ABSTRACT
To assess the prevalence of diabetic retinopathy (DR) among persons with diabetes and prediabetes participating in the 2018 Population Health Survey in Greenland (B2018), a follow-up survey of three previous health surveys. Participants were invited to a diabetes complication screening. We assessed the prevalence of DR using Optos Daytona Ultra-wide field fundus camera and assessed differences in prevalence according to demographic and clinical characteristics using chi square test and a t-test and assessed DR based on ethnicity. The overall prevalence of DR was 2% (10/483). Among participants with HbA1c ≥48 mmol/mol (6.5%) DR prevalence was 9% (9/91), compared with <1% (1/382) among participants with HbA1c <48 mmol/mol (6.5%). All participants with DR lived in towns. The mean Inuit genetic admixture was lower among participants with DR. The prevalence of DR is low in Greenland and almost non-existent among persons with HbA1c below the diabetes threshold.
KEYWORDS:
Introduction
The prevalence of type 2 diabetes in Greenland has increased markedly over the past decades. Based on results from population-based health surveys approximately 10% of the adult population have diabetes, and additionally 20% have prediabetes [Citation1,Citation2]. The increasing prevalence of diabetes can, besides cultural and social changes, be explained by several genetic and epigenetic traits, where ethnicity seems to play an important role [Citation3]. In a study investigating the genetic history of the adult Greenlandic population (covering 10% of the total population), more than 80% of the Greenlandic population, on average had 25% European ancestry, and the amount of European gene flow varied across Greenland. In the more historically isolated areas, such as small settlements in South Greenland and in Qaanaaq, North Greenland, the Inuit gene pool was higher [Citation4].
Further, a study conducted in Greenland, found a genetic variant, TBC1D4, among Inuit to be strongly associated with type 2 diabetes. Approximately 4% of the Greenlandic population are homozygous carriers of the variant, having an odds ratio of 10.3 for developing diabetes and the variant accounts for approximately 15% of all diabetes in Greenland [Citation5].
The emerging prevalence of diabetes and prediabetes in Greenland is worrying due to the risk of developing long-term diabetes complications. Long-term diabetes complications develop gradually over time and can be classified as either micro or macrovascular complications. Microvascular complications affect the small blood vessels and typically include diseases such as retinopathy, nephropathy or neuropathy [Citation6].
Among microvascular complications, diabetic retinopathy (DR) is one of the most frequently occurring complications of diabetes causing blindness in working-age individuals, and affecting approximately 28% of people with diabetes [Citation7]. DR is largely asymptomatic characterised by extensive vascular lesions by the time worsening vision is experienced. Thus, regular screening for DR is necessary [Citation7,Citation8].
In Greenland, the geographical distance between town and settlements is a challenge as each destination can only be reached by plane or boat resulting in limited access to DR screening. The daily healthcare services range from consulting a health worker with very little formal health training in the smaller settlements with around 3–500 inhabitants, to consulting a specialist at the national hospital in Nuuk with 15.000 inhabitants [Citation9].
The aim of this study was to estimate the prevalence of DR in individuals with diabetes and prediabetes identified in population health surveys in Greenland, as representative estimates of DR are not available in Greenland.
Research design and methods
Settings and study population
We assessed the prevalence of DR among all persons with known diabetes, newly diagnosed diabetes or prediabetes participating in the 2018 Population Health Survey in Greenland (B2018), a follow-up survey of three previous health surveys conducted in 1999–2001, 2005–10 and 2014. In short, 2539 Greenlandic citizens from 12 towns and 8 settlements (current administrative definition) participated in B2018.
Upon participation, blood samples (HbA1c, genetic profile on ethnicity, etc.) were taken and anthropometrics measured [Citation10]. Information on place of residence, socio-economic and lifestyle factors were obtained from questionnaires. Participants with an HbA1c measurement at the date of study of ≥42 mmol/mol (IFCC standard) (6.0%) or with known diabetes or previously screen-detected with prediabetes (Impaired fasting glucose (6.1–6.9 mmol/L) or impaired glucose tolerance 120 minutes plasma glucose of 7.8–11.0 mmol/L after OGTT) or diabetes (fasting plasma glucose 7.0 mmol/l and/or 2-h plasma glucose 11.1 mmol/l) in one of the previous population health surveys, were invited to participate in a diabetes complication screening.
Diabetes complication screening
Participants were screened for cardiovascular autonomic neuropathy (CAN), peripheral neuropathy and DR.
CAN was assessed from 5-min resting ECG recordings after 5 min of initial rest. Subsequent to the 5-min ECG recording following three standard cardiovascular autonomic reflex tests were performed: the lying-to-standing test (30/15), the deep breathing test (E/I ratio) and the Valsalva manoeuvre. These three tests reflect the overall condition of the parasympathetic nerve fibres and were recorded by trained personnel using a Vagus® device (Medicus Engineering, Aarhus, Denmark) [Citation11].
