ABSTRACT
Non-alcoholic fatty liver disease (NAFLD) is the most common liver disease worldwide due to its close association to the metabolic syndrome of type 2 diabetes mellitus (T2DM), obesity and insulin resistance. However, the prevalence and severity of NAFLD in Greenland remain unexplored. We aimed to estimate the prevalence of liver steatosis and fibrosis among Greenlanders and Danes with T2DM living in Greenland using biochemical surrogate markers. We included 1409 Greenlanders and 182 Danes with T2DM in this register-based cross-sectional study. Greenlanders had higher BMI and plasma lipid levels and lower HbA1c levels compared with Danes (p<0.05). Their median alanine aminotransferase (ALAT) levels were similar. However, more Greenlanders had elevated ALAT levels (20.5% vs. 11.5%, p<0.05). Greenlanders had lower FIB-4 scores than Danes, 0.91 (IQR: 0.66–1.27) vs. 0.97 (IQR: 0.78–1.34), without difference in FIB-4 score categories (p=0.27). The prevalence of advanced fibrosis was low in both populations (1.7–2.6%). In conclusion, Greenlanders with T2DM had better glycaemic control despite higher BMI and plasma lipids. A larger proportion of Greenlanders had elevated plasma ALAT levels, while FIB-4 scores were lower than Danes. These findings suggest that Greenlanders with T2DM may be less likely to develop liver complications than Danes with T2DM in Greenland.
Introduction
Non-alcoholic fatty liver disease (NAFLD) is one of the most common liver diseases in the world [Citation1]. NAFLD is closely related to obesity, type 2 diabetes mellitus (T2DM) and insulin resistance and may be considered the hepatic manifestation of the metabolic syndrome [Citation2,Citation3].
An essential immunomodulatory event driving NAFLD disease progression is hepatic lipid accumulation (steatosis). If the hepatic metabolic capacity is surpassed, toxic lipid intermediates induce hepatic inflammation, referred to as steatohepatitis, which eventually promotes liver fibrogenesis [Citation4]. Continued fibrogenesis may ultimately lead to manifest cirrhosis, with approximately 20% of patients with NAFLD as rapid progressors [Citation5].
The population in Greenland consists primarily of Inuit (approximately 90%) with Danes as the second largest population of approximately 10% [Citation6]. For the indigenous Arctic populations, data on NAFLD prevalence are sparse and non-existent for the Greenland Inuit population [Citation7]. However, the BMI among the indigenous Greenlanders has increased during the past half century [Citation8]. Moreover, although T2DM used to be a rare condition in the Greenland Inuit population, the prevalence now follows the global trend, with an increased pace that may concern healthcare professionals and challenge the healthcare system [Citation9,Citation10].
This growing prevalence of obesity and T2DM [Citation11] demands the burden of related chronic diseases, including NAFLD, to be elucidated among the Greenlandic population. Recently, it was observed that microalbuminuria and especially retinopathy are less common among Greenlanders compared with non-Greenlanders with T2DM [Citation12]. A genetic mutation in the TBC1D1 gene is present in 17% of Greenlanders and known to cause insulin resistance but without the usual association to known diabetes risk factors [Citation10]. Thus, the frequency of diabetic complications may, to some extent, be explained by genetic variants, but whether other diabetic complications and diabetes-related diseases, including NAFLD, also occur less frequently among Greenlanders remains to be investigated.
Due to large travel distances for basic healthcare services, a characterisation of the prevalence of liver disease in T2DM patients in Greenland may have a large impact on patient monitoring and early intervention.
We aimed to estimate the prevalence of liver steatosis and fibrosis through the use of biochemical surrogate markers among all patients diagnosed with T2DM living in Greenland and to analyse the association between liver disease and relevant characteristics of the metabolic syndrome. We hypothesised that Greenlanders with T2DM are less likely to develop liver steatosis and fibrosis than Danes with T2DM.
Materials and methods
We performed a register-based cross-sectional study on anthropometric, biochemical, and pharmacological data obtained from the electronic medical records (EMRs) in Greenland. The data were extracted on 8 April 2021 after approval by the Ethics Committee in Greenland (KVUG 2020–22) and the Agency for Health and Prevention in Greenland.
