ABSTRACT
Like other Indigenous Circumpolar populations, Alaska Native (AN) people experience different patterns of cancer than their non-Indigenous counterparts. Every 5 years, the Alaska Native Tumour Registry releases a comprehensive report on cancer among AN people; this study provides 50 years of cancer surveillance data. Five-year annual-average age-adjusted incidence rates were calculated for time-periods ranging 1969–2018. AN data were compared with data for US whites (SEER 9). Mortality rates were calculated for 1994–2018 using data from the National Center for Health Statistics. During 2014–2018, there were 2,401 cases of invasive cancer among AN people. Among these, the most commonly diagnosed cancers were colorectal (405 cases, 17% of all cancers), lung and bronchus (373 cases, 16% of all cancers), and female breast (340 cases, 14% of all cancers). Lung cancer was the leading cause of cancer death, followed by colorectal and female breast cancers. These leading cancers are screenable, and preventable through lifestyle modifications including tobacco cessation, healthy eating and engaging in physical activity. These data provide important information to support cancer prevention and control among AN people. Cancer surveillance has been a valuable tool throughout the Circumpolar North to support reducing the burden of cancer among Indigenous populations.
Abbreviations: ANAI: Alaska Native/American Indian; AN: Alaska Native; USW: U.S. White(s); ANMC: Alaska Native Medical Center; ANTR: Alaska Native Tumour Registry; IR: Incidence Rate; CI: Confidence Interval; RR: Rate Ratio; ICD-O-3: International Classification of Diseases for Oncology – Third Edition; SEER: Surveillance, Epidemiology and End Results.
Introduction
Indigenous people throughout the Circumpolar North have a unique pattern of cancer incidence and mortality [Citation1–4]. The Alaska Native Tumour Registry (ANTR) was established for cancer surveillance among Alaska Native and American Indian (ANAI) people living in Alaska so that those patterns could be better understood; data are available going back to 1969 [Citation5,Citation6]. The ANTR is one of very few registries, in the USA or in the Circumpolar region, with such a long history of data collection; thus, it provides a unique resource to support disease monitoring [Citation6–11], public health programme evaluation [Citation12], and research [Citation13–16] to understand and address the burden of cancer – the leading cause of death – among Alaska Native (AN) people. For example, data published by the registry indicated that colorectal cancer (CRC) rates were twice as high among AN people relative to US white people (USW), and that this disparity had existed for over 30 years [Citation17]. Furthermore, CRC rates among younger people (aged <50 years) were shown to be significantly higher among AN people compared to USW [Citation7]. In response to these findings, researchers in the Alaska Tribal Health System initiated public health programmes and research studies to investigate both primary and secondary prevention of CRC [Citation18–21]. In addition, in 2013, the Alaska Native Medical Center (ANMC) changed its guidelines to recommend screening starting at age 40 years, 10 years earlier than the national recommendation to begin screening at 50 years. Recently, we used ANTR data to show how these programmes had impacted incidence and mortality rates among AN people over the last 15 years, with incidence decreases among those of screenable age, but a surprising increase in mortality over the time-period [Citation22]. These examples highlight the power of high-quality, long-term cancer surveillance data to support and evaluate the impact of cancer prevention and control efforts.
Every 5 years, the ANTR releases a report with the most recent cancer data for AN people [Citation5,Citation6,Citation17]. In 2021, the ANTR celebrated 50 years of cancer surveillance among AN people [Citation23], a notable milestone. This manuscript provides a summary of the 50-year report data with a focus on the most recent 5-year period. Here, we describe the most salient results from those analyses, including a brief description of the leading cancers among AN people, cancer mortality and long-term cancer trends. We hope that this manuscript will provide useful data for those interested in cancer epidemiology among Indigenous peoples of Alaska specifically, and the Circumpolar region more broadly.
Methods
Study population
An estimated 144,274 ANAI people resided in Alaska in 2015 (individuals reporting ANAI identity alone, or in combination with another racial identity), comprising 19.5% of the Alaskan population [Citation24]. Almost 90% of ANAI people living in Alaska identify as AN [Citation25]; therefore, hereafter we will refer to ANAI people residing in Alaska as “Alaska Native (AN) people”. Healthcare for AN people residing in Alaska is provided by over 30 regional Tribal health organisations, and the Alaska Native Tribal Health Consortium, which provides statewide speciality care services [Citation26].
Ethical review
This study did not require IRB review because the ANTR collects data for the purposes of public health surveillance and these data are available publicly through the National Cancer Institute’s Surveillance, Epidemiology and End Results (NCI SEER) Program [Citation27]. The authors received Tribal approval for publication of this manuscript from the Alaska Native Tribal Health Consortium.
