citation44]. However, the daily SPF use in an adequate dose does not necessarily compromise VD synthesis [Citation45]. Considering this, Antarctica is an ideal ambient to test the association between sun exposure and the risk of generating VD deficiency. Hence, our report is consistent with the previous investigations that concluded an association of living in sun deprivation and worse VD status, highly probably due to factors previously mentioned.
This article is the first report that systematically summarises the sparse literature about the adverse effects of residence in Antarctica on VD status of its transient population. We included the most articles published in order to add strength to our study. However, few data are reported about VD status and stays in Antarctica and even less research with VD supplementation treatment. Despite this limitation, the meta-analysis about completed and winter-stays shows a strong significance and without observable publication bias favouring the association between worsen VD level and sun deprivation in the white continent.
The meta-analyses performed in this review show high heterogeneity due to large clinical variability among studies. The main differences are related to the populations’ characteristics involving different baseline VD status, which might differentially change the effect of lack of sunlight exposure on the studies’ participants. Regarding the 25(OH)D at baseline, higher baseline VD serum concentrations might protect from subsequent deficiencies after completing extended stays in Antarctica [Citation30,Citation32,Citation36]. Likewise, deficient and insufficient VD levels might be unfavourable conditions to worsen VD status after living in Antarctica. However, the reported evidence is not enough to get a significant conclusion in a subgroup analysis according to baseline status.
Possible explanations of why summer stays in Antarctica did not significantly affect 25(OH)D levels probably include the higher solar radiation that is proper of this season and the formation of the ozone layer hole every austral spring. UV radiation reaching the surface in the Antarctic summer is highly variable, and UV index in spring and summer may be comparable to that of northern European countries in this same season [Citation46]. This it is possible that summer Antarctic inhabitants from high latitude countries are not affected by a significant change in UV exposure. However, the results of our meta-analysis of 25(OH)D change in summer could also be influenced by the small number of reported investigations, the small size of the studies, and differences in their design. Moreover, the visual analysis of publication bias supports scarcity of information and potential methodological issues. The largest study evaluating change in VD status in summer showed a worsening 25(OH)D concentration after a summer residence in those with VD deficiency [Citation37], dropping from 48.4 ± 15.0 nmol/L to 39.0 ± 10.8 nmol/L in subjects evaluated in January (n = 31) and March (n = 43) of 2007 to 2012. Noteworthy, from January to March VD deficiency increased from 3.2% to 14% in this report. Given the large sample size and the multiple measurements presented in this study, it could be considered strong support to investigate, with the right study design, how seasons and baseline status of populations impact VD status during Antarctic residence and to test the hypothesis of whether a summer residence affects VD status as well.
To prevent the worsening of VD status that is generated by living in Antarctica, oral VD supplementation is an easy, low cost, and effective intervention for this population. Our meta-analysis supports the intervention in people who reside in Antarctica and receive treatment for at least 120 days especially in subjects with low baseline 25(OH)D levels. VD dosing and frequency determine the impact of VD supplementation. Our study did not compare the effect of different doses or dosing regimens in Antarctic population. The optimal dose and frequency of supplementation remain unclear, as the few supplementation studies evaluated are too heterogenous to provide an universally applicable recommendation on VD dosing and frequency for Antarctic inhabitants. Thus, despite the significant result of the meta-analysis regarding the effect of VD supplementation during Antarctic residence, more interventional clinical trials appear necessary. Another alternative to improve VD status of Antarctic residents is increasing their intake of VD-rich foods (i.e. oily fish) or preferentially importing VD-fortified foods to Antarctic bases. Our research did not include the dietary assessments of VD intake because these are not systematically reported in the reviewed studies. Further research on Antarctic residents’ diet also seems necessary to address this issue.
Conclusions
This meta-analysis shows that VD status significantly worsens after residence in Antarctica, particularly when over-wintering. Our findings also suggest that supplementation with VD is beneficial to humans in Antarctica by improving VD status and suggests it may be necessary for expeditions to this continent and other places with limited access to UVB light. Besides oral VD supplementation, another strategy that might be considered for preventing VD deficiency in this population, is incorporating VD food fortification to products brought to Antarctica. Likewise, people that will move to this continent might be advised to reach a sufficiency level of 25(OH)D before departure to avoid unhealthy risk consequences related to VD deficiency. Future studies are needed to clarify the effect of living in Antarctica during summer on VD status, optimal dosing and frequency of VD supplementation, the efficacy of VD treatment related to the length of stay, and the impact of Antarctic residence on the risk of developing VD deficiency-associated conditions.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Roth DE, Abrams SA, Aloia J, et al. Global prevalence and disease burden of vitamin D deficiency: a roadmap for action in low- and middle-income countries. Ann N Y Acad Sci. 2018;1430(1):44–14.