Study population
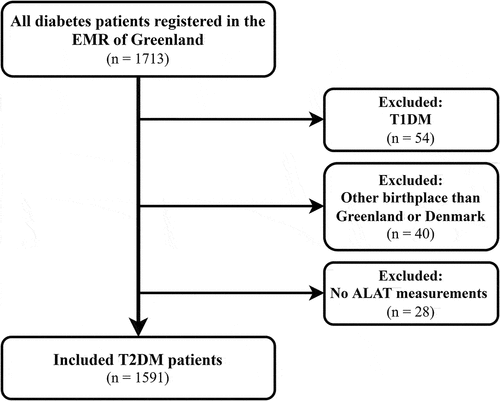
A total of 1713 patients diagnosed with diabetes mellitus and with permanent residence in Greenland were registered in the EMR. We excluded 54 patients (3.2%) with type 1 diabetes mellitus (T1DM), 40 patients (2.3%) with T2DM who were not born in Greenland or Denmark, and 28 patients (1.6%) without plasma alanine aminotransferase (ALAT) measurements. A diagnosis of T1DM and T2DM was determined in the EMR through diagnosis codes in accordance with the International Classification of Primary Care, 2nd edition [Citation13]. Consequently, 1591 patients with T2DM born in Greenland or Denmark were included in the study ().
Variables and analysis
From the EMR, data on birthplace, current residence in Greenland, age, sex, height, weight, systolic blood pressure, diastolic blood pressure, glycated haemoglobin (HbA1c), liver and lipid biochemistry, hepatitis B and C virus test results and anti-lipidemic, anti-hypertensive and diabetes treatments were extracted. All anthropometric and biochemical tests were performed between 5 January 2016 and 30 March 2021. Ethnicity was categorised according to birthplace as either “Greenland” or “Denmark”. The residential location was included for two purposes: (1) to assess differences in healthcare access and (2) to explore differences in the prevalence of liver disease among T2DM patients in Greenland with an urban residence (capital Nuuk) vs. rural residence (elsewhere in the country). Height and weight were used to calculate the body mass index (BMI), and patients were grouped according to the World Health Organization conventional cut-offs (underweight <18.5 kg/m2, normal weight 18.5–24.9 kg/m2, overweight 25–29.9 kg/m2 and obese ≥30 kg/m2). Standard procedures for height, weight and blood pressure measurements have been described previously [Citation12]. Details regarding alcohol consumption were unavailable.
Lipid biochemistry comprised plasma low-density lipoprotein-cholesterol (LDL), high-density lipoprotein-cholesterol (HDL), total cholesterol (TC) and triglycerides (TG). Liver biochemistry included plasma ALAT, aspartate aminotransferase (ASAT) and platelet count. In Greenland, the upper reference levels for ALAT are >35 U/L in women and >50 U/L in men aged 50 years or younger and >70 U/L in all individuals more than 50 years. An ALAT concentration above the upper reference limit was used as a surrogate marker for liver steatosis. The upper limits of ASAT were 35 U/L for women and 45 U/L for men.
Two non-invasive scoring tools, the Fibrosis-4 (FIB-4) score and ASAT to Platelet Ratio Index (APRI), were used to quantify the level of liver fibrosis. FIB-4 includes age, ASAT, platelet count and ALAT. FIB-4 scores <1.45 have a high negative predictive value of advanced liver fibrosis and scores >2.67 have a high positive predictive value of advanced liver fibrosis [Citation14,Citation15].
APRI includes ASAT, the upper limit of the reference interval of ASAT for the given patient, and the platelet count (109/L). The level of hepatic fibrosis was assessed based on conventional cut-off values, with APRI <0.7 likely ruling out fibrosis, values between 0.7 and 1.0 suspicious of fibrosis and >1.0 highly predictive for cirrhosis [Citation16,Citation17].
Finally, we used the following five criteria for the metabolic syndrome: T2DM, BMI >30 kg/m2, triglycerides >1.7 mmol/l or anti-lipidemic treatment, HDL <1.0 mmol/l for men or <1.3 mmol/l for women or anti-lipidemic treatment and systolic blood pressure >130 mmHg or diastolic blood pressure >85 mmHg or anti-hypertensive treatment. Fulfilling at least three of these was considered diagnostic for the metabolic syndrome [Citation18].
Statistics
Cohort characteristics were described using medians with interquartile ranges or proportions. We used the Wilcoxon rank-sum test to study differences between groups of non-normally distributed data and Student’s t-test for normally distributed data. Prior to non-parametric tests, histograms and QQ-plots were used to check for normality. To assess the differences in proportions, χ2-tests or Fisher’s exact tests were used. We performed the non-parametric Spearman’s rank correlation to test the association of both fibrosis scores and steatosis markers related to T2DM and metabolic syndrome variables. Linear regression was applied to assess relationships of both fibrosis scores and steatosis (after logarithmic transformation) to relevant continuous variables. The model was checked by diagnostic plots of the residuals. The number of metabolic syndrome criteria fulfilled was treated as a continuous variable in the linear regression analyses. p-values <0.05 were considered statistically significant for all tests performed. The statistical software Stata, version 17 (StataCorp, Texas, USA), was used for all data analyses.