Data sources
Cancer data were collected by the ANTR a population-based central cancer registry that records information on AN people who meet eligibility requirements for Indian Health Service benefits, have been diagnosed with cancer in Alaska since 1969, and resided in Alaska at the time of diagnosis. The ANTR has been collecting cancer information according to NCI SEER Program standards since its inception in 1969, and has been a full member special population registry of the SEER Program since 1999.
Cases were ascertained following standard ANTR casefinding procedure from a variety of sources, including (1) hospital discharge diagnoses for Tribal and non-Tribal health facilities in Alaska; (2) tumour registry and pathology files of the ANMC and other in-state healthcare facilities; (3) linkage to the Alaska Cancer Registry and the Washington State Cancer Registry, which assures all cases of cancer diagnosed among AN people in Alaska are captured irrespective of where they receive their care; and (4) death certificates (<1% cases were registered solely on the basis of information from a death certificate).
This report describes information on cancers diagnosed among AN people from 1969 through 2018. Cancer incidence and mortality data for the USW population are included in this report to provide a standard point of reference for comparison. Cancer data for USW were taken from the SEER 9 data set, which includes the following registries: Iowa, New Mexico, Connecticut, Detroit, San Francisco, Hawaii, Seattle-Puget Sound and Utah. At the time of analysis, data were available for USW for only the years 1973–2017. Therefore, in 5-year rate comparisons, data for USW in the most recent time-period include only the 4 years (2014–2017). For longer term trends, data for USW include the years 1975–2017, to align with 5-year date ranges based on ANTR data. Population estimates for both the AN and USW populations originate from the US Census (1970, 1980, 1990, 2000 and 2010), as well as from the National Center for Health Statistics’ bridged population series for AN people, 1990–2018, available from the SEER Program through SEER*Stat [Citation28]. Mortality data, also accessed through SEER*Stat, were provided by linkage to the National Death Index Plus, which is maintained by the National Center for Health Statistics; mortality data were available for the years 1990–2018; data from 1994–2018 were included in this report to align with 5-year date ranges based on ANTR data.
Primary cancer site of origin, pathology, behaviour, and grade coding followed the International Classification of Diseases for Oncology, 3rd edition [Citation29]. Cancer sites of origin were grouped according to SEER primary site groups, ICD-O-3/WHO 2008 recode [Citation30]. Directly corresponding stage variables were not available for AN and USW data sets. While the available stage variables use the same classifications for stage at diagnosis (local, regional, distant, unknown, etc.) the specific rules used to make those classifications vary slightly. For AN data several stage classifications were available; we used a combination of two classifications that were most complete and covered the desired date range: “Derived SS2000“ and “Derived Summary Stage 2018“. For USW data we chose to use ”SEER Combined Summary Stage (2004+)” because it was largely compatible with ”Derived SS2000.”
Statistical analysis
Age-adjusted incidence rates (IR), rate ratios (RR), and confidence intervals were computed using R version 3.6.3 (2020–02-29) and cross-checked against output from SEER*Stat version 8.3.8 (Statistical Methodology and Applications Branch, Surveillance Research Program, National Cancer Institute). Age-adjusted mortality rates (MR) were calculated directly using SEER*Stat version 8.3.8. All rates for AN people and USW were age-adjusted to the U.S. Census 2000 standard population using the direct method. RRs are expressed as the AN rate divided by the USW rate. Age-specific statistics were calculated in the following age groups: 0–39 years, 40–59 years, 60–79 years and 80+ years. Trends were assessed by calculating rates for 5-year time-periods 1969–2018 (AN people) and 1975–2017 (USW people); annual percent change (APC) and significance of trends were analysed using Joinpoint version 4.9.0.0 (Statistical Methodology and Applications Branch, Surveillance Research Program, National Cancer Institute). IRs and case counts are not given where cell sizes were <5 to protect individuals’ privacy.
Results
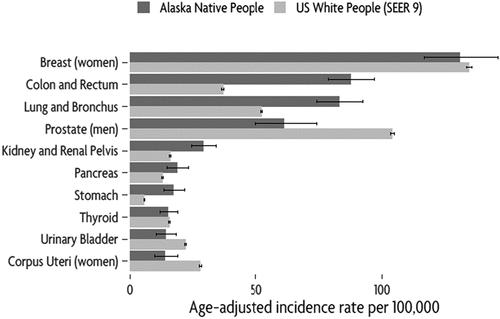
During 2014–2018, there were 2,401 cases of invasive cancer diagnosed among AN people, which was an increase compared with the 2,123 diagnosed during 2009–2013. Age-adjusted incidence of leading cancers is shown in ; rates for all cancer sites are given in Supplementary. During 2014–2018, the most commonly diagnosed cancers were female breast (340 cases, 14% of all cancers; IR 130.8 [116.7, 146.0]), colorectal (405 cases, 17% of all cancers, IR 87.6 [78.8, 97.1]), lung and bronchus (373 cases, 16% of all cancers, IR 83.0 [74.3, 92.4]), prostate (126 cases, 5% of all cancers, IR 61.2 [49.9, 74.2]), and kidney and renal pelvis (150 cases, 6% of all cancers, IR 28.9 [24.3, 34.1]). This pattern was identical to both the previous 5-year period, and the 50-year period 1969–2018. As seen in , rates of colorectal, lung and stomach cancers were higher among AN people than USW; rates of prostate (men) and corpus uteri cancers (women) were lower among AN people than USW.