- Holick MF. Vitamin D deficiency. N Engl J Med. 2007;357(3):266–281.
- Christakos S, Dhawan P, Verstuyf A, et al. Vitamin D: metabolism, molecular mechanism of action, and pleiotropic effects. Physiol Rev. 2016;96(1):365–408.
- Martineau AR, Jolliffe DA, Hooper RL, et al. Vitamin D supplementation to prevent acute respiratory tract infections: systematic review and meta-analysis of individual participant data. BMJ. 2017;356:i6583.
- Borzutzky A, Camargo CA Jr. Role of vitamin D in the pathogenesis and treatment of atopic dermatitis. Expert Rev Clin Immunol. 2013;9(8):751–760.
- Kheiri B, Abdalla A, Osman M, et al. Correction to: vitamin D deficiency and risk of cardiovascular diseases: a narrative review. Clin Hypertens. 2018;24(1):19.
- Marino R, Misra M. Extra-skeletal effects of Vitamin D. Nutrients. 2019;11:7.
- Murdaca G, Tonacci A, Negrini S, et al. Emerging role of vitamin D in autoimmune diseases: an update on evidence and therapeutic implications. Autoimmun Rev. 2019;18(9):102350.
- Dahl SR, Thorsby PM. [How to measure vitamin D status?]. Tidsskr Nor Laegeforen. 2014;134(7):729–731.
- Giustina A, Bouillon R, Binkley N, et al. Controversies in Vitamin D: a statement from the Third International Conference. JBMR Plus. 2020;4(12):e10417.
- Holick MF, Binkley NC, Bischoff-Ferrari HA, et al. Evaluation, treatment, and prevention of vitamin D deficiency: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2011;96(7):1911–1930.
- Edvardsen K, Brustad M, Engelsen O, et al. The solar UV radiation level needed for cutaneous production of vitamin D3 in the face. A study conducted among subjects living at a high latitude (68 degrees N). Photochem Photobiol Sci. 2007;6(1):57–62.
- Webb AR, Kline L, Holick MF. Influence of season and latitude on the cutaneous synthesis of vitamin D3: exposure to winter sunlight in Boston and Edmonton will not promote vitamin D3 synthesis in human skin. J Clin Endocrinol Metab. 1988;67(2):373–378.
- Bais AF, Lucas RM, Bornman JF, et al. Environmental effects of ozone depletion, UV radiation and interactions with climate change: UNEP Environmental Effects Assessment Panel, update 2017. Photochem Photobiol Sci. 2018;17(2):127–179.
- Cordero RR, Damiani A, Seckmeyer G, et al. Satellite-derived UV climatology at Escudero Station, Antarctic Peninsula. Antarctic Science. 2013;25(6):791–803.
- Tsiaras WG, Weinstock MA. Factors influencing vitamin D status. Acta Derm Venereol. 2011;91(2):115–124.
- Iuliano S, Ayton J. Dietary intakes of expeditioners during prolonged sunlight deprivation in polar enviroments do not support bone health. Int J Circumpolar Health. 2015;74(1):27965.
- Raulio S, Erlund I, Mannisto S, et al. Successful nutrition policy: improvement of vitamin D intake and status in Finnish adults over the last decade. Eur J Public Health. 2017;27(2):268–273.
- Iuliano-Burns S, Ayton J, Hillam S, et al. Skeletal and hormonal responses to vitamin D supplementation during sunlight deprivation in Antarctic expeditioners. Osteoporos Int. 2012;23(10):2461–2467.
- Lisbona Gil A, Fernandez Riestra FA, Contreras Fernandez R, et al. [Concentrations of 25-hydroxyvitamin D3 in Antarctica]. Med Clin (Barc). 1992;99(6):206–209.
- Oliveri B, Zeni S, Lorenzetti MP, et al. Effect of one year residence in Antarctica on bone mineral metabolism and body composition. Eur J Clin Nutr. 1999;53(2):88–91.
- Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ. 2009;339:b2535.
- Correa MP, Yamamoto ALC, Moraes GR, et al. Changes in the total ozone content over the period 2006 to 2100 and the effects on the erythemal and vitamin D effective UV doses for South America and Antarctica. Photochem Photobiol Sci. 2019;18(12):2931–2941.
- Bengtson Nash SM, Schlabach M, Nichols PD. A nutritional-toxicological assessment of Antarctic krill oil versus fish oil dietary supplements. Nutrients. 2014;6(9):3382–3402.