Results
Background characteristics
The distribution of T2DM in Greenland in different age groups was 55 (3.3%) among <39 years old, 550 (33.2%) among 40–59 years old, 949 (57.2%) among 60–79 years old and 105 (6.3%) among 80+ years old, regardless of birthplace [Citation6]. Of the 1591 patients with T2DM included in this study, 1409 were born in Greenland and 182 were born in Denmark (, ).
Table 1. Background characteristics of the cohort
The median age was similar in Greenlanders and Danes. The male-to-female ratio was approximately 1:1 in the Greenland population, while in the Danish subgroup, there was a marked gender difference with a male-to-female ratio of approximately 10:1.
Greenlanders had a lower median height and weight, but higher BMI than Danes. A higher proportion of Greenlanders had BMI ≥30 kg/m2 compared with Danes (63.2% vs. 52.9%). Furthermore, the Greenlanders had lower HbA1c levels and higher levels of total cholesterol, LDL, HDL and platelets compared with Danes. Fewer Greenlanders were prescribed diabetes medication compared with Danes. Finally, a larger proportion of patients in the Danish subgroup were living in the urban capital Nuuk compared with the Greenlanders ().
Transaminasemia and liver steatosis
The median plasma ALAT concentration was similar in the two groups. However, proportionately more Greenlandic patients had elevated ALAT compared with Danish patients (289 (20.5%) vs. 21 (11.5%), p=0.004). The median ASAT was higher in Greenlanders than Danes ().
In Greenland Inuit, we found a positive association between ALAT and BMI, HbA1c, triglycerides, total cholesterol, LDL and diastolic blood pressure. We also found a negative association between ALAT and HDL in this group ().
Table 2. Univariable associations between ALAT and individual risk factors of the metabolic syndrome
For the small Danish group, only a positive association between ALAT and BMI was identified ().
For both groups, BMI had the strongest correlation with ALAT, while other correlations were modest at best.
Among Greenlanders, median ALAT concentrations were higher for patients with the metabolic syndrome (at least three criteria fulfilled) compared with those without metabolic syndrome (42 vs. 35 U/L, p<0.001), while the ALAT concentration was similar in patients with and without metabolic syndrome in the Danish group (p=0.37). In the linear regression analysis, no interaction of ethnicity was identified, and in the subsequent regression analysis, ALAT increased by 8.0% (95% CI: 5.2–10.9%, p<0.001) for each metabolic syndrome criteria fulfilled when adjusting for birthplace. Among T2DM patients fulfilling the criteria for metabolic syndrome, median HbA1c remained lower (53 vs. 56 mmol/mol, p=0.002) and median BMI remained higher (32.4 vs. 30.7 kg/m2, p<0.001) in Greenlanders compared with Danes and their plasma lipid profiles persisted.
Liver fibrosis
Danes with T2DM had a higher FIB-4 score than Greenlanders (). However, there was no difference between Danes and Greenlanders within FIB-4 score categories. Notably, for both populations, 1.7–2.6% had an FIB-4 score of >2.67 suggesting advanced fibrosis and 13–18% had an intermediate risk of significant fibrosis (FIB-4 score 1.45–2.67); furthermore, 80–84% had a very low risk of significant fibrosis (FIB-4 score <1.45). Similarly, only few patients had an APRI-score in the interval for advanced fibrosis or cirrhosis.
In univariate analysis, we identified negative correlations of FIB-4 scores to HbA1c, triglycerides, LDL and diastolic blood pressure, as well as positive correlations of FIB-4 scores to HDL and systolic blood pressure in Greenlanders (). No significant correlations between FIB-4 and the metabolic syndrome-related variables were identified in Danes (). To further investigate these correlations, we performed regression analyses to adjust for relevant medications. However, the associations persisted. After adjustment for birthplace, we did not find any significant increase in FIB-4 score with an increasing number of fulfilled metabolic syndrome criteria (p=0.84).