Table 1. Leading causes of cancer death among AN people, 2014–2018. Data from the Alaska Native Tumour Registry (AN people) and SEER 9 registries (USW)
Figure 1. Five-year incidence per 100,000 population for the leading cancers diagnosed among AN people, 2014–2018, compared to USW
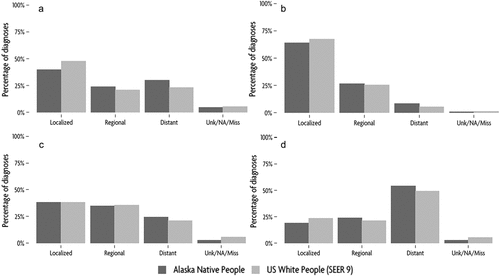
We examined stage at diagnosis for all sites and the most common cancers diagnosed 2014–2018 (). The majority of cancers (40%) were diagnosed at local stage, with 24% diagnosed at regional stage, and 30% at distant stage. However, the pattern varied by cancer site. For female breast cancer (), almost two thirds of cases were diagnosed at local stage, with a quarter at regional stage and one tenth at distant stage. Among colorectal cancers, just over one third were diagnosed at local and regional stages each, with one quarter diagnosed at distant stage (). Over half of lung cancers were diagnosed at distant stage (). For those sites not presented in : among pancreatic cancers, 54% were diagnosed at distant stage, with 24% at regional stage and 14% local stage. Among stomach cancers, 60% were diagnosed at distant stage, with 16% at regional stage and 22% local stage.
Figure 2. Frequency of diagnosis by SEER summary stage for (a) All sites, (b) female breast, (c) colorectal, (d) lung cancers, among AN people compared to USW, 2014–2018
Overall, the incidence of cancer (all sites) was similar among AN people and USW during the last 5 years (). Several leading cancers were more common among AN people than among USW, including colorectal; lung and bronchus, and kidney and renal pelvis cancers. We also observed differences in other, less common cancers. The greatest difference we observed was for cancers of the nasopharynx. While these cancers are diagnosed relatively infrequently (there were 28 cases diagnosed 2014–2018), the IR was 15.2 times higher among AN people compared with USW. Other cancers that were more common among AN people include penis; Kaposi sarcoma; stomach; oesophageal; gallbladder; and cervix uteri. There were also several cancers that were less common among AN people compared with USW, including cancers of the prostate; urinary bladder; eye and orbit; brain; Hodgkin lymphoma; non-Hodgkin lymphoma; and melanoma of the skin.
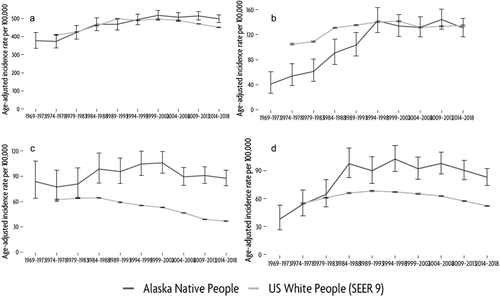
shows long-term (50-year) trends in the leading cancer sites, comparing AN people with USW. Age-adjusted IRs for all cancers among AN people increased slightly from the 1970s to the late 1990s (; APC: 1.08, statistically significant). Since that time, rates have remained fairly stable (APC: −0.27, not significant) although there have been some random variations – as is expected, especially among small populations. Trends varied by cancer site. Among AN women, we observed increasing breast cancer rates during the 1970s and 1980s (; APC: 4.98, statistically significant); at this time, AN rates were much lower than USW rates. However, since the mid 1990s rates have remained generally stable (APC: −0.12, not significant), and similar to those seen among USW women. CRC rates (AN men and women combined; ) have remained relatively constant over the 50 years of surveillance (no significant Joinpoints). In contrast, USW have seen declines in their CRC rates, resulting in a widened disparity between AN and USW populations. Finally, lung cancer rates () increased consistently between the 1970s and the late 1990s (APC: 6.72, significant), at which time they stabilised (APC: −0.41, not significant), potentially trending towards a decline in the most recent 5-year period.