Peripheral neuropathy was assessed as part of a clinical foot examination. The examination included a basic check of both feet for deformities and pulse. Sensibility check was performed using a monofilament, three different places on each foot and vibration sensation was measured by biotensiometry at the distal tip of the first toe on each foot, in turn, using a Bio-Thesiometer (Bio-medical instruments, Ohio, USA) [Citation12].
For the assessment of DR, two retina fundus photos of each eye were taken using Optos Daytona ultra-widefield ophthalmoscope [Citation13,Citation14]. Participants with known diabetes, who within a year had had an eye screening in the public health care system, were not imaged but gave a written consent to look them up in the system from the Greenlandic Electronic Medical Records (EMR). All retina fundus photos were uploaded to a central server in Greenland. The images were double graded by two specialist ophthalmologist nurses at Steno Diabetes Center Copenhagen and Glostrup University hospital and discrepancies between two grading’s were resolved by a senior ophthalmologist.
All images were graded according to the International Clinical Diabetic Retinopathy diseases severity scale (ICDR) [Citation15].
Participants were informed about the screening results for CAN and peripheral neuropathy at the examination place and informed they would receive a letter of the retina screening results approximately 2–3 months after the examination.
Statistical analysis
We assessed the prevalence of DR using Pearson's chi-square test to assess the effects of sex, diabetes defined by HbA1c in percentage and place of residence. Furthermore, we assessed the level of Inuit genetic admixture according to DR using t-test to see if DR could be explained by genetic traits.
Ethics
The ethical review committee for Greenland approved the study (KVUG 2017–10). Participants were informed about the study objectives and procedures orally and in writing and gave their informed consent in writing. Participants were referred for treatment and/or check-up if any results in the survey were found to be abnormal. Qualitative interviews in Greenland (Sharing Circles) have supported the conduct of genetic studies in the field of diabetes [Citation16].
Results
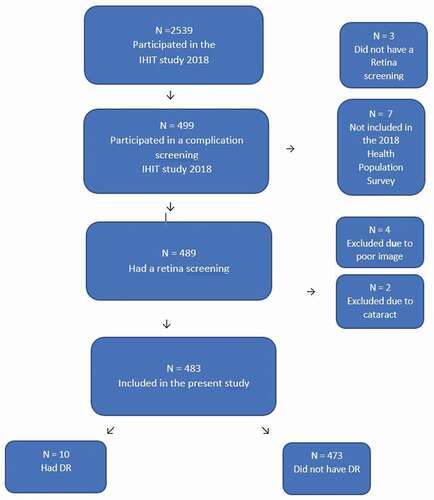
A total of 499 participants with diabetes or prediabetes accepted to participate in the diabetes complication screening. We excluded 3 participants from the study, who did not have a retina screening, due to blindness and difficulties in cooperating for the eye screening. Further, 7 participants with normal glucose tolerance were excluded, as they, by mistake, were included in the diabetes complication screening. Finally, 489 had a retina screening. We excluded 4 participants due to poor image quality and further excluded 2 participants due to cataract.
Thus, our final study population comprised 483 participants. Overview of participants can be seen in .
A total of 10 participants, equivalent to a prevalence at 2% had DR. Only mild or moderate DR was found (ICDR grading of 1 or 2). All baseline characteristics stratified by DR can be seen in . The study comprised 58% women (280/483) and 42% (203/483) men, but the prevalence of DR was almost equally distributed corresponding to a prevalence of 2.1% among women compared to 1.9% among men. Among participants with an HbA1c ≥48 mmol/mol (6.5%) the prevalence of DR was 9% (9/91), compared to a prevalence <1% (1/382) among participants with HbA1c ≤48 mmol/mol (6.5%). All participants with DR lived in towns – none of the participants living in settlements had DR. Finally, the mean Inuit genetic admixture was lower among participants with DR.
Table 1. Baseline characteristics and results stratified by DR
Conclusion
The prevalence of DR is low in Greenland and almost non-existent among participants with HbA1c below the diabetes threshold <48 mmol/mol (6.5%). We found no DR in settlements, although diabetes generally is more prevalent in settlements in Greenland [Citation17]. Finally, participants with DR had a lower level of Inuit genetic admixture indicating a higher proportion of European ancestry. According to a study conducted in Greenland, Greenlandic ethnicity seems to be related to a lower risk of diabetes-related complications [Citation18]. This is the first study to assess the general DR prevalence in a representative sample of the Greenlandic population, based on Health Population Surveys.
The low prevalence of DR can be explained partly by a short diabetes duration as we examined participants with screen-detected diabetes and prediabetes. However, our results corroborate findings from a previous register-based study conducted in the capital Nuuk where the prevalence of DR was 7% among ethnic Greenlanders compared with 21.4% among non-Greenlanders, suggesting that Greenlanders may have a different risk of developing DR than non-Greenlanders [Citation19].
Another study found that diabetes was not associated with the expected risk of macrovascular complications, probably due to the unique genetic characteristics of this population with the
TBC1D4 variant and other yet undiscovered variants [Citation20]. The genetic contribution to diabetes in Greenland is so potent that the condition is different from diabetes that we know from Europe and other North American white populations. The diabetes type associated with the TBC1D4 variant underlines the importance of not uncritically inferring disease associations, treatments or screening intervals from one population to another.