- Pitson GA, Lugg DJ, Muller HK. Seasonal cutaneous immune responses in an Antarctic wintering group: no association with testosterone, vitamin D metabolite or anxiety score. Arctic Med Res. 1996;55(3):118–122.
- Smith SM, Zwart SR, Ploutz-Snyder RJ, et al. Response to vitamin D intake: from the Antarctic to the Institute of Medicine. J Nutr. 2011;141(5):985–986.
- Griffiths AP, Fairney A. Effect of phototherapy on serum 25-hydroxyvitamin D in the Antarctic. Eur J Appl Physiol Occup Physiol. 1989;59(1–2):68–72.
- Griffiths P, Fairney A. Vitamin D metabolism in polar vertebrates. Comp Biochem Physiol B. 1988;91(3):511–516.
- Zerath E, Holy X, Gaud R, et al. Decreased serum levels of 1,25-(OH)2 vitamin D during 1 year of sunlight deprivation in the Antarctic. Eur J Appl Physiol Occup Physiol. 1999;79(2):141–147.
- Fairney A, Fry J, Lipscomb A. The effect of darkness on vitamin D in adults. Postgrad Med J. 1979;55(642):248–250.
- Iuliano-Burns S, Wang XF, Ayton J, et al. Skeletal and hormonal responses to sunlight deprivation in Antarctic expeditioners. Osteoporos Int. 2009;20(9):1523–1528.
- Pitson GA, Lugg DJ, Roy CR. Effect of seasonal ultraviolet radiation fluctuations on vitamin D homeostasis during an Antarctic expedition. Eur J Appl Physiol Occup Physiol. 1996;72(3):231–234.
- Oliveri MB, Mautalen C, Bustamante L, et al. Serum levels of 25-hydroxyvitamin D in a year of residence on the Antarctic continent. Eur J Clin Nutr. 1994;48(6):397–401.
- Premkumar M, Sable T, Dhanwal D, et al. Vitamin D homeostasis, bone mineral metabolism, and seasonal affective disorder during 1 year of Antarctic residence. Arch Osteoporos. 2013;8(1–2):129.
- Smith SM, Gardner KK, Locke J, et al. Vitamin D supplementation during Antarctic winter. Am J Clin Nutr. 2009;89(4):1092–1098.
- Zwart SR, Mehta SK, Ploutz-Snyder R, et al. Response to vitamin D supplementation during Antarctic winter is related to BMI, and supplementation can mitigate Epstein-Barr Virus Reactivation. J Nutr. 2011;141(4):692–697.
- Steinach M, Kohlberg E, Maggioni MA, et al. Changes of 25-OH-Vitamin D during overwintering at the German Antarctic Stations Neumayer II and III. PLoS One. 2015;10(12):e0144130.
- Wilske J. Plasma concentrations of 25-hydroxyvitamin-D3 (calcifediol) as an indicator of ultraviolet radiation. Arctic Med Res. 1993;52(4):166–169.
- Yonei T, Hagino H, Katagiri H, et al. Bone metabolic changes in Antarctic wintering team members. Bone. 1999;24(2):145–150.
- Chen TC, Chimeh F, Lu Z, et al. Factors that influence the cutaneous synthesis and dietary sources of vitamin D. Arch Biochem Biophys. 2007;460(2):213–217.
- Greene-Finestone LS, Berger C, De Groh M, et al. 25-Hydroxyvitamin D in Canadian adults: biological, environmental, and behavioral correlates. Osteoporos Int. 2011;22(5):1389–1399.
- Klingberg E, Olerod G, Konar J, et al. Seasonal variations in serum 25-hydroxy vitamin D levels in a Swedish cohort. Endocrine. 2015;49(3):800–808.
- Reeder AI, Gray AR, Liley JB, et al. Factors associated with photoprotection by body clothing coverage, particularly in non-summer months, among a New Zealand community sample. Photochem Photobiol Sci. 2016;15(3):389–397.
- Skotarczak K, Osmola-Mankowska A, Lodyga M, et al. Photoprotection: facts and controversies. Eur Rev Med Pharmacol Sci. 2015;19(1):98–112.
- Passeron T, Bouillon R, Callender V, et al. Sunscreen photoprotection and vitamin D status. Br J Dermatol. 2019;181(5):916–931.
- Cordero RR, Damiani A, Ferrer J, et al. UV irradiance and albedo at Union Glacier Camp (Antarctica): a case study. PLoS One. 2014;9(3):e90705.