Figure 3. Trends in5-year average annual age-adjusted incidence for (a) All sites, (b) female breast, (c) colorectal, (d) lung cancers, among AN people compared to USW, 1969–2018
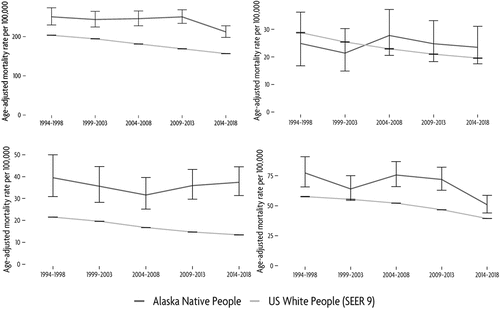
presents the leading causes of cancer death during 2014–2018. Lung cancer remained the leading cause of cancer death, followed by cancers of the colon and rectum, and female breast cancers. Comparing mortality rates among AN people to those among USW, we observed several differences. Among our leading cancers, lung cancer mortality was 1.3 times higher among AN people, and colorectal cancer 2.8 times higher among AN people. Female breast cancer mortality rates were not significantly different between AN people and USW. There were several less common cancers for which mortality was higher among AN people than USW. These included cancers of the stomach, and oesophagus. Because numbers of cancer deaths are very small for certain cancer sites, we also examined 25-year cancer mortality rates, which enabled us to generate rates for additional less common cancer sites (Supplementary Table 2). During this time-period, mortality rates were higher among AN people than USW for the following additional cancer sites: pancreatic, gallbladder, intrahepatic bile duct and liver. Mortality rates were lower among AN people for the following additional cancer sites: melanoma of the skin, brain and other nervous system, non-melanoma skin, acute myeloid leukaemia, uterus and ovary.
shows longer-term mortality data for the leading cancers; there was not sufficient data for Joinpoint to locate inflection points in the mortality data. Overall, there was a decrease in cancer death between the current and previous 5-year periods. We observed a 30% decrease in lung cancer mortality between the previous and current 5-year periods. Colorectal and female breast cancer mortality did not change, remaining relatively steady for both cancer sites for the 25 years of surveillance.
Discussion
The ANTR collects cancer surveillance information to support cancer control programming, monitoring, programme evaluation and research among AN people. The present manuscript provides information on 50-year cancer incidence trends among AN people, as well as more detailed information from the most recent 5-year period. Our key findings were thus: leading cancers 2014–2018 were female breast, colorectal and lung cancers. Leading causes of cancer death were lung cancer, colorectal cancers and female breast cancer. These leading cancers, and leading causes of cancer deaths, are unchanged from the prior 5-year period [Citation17].
The leading cancers among AN people may be potentially preventable. Female breast, colorectal and lung cancers are all screenable within the Alaska Tribal Health System; increased efforts should be made to support all AN men and women of appropriate ages to receive screening. In a recent study by our group, we showed that increases in CRC screening prevalence occurred concurrently with increases in CRC mortality [Citation22]. Reasons for this should be elucidated in further research, but these data indicate a potential disconnect between CRC screening, treatment and outcomes. We showed that a large proportion of lung cancers were diagnosed at distant stage among AN people, likely contributing to the high mortality from this malignancy. Lung cancer screening became available within the Alaska Tribal Health System for those who meet eligibility criteria per Centers for Medicare and Medicaid Services guidelines in 2019; we hope to see a decrease in mortality and late-stage diagnoses as screening prevalence increases over time [Citation31]. Furthermore, risk of these leading cancers has been linked to lifestyle and health-related behaviours. Smoking has been comprehensively linked to increased risk of lung and colorectal cancers [Citation32], and physical activity [Citation33,Citation34] and dietary components [Citation35] have been linked to decreased risk of all three leading cancers. Smoking prevalence is twofold higher among AN people than USW [Citation36–38]; further, among some, particularly rural AN communities there may be lower access to healthy foods and opportunities to physical activity [Citation39–43]. Thus, a comprehensive approach to reduce the burden of cancer among AN people should incorporate both primary and secondary prevention approaches.