In 2008, a national diabetes programme was initiated to improve detection of undiagnosed diabetes and to improve the care for patients diagnosed with diabetes in Greenland. In relation to DR, Optos wide-field cameras were installed in nine towns replacing the former retinal fundus camera in 2015, and making screening available in more locations [Citation18]. This type of equipment does not require specialist staff and provides non-mydriatic diagnosis. This setup, in combination with a systematic approach to monitoring quality of diabetes care, is particularly suitable in sparsely populated areas like the remote villages and small towns in Greenland. In conclusion, we found a low prevalence of DR in Greenland. However, continued monitoring of DR is needed, and the effects of genetic diabetes variants are still warranted.
Disclosure of potential conflicts of interest
No potential conflict of interest was reported by the author(s).
Acknowledgement
We are grateful to OPTOS Scotland for the opportunity to borrow the Daytona OPTOS camera for the examinations conducted in the study.
Additional information
Funding
References
- Jørgensen ME, Bjeregaard P, Borch-Johnsen K. Diabetes and impaired glucose tolerance among the inuit population of Greenland. Diabetes Care. 2002 Oct;25(10):1766–5. . Erratum in: Diabetes Care. 2002 Dec;25(12):2372.PMID: 12351475
- Viskum ES, Pedersen ML. Prevalence of diagnosed diabetes and quality of care among Greenlanders and non-Greenlanders in Greenland. Diabetes Res Clin Pract. 2016 Nov;121:91–98. Epub 2016 Sep 21. PMID: 27690318.
- Jeppesen C, Bjerregaard P, Jørgensen ME. Dietary patterns in Greenland and their relationship with type 2 diabetes mellitus and glucose intolerance. Public Health Nutr. 2014 Feb;17(2):462–470. . Epub 2013 Feb 11. PMID: 23399043
- Moltke I, Fumagalli M, Korneliussen TS, et al. Uncovering the genetic history of the present-day Greenlandic population. Am J Hum Genet. 2015 Jan 8;96(1):54–69. . Epub 2014 Dec 31. PMID: 25557782; PMCID: PMC4289681.
- Moltke I, Grarup N. A common Greenlandic TBC1D4 variant confers muscle insulin resistance and type 2 diabetes. Nature. 2014 Aug 14;512(7513):190–193. .
- Fowler MJ. Microvascular and macrovascular complications of diabetes. Clin Diabetes. 2008 Apr;26(2):77–82. .
- Stitt A, Lois N, Medina R, et al. Advances in our understanding of diabetic retinopathy. Clin sci. 2013;125:1–17.
- Behnam- Rassouli M, Ghayour MB, Ghayour N. Microvascular complications of diabetes. J Biol Sci. 2010;10:411–423. .
- Niclasen B, Mulvad G. Health care and health care delivery in Greenland. Int J Circumpolar Health. 2010;69:437–447.
- Bjerregaard P, 2019. In.: SIF, Befolkningsundersøgelsen i Grønland 2018. Levevilkår, livsstil og helbred. Oversigt over indikatorer for folkesundheden
- Fleischer J, Yderstraede K, Gulichsen E, et al. Cardiovascular autonomic neuropathy is associated with macrovascular risk factors in type 2 diabetes: new technology used for routine large-scale screening adds new insight. J Diabetes Sci Technol. 2014;8(4):874–880.
- Boulton A. Management of diabetic peripheral neuropathy. Clin Diabetes. 2005;23:9–15.
- Brown DM. Advancing the detection and management of diabetic retinopathy with ultra-widefield retinal imaging. US Ophthal Rev. 2017;10:23. .
- Optos. Daytona, Brochure. Scotland. Scotland: Optos; 2018.
- American association of ophthalmology. International clinical diabetes retinopathy disease severity scale. Am Acad Ophalmol. 2002.
- Available from: https://www.sdu.dk/da/sif/rapporter/2020/sharing_circle_dk
- Jørgensen ME, Borch‐Johnsen K, Witte DR, et al. Diabetes in Greenland and its relationship with urbanization. Diabetic Med. 2012;29:755–760.
- Pedersen ML. Diabetes care in the dispersed population of Greenland. A new model based on continued monitoring, analysis and adjustment of initiatives taken. Int J Circumpolar Health. 2019;78(sup1):1709257. . PMID: 31996108; PMCID: PMC7034430
- Pedersen M. Microvascular complications in Nuuk, Greenland, among Greenlanders and non-Greenlanders diagnosed with type 2 diabetes. Diabetes Res Clin Pract. 2017;136:1–6.
- Overvad M, Diaz LJ, Bjerregaard P, et al. The effect of diabetes and the common diabetogenic TBC1D4 p.Arg684Ter variant on cardiovascular risk in Inuit in Greenland. Sci Rep. 2020 Dec 16;10(1):22081. .