Where data were available, we compared average annual age-adjusted cancer IRs among AN people to those among USW for the same time-periods. Such comparisons help us to describe similarities and differences in cancer patterns, which in turn can help identify areas of strength, and opportunities for improvement. There were several disparities of note. The largest disparity was observed for nasopharyngeal cancer, which is relatively rare. Although we observed only 28 cases in the 5-year period 2014–2018, AN incidence was over 15 times greater than that among USW, and mortality 29 times greater. Reasons for this disparity are unknown; previous research has indicated a potential familial link, a role for Epstein-Barr virus, or perhaps both [Citation44–47]. Stomach cancer incidence was 3 times greater, and mortality 4.5 times greater among AN people than USW. Stomach cancer rates are known to be high across ANAI people nationwide: a recent study by Melkonian and colleagues reported high rates in several Indian Health Service regions, particularly Alaska, the Southwest, and Northern Plains regions [Citation48]. Prevalence of Helicobacter pylori infection is high in Alaska [Citation49], and antimicrobial resistance and treatment failure have also been documented [Citation49–53]. Prevalence of other known risk factors including smoking, dental disease, and consumption of salted and smoked foods is high [Citation54], and may contribute to the increased burden of this malignancy. A recent community-researcher summit discussed these issues and provided recommendations for strategies that may move towards a lower burden of this malignancy among AN people, including both research and clinical programmes [Citation55]. In the present study, we also identified colorectal cancer as a leading disparity, with AN people experiencing among the highest recorded rates in the world [Citation56]. The Alaska Native Tribal Health Consortium and its Tribal health partners statewide are engaged in a breadth of research and programming to inform both primary prevention [Citation19,Citation54], and increased screening [Citation20,Citation54,Citation57,Citation58] among AN people.
While we did not directly compare rates among AN people to those among other Indigenous Circumpolar populations in this report, we can place our findings in the context of previous reports focused on the region. For example, we reported lung and colorectal cancers as the leading causes of cancer death among AN people. In 2016, Young and colleagues identified colorectal and lung cancers as of increasing public health concern across Arctic Indigenous peoples, showing that incidence was higher than the GLOBOCAN average for Arctic Indigenous peoples spanning Alaska, Northwest Territories, Nunavut and Greenland [Citation4]. Herein, we also describe stomach cancer disparities among AN people. A recent study by Simkin and colleagues demonstrated that, despite some recent declines in stomach cancer incidence across several Circumpolar Indigenous populations, many regions continued to experience high rates and large disparities [Citation2]. A recent examination of health indicators (health status, health determinants, and health care) across Alaska, Greenland and the northern regions of Canada, Russia and the Nordic countries showed that Indigenous peoples generally fared worse than their non-Indigenous counterparts [Citation59], which may contribute to these cancer disparities. However, the authors also noted the lack of consistent health status data with the exception of for Alaska, and called for increased and ongoing health monitoring across the region. Our data, as well as other studies from the ANTR that combine registry data with information on health status and behaviours [Citation22,Citation60], indicate how powerful health surveillance data can be in monitoring and programme evaluation among AN people, and may provide an example for other Arctic nations and Indigenous peoples interested in improving the health of their communities.
Yet, while comparisons to other populations can be helpful, it is also very informative to focus only on AN people, for example examining how rates vary over time. Examining trends in cancer incidence can show us whether, and how, we are making progress; where we are being successful in our efforts to decrease cancer burden among AN people; and where we still have room to improve. Having 50 years of surveillance data is uncommon, and provides a rare opportunity to examine very long-term trends among AN people. In the present manuscript, we focused on trends among all cancer sites, as well as the three leading cancers of focus. We observed increases in female breast cancer incidence among AN women during the 1970s and 1980s, during which time rates increased from being substantially lower than that observed among USW, to almost identical; more detailed information on this malignancy was reviewed in a recent paper on this topic by our group [Citation61]. Colorectal cancer rates have remained steadily high for the entire period of surveillance, despite increases in colorectal cancer screening [Citation22]. Of note, rates among USW have declined slowly but steadily over the last decades but we have not seen these same declines among AN people; this leads to increasing disparity. Efforts to address this issue are described above. National and global lung cancer trends have shown strong correlations with smoking prevalence [Citation62], as smoking can be linked to 85% of lung cancer cases [Citation63]. We observed slight declines in lung cancer incidence over time among AN people. Data from the Alaska Behavioural Risk Factor Surveillance System indicate that smoking prevalence decreased among AN people from 44% during 1991–1993 to 36% during 2015–2017 [Citation38]. Together, these data indicate continuing need for further work in research, programming and policy towards cancer prevention and control within the Alaska Tribal Health System.
This study had several strengths and limitations that warrant consideration. A key strength of this work is the long history of data collected by the ANTR about and for AN people. This provides a rare opportunity to examine long-term cancer trends among AN people. The ANTR has followed SEER’s data collection protocols since its inception; therefore, it represents a high-quality source of cancer data. A key limitation is that because the AN population is small, case counts and numbers of deaths even from the leading cancers were small. To maximise the number of cases for each cancer site, we aggregated data over several years. However, there were still many cancer sites for which we were unable to present data due to low case counts. Regardless, the small number of cases do not diminish the importance of such research [Citation64], as providing population-specific data is critical.
This study presents the most up-to-date information on cancer among AN people, and celebrates 50 years of cancer surveillance among AN people. These data are unique across the Circumpolar North, and provide important information for clinicians, tribal health leaders, and public health practitioners to support cancer prevention and control among AN people. Monitoring cancer rates and trends is an essential tool for understanding the health status of Arctic Indigenous populations, identifying potential areas for preventive programmes and policies, and for gaining insight into the efficacy of policy changes or public health campaigns. The ANTR’s regular publication of these data every 5 years allows for continued benchmarking of progress towards reducing the burden of cancer among AN people.
Supplemental Material
Download Zip (41 KB)Disclosure statement
No potential conflict of interest was reported by the author(s).
Supplementary material
Supplemental data for this article can be accessed here.
Additional information
Funding
References
- Kelly JJ, Lanier AP, Schade T, et al. Cancer disparities among Alaska native people, 1970-2011. Prev Chronic Dis. 2014;11:E221.
- Simkin J, Nash SH, Barchuk A, et al. Stomach Cancer incidence and mortality trends among circumpolar nations. Cancer Epidemiol Prev Biomarkers. 2021;30(5):845–10.
- Simkin J, Woods R, Elliott C. Cancer mortality in Yukon 1999-2013: elevated mortality rates and a unique cancer profile. Int J Circumpolar Health. 2017;76(1):1324231.
- Young TK, Kelly JJ, Friborg J, et al. Cancer among circumpolar populations: an emerging public health concern. Int J Circumpolar Health. 2016;75(1):29787.
- Lanier AP, Kelly JJ, Holck P, et al. Cancer incidence in Alaska Natives thirty-year report 1969-1998. Alaska Med. 2001 Oct-Dec;43(4):87–115.
- Lanier AP, Kelly JJ, Maxwell J, et al. Cancer in Alaska Native people, 1969-2003. Alaska Med. 2006 Jul-Sep;48(2):30–59.
- Kelly JJ, Alberts SR, Sacco F, et al. Colorectal cancer in Alaska native people, 2005-2009. Gastrointest Cancer Res. 2012 Sep;5(5):149–154.
- Kelly JJ, Day GE, Lanier AP, et al. Women’s cancers among Alaska Natives: [corrected] 1969-2003. Alaska Med. 2007 Apr-Jun;49(2):55–61.
- Lanier AP, Kelly JJ, Smith B, et al. Alaska Native cancer update: incidence rates 1989-1993. Cancer Epidemiol Biomarkers Prev. 1996 Sep;5(9):749–751.
- Alberts SR, Kelly JJ, Ashokkumar R, et al. Occurrence of pancreatic, biliary tract, and gallbladder cancers in Alaska Native people, 1973-2007. Int J Circumpolar Health. 2012;71(1):17521.
- Alberts SR, Kelly JJ, Lanier AP, et al. Occurrence of esophageal and gastric cancer in Alaska Natives, 1969-2003. Alaska Med. 2006 Apr-Jun;48(1):2–11.
- Lanier AP, Kelly JJ, Holck P. Pap prevalence and cervical cancer prevention among Alaska Native women. Health Care Women Int. 1999 Sep-Oct;20(5):471–486.
- Boardman LA, Lanier AP, French AJ, et al. Frequency of defective DNA mismatch repair in colorectal cancer among the Alaska Native people. Cancer Epidemiol Biomarkers Prev. 2007 Nov;16(11):2344–2350.
- Slattery ML, Schumacher MC, Lanier AP, et al. A prospective cohort of American Indian and Alaska Native people: study design, methods, and implementation. Am J Epidemiol. 2007 Sep 01;166(5):606–615.
- Kelly JJ, Unger ER, Dunne EF, et al. HPV genotypes detected in cervical cancers from Alaska Native women, 1980-2007. Int J Circumpolar Health. 2013;72(1):21115.
- Nash SH, Hiratsuka V, Day G, et al. Cancer among participants of the Alaska EARTH study: prevalence, incidence, and associations with known risk factors. In review.
- Carmack A, Schade TL, Sallison I, et al. Cancer in Alaska Native People: 1969-2013, The 45 Year Report (Anchorage, AK: Alaska Native Tribal Health Consortium). 2015.
- Nash SH, Greenley R, Dietz-Chavez D, et al. Incorporating Participant and Clinical Feedback into a Community-Based Participatory Research Study of Colorectal Cancer Among Alaska Native People. J Community Health. 2020;45(1):1–9.
- Nash SH, Peters U, Redwood D. Developing an Epidemiologic Study to Investigate Risk Factors for Colorectal Cancer Among Alaska Native People. J Public Health Manag Pract. 2019 Apr 5;25(5):S54–S60.
- Redwood DG, Asay ED, Blake ID, et al. Stool DNA Testing for Screening Detection of Colorectal Neoplasia in Alaska Native People. Mayo Clin Proc. 2016 Jan;91(1):61–70.
- Redwood DG, Blake ID, Provost EM, et al. Alaska Native Patient and provider perspectives on the multitarget Stool DNA test compared with colonoscopy for colorectal cancer screening. J Prim Care Community Health. 2019Jan-Dec;10:2150132719884295.
- Nash SH, Britton C, Redwood D. Characteristics of colorectal cancers among Alaska Native people before and after implementing programs to promote screening. J Cancer Policy. 2021;29:100293.
- Zimpelman G, Miller KN, Carlo DD, et al. Cancer in Alaska Native people: the 50 year report (Anchorage, Alaska: Alaska Native Tribal Health Consortium). 2021.
- Alaska Department of Labor and Workforce Development Alaska Population Overview: 2013 Estimates. 2015. Anchorage, AK: State of Alaska.
- Bureau USC. 2010. Census summary file 1. cited 2017 March 23. https://factfinder/census.gov
- Sherry P. Health care delivery for Alaska Natives: a brief overview. Int J Circumpolar Health. 2004;63(sup2):54–62.
- McLaughlin RH, Gomez SL, Deapen D, et al. Human subjects protection and cancer surveillance research: revised regulations, expanded opportunities. Cancer Res. 2017 Jun 15;77(12):3140–3143.
- Surveillance E, and End Results (SEER) Program. Data from: SEER*Stat Database: incidence - SEER 9 regs research data, Nov 2020 Sub (1973-2018) <Katrina/Rita population adjustment> - Linked To County Attributes - Total U.S., 1969-2018 Counties. Released April 2021, based on the November 2020 submission. https://seer.cancer.gov/data/citation.html
- World Health O. ICD-10: international statistical classification of diseases and related health problems: tenth revision. 2nd ed. Geneva: World Health Organization; 2004.
- National Cancer Institute SRP. Site Recode ICD-O-3/WHO 2008 definition. cited 2021 November 18. https://seer.cancer.gov/siterecode/icdo3_dwhoheme/index.html
- Hoffman RM, Atallah RP, Struble RD, et al. Lung cancer screening with low-dose CT: a meta-analysis. J Gen Intern Med. 2020 Oct;35(10):3015–3025.
- Health UDo, Services H. The health consequences of smoking—50 years of progress: a report of the Surgeon General. Atlanta, GA: US Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health. 2014; 17
- McTiernan A, Friedenreich CM, Katzmarzyk PT, et al. Physical activity in cancer prevention and survival: a systematic review. Med Sci Sports Exerc. 2019 Jun;51(6):1252–1261.
- Patel AV, Friedenreich CM, Moore SC, et al. American College of Sports medicine roundtable report on physical activity, sedentary behavior, and cancer prevention and control. Med Sci Sports Exerc. 2019 Nov;51(11):2391–2402.
- World Cancer Research Fund and American Institute for Cancer Researc. Diet, nutrition, physical activity and cancer: a global perspective: a summary of the Third Expert Report. World Cancer Research Fund and American Institute for Cancer Research; 2018 dietandcancerreport.org.
- Smith JJ, Ferucci ED, Dillard DA, et al. Tobacco use among Alaska Native people in the EARTH study. Nicotine Tob Res. 2010 Aug;12(8):839–844.
- Redwood D, Lanier AP, Renner C, et al. Differences in cigarette and smokeless tobacco use among American Indian and Alaska Native people living in Alaska and the Southwest USA. Nicotine Tob Res. 2010 Jul;12(7):791–796.
- Center ANE. Adult Current Smoking. Alaska Native tribal health consortium. cited 2018 Apr 26th. <http://anthctoday.org/epicenter/healthData/factsheets/adult_current_smoking_statewide_1_10_2017.pdf>
- Bersamin A, Zidenberg-Cherr S, Stern JS, et al. Nutrient intakes are associated with adherence to a traditional diet among Yup’ik Eskimos living in remote Alaska Native communities: the CANHR Study. Int J Circumpolar Health. 2007 Feb;66(1):62–70.
- Bersamin A, Luick BR, Ruppert E, et al. Diet quality among Yup’ik Eskimos living in rural communities is low: the Center for Alaska Native health research pilot study. J Am Diet Assoc. 2006 Jul;106(7):1055–1063.
- Bersamin A, Luick BR, King IB, et al. Westernizing diets influence fat intake, red blood cell fatty acid composition, and health in remote Alaskan Native communities in the center for Alaska Native health study. J Am Diet Assoc. 2008 Feb;108(2):266–273.
- Redwood DG, Ferucci ED, Schumacher MC, et al. Traditional foods and physical activity patterns and associations with cultural factors in a diverse Alaska Native population. Int J Circumpolar Health. 2008 Sep;67(4):335–348.
- Redwood DG, Day GM, Beans JA, et al. Alaska Native Traditional food and harvesting activity patterns over 10 years of follow-up. Curr Dev Nutr. 2019 Nov;3(11):nzz114.
- Lanier A, Bender T, Talbot M, et al. Nasopharyngeal carcinoma in alaskan eskimos, indians, and aleuts: a review of cases and study of epstein‐barr virus, HLA, and environmental risk factors. Cancer. 1980;46(9):2100–2106.
- Ireland B, Lanier AP, Knutson L, et al. Increased risk of cancer in siblings of Alaskan native patients with nasopharyngeal carcinoma. Int J Epidemiol. 1988 Sep;17(3):509–511.
- Lanier A, Bender T, Talbot M, et al. Epstein-Barr virus D.N.A. in tumour tissue from native Alaskan patients with nasopharyngeal carcinoma. Lancet. 1978 Nov 18;2(8099):1095.
- Lanier AP, Bornkamm GW, Henle W, et al. Association of Epstein-Barr virus with nasopharyngeal carcinoma in Alaskan native patients: serum antibodies and tissue EBNA and DNA. Int J Cancer. 1981 Sep 15;28(3):301–305.
- Melkonian SC, Pete D, Jim MA, et al. Gastric cancer among American Indian and Alaska Native populations in the USA, 2005-2016. Am J Gastroenterol. 2020 Dec;115(12):1989–1997.
- Keck JW, Miernyk KM, Bulkow LR, et al. Helicobacter pylori infection and markers of gastric cancer risk in Alaska Native persons: a retrospective case-control study. Can J Gastroenterol Hepatol. 2014 Jun;28(6):305–310.
- Martinson HA, Mallari D, Richter C, et al. Molecular Classification of Gastric Cancer among Alaska Native People. Cancers (Basel). 2020 Jan 13;12(1):198.
- McMahon BJ, Bruce MG, Hennessy TW, et al. Reinfection after successful eradication of Helicobacter pylori: a 2-year prospective study in Alaska Natives. Aliment Pharmacol Ther. 2006 Apr 15;23(8):1215–1223.
- McMahon BJ, Bruce MG, Koch A, et al. The diagnosis and treatment of Helicobacter pylori infection in Arctic regions with a high prevalence of infection: expert Commentary. Epidemiol Infect. 2016 Jan;144(2):225–233.
- McMahon BJ, Hennessy TW, Bensler JM, et al. The relationship among previous antimicrobial use, antimicrobial resistance, and treatment outcomes for Helicobacter pylori infections. Ann Intern Med. 2003 Sep 16;139(6):463–469.
- Nolen LD, Vindigni SM, Parsonnet J. Combating Gastric Cancer in Alaska Native people: an expert and community symposium. Gastroenterology. 2020 Apr;158(5):1197–1201.
- Nolen LD, Vindigni SM, Parsonnet J, et al. Combating gastric cancer in Alaska native people: an expert and community symposium. Gastroenterology. 2020;158(5):1197–1201.
- Arnold M, Sierra MS, Laversanne M, et al. Global patterns and trends in colorectal cancer incidence and mortality. Gut. 2017 Apr;66(4):683–691.
- Redwood D, Provost E, Asay E, et al. Giant inflatable colon and community knowledge, intention, and social support for colorectal cancer screening. Prev Chronic Dis. 2013;10:E40.
- Redwood D, Provost E, Lopez ED, et al. A process evaluation of the Alaska Native colorectal cancer family outreach program. Health Educ Behav. 2016 Feb;43(1):35–42.
- Young TK, Broderstad AR, Sumarokov YA, et al. Disparities amidst plenty: a health portrait of Indigenous peoples in circumpolar regions. Int J Circumpolar Health. 2020;79(1):1805254.
- Nash SH, Redwood DG. . In: Cancer Health Disparities. ; 2017 2: e1-e15. doi:10.9777/chd.2018.10001
- Nash SH, Zimpelman G, Stillwater B, et al. Invasive breast cancer among Alaska Native women in Alaska. Int J Circumpolar Health. 2019;78(1):1633190.
- Islami F, Torre LA, Jemal A. Global trends of lung cancer mortality and smoking prevalence. Transl Lung Cancer Res. 2015;4(4):327.
- Islami F, Goding Sauer A, Miller KD, et al. Proportion and number of cancer cases and deaths attributable to potentially modifiable risk factors in the USA. CA Cancer J Clin. 2017 68: 31–54 .
- Srinivasan S, Moser RP, Willis G, et al. Small is essential: importance of subpopulation research in cancer control. Am J Public Health. 2015 Jul;105(Suppl 3):S371–3.