Table 3. Univariable associations between FIB-4 or APRI score and individual risk factors of the metabolic syndrome
For the APRI score, a positive correlation was present with BMI for both ethnic groups. Moreover, in Greenlanders, APRI correlated negatively with HbA1c and positively with diastolic blood pressure (). The APRI score increased with 0.9% (95% CI: 0.4–1.4%, p<0.001) for each 1.0 kg/m2 increase in BMI after adjustment for birthplace and after ruling out effect modification by birthplace. Finally, the APRI score increased by 3.1% (95% CI: −0.02–6.4%, p=0.052) for each metabolic syndrome criteria fulfilled after adjusting for birthplace.
Residence
While 61% of ethnic Danes with T2DM were residing in the capital Nuuk, only 29% of Greenland Inuit patients with T2DM were living in Nuuk. We searched for differences in liver steatosis (ALAT) and fibrosis (FIB-4 and APRI scores) among patients living in Nuuk compared with patients living elsewhere in the country. For ethnic Greenlanders living in Nuuk, median ALAT was higher (43 vs. 40 U/L, p=0.001), median FIB-4 score was lower (0.84 vs. 0.97, p<0.001) and median APRI was similar (0.22 vs. 0.23, p=0.17) compared with Greenlanders residing outside the capital.
We did not observe any differences in ethnic Danes based on whether they lived in Nuuk or elsewhere in Greenland.
For both groups, the number of metabolic syndrome criteria fulfilled was unaffected by place of residence. However, all variables included in the FIB-4 score were available in 87% of Greenlanders living in Nuuk compared with only 51% in Greenlanders living elsewhere in the country (p<0.001).
Discussion
This is the first study to systematically investigate biochemical surrogate markers of NAFLD prevalence and severity in the Greenlandic population of patients with T2DM of both Greenland Inuit and Danish background. Evidently, Greenlanders with T2DM had better glycaemic control despite higher BMI and plasma lipid levels than Danes living in Greenland. Furthermore, more Greenlanders had elevated ALAT levels, but lower FIB-4 fibrosis scores compared with Danes, suggesting that Greenlanders may tolerate a higher level of liver steatosis than Danes before they develop liver fibrosis. These findings may be highly relevant for patients and clinicians and help to identify specific healthcare policies to be implemented in the Greenlandic healthcare system. Regular evaluations of liver biochemistry and introduction of non-invasive fibrosis scores in the monitoring of T2DM patients in all areas of Greenland will improve the awareness and identification of NAFLD among T2DM patients.
Greenlanders with T2DM had higher BMI than Danes. This may partly be explained by the fact that Greenlanders have a relatively larger trunk compared with total body length than Danes, thus affecting estimates of BMI [Citation19]. Furthermore, the distribution and storage of adipose tissue vary from Europeans to Inuit, with Europeans having relatively more visceral adipose tissue and Inuit having relatively more subcutaneous adipose tissue [Citation20]. This does not affect BMI measures per se, but it affects how BMI can be interpreted as a risk factor [Citation21].
Greenlanders with T2DM had better glycaemic control than Danes, and the prescription rate of diabetes medications was lower for Greenlanders. This may be due to different fat storage patterns. A relationship between the distribution of visceral vs. subcutaneous adipose tissue and glucose intolerance has been described in favour of the phenotype attributed to Inuit [Citation20]. Furthermore, the current work confirms previous findings regarding Greenlanders having higher plasma lipid levels than Danes. Nevertheless, the prescription rate of anti-lipidemic drugs was similar in Greenlanders and Danes with T2DM. These characteristics may be attributed to metabolic differences and westernisation of the Inuit culture and lifestyle [Citation22–25]. Whether this plays a role in the development of NAFLD is uncertain.
Previous studies on either liver disease or diabetes suggest that the Inuit may carry protective factors that decrease their likelihood of developing complications to T2DM [Citation12,Citation20,Citation26–28]. The differences in metabolic variables between Greenlanders and Danes may be explained within the fields of evolutionary genetics and molecular anthropology. The Inuit population have developed a distinct fatty acid metabolism with a variation in the “carnitine palmitoyl transferase 1”-gene (CPT1-gene), which supports survival in the harsh Arctic environment on a carnivorous and low carbohydrate diet [Citation29]. Further studies have identified a gene variation in the TBC1D4-gene among Greenlanders. This variant is associated with impaired glucose tolerance and a higher risk of developing T2DM among homozygous carriers in the Greenlandic population [Citation30]. Unfortunately, genetic testing was impossible for the present register-based study.
We estimated liver steatosis by assessing elevated plasma ALAT levels as previously shown [Citation31]. Furthermore, plasma ALAT is in general accepted as a surrogate marker of liver steatosis in epidemiological studies [Citation32], with the cut-off value of 36 U/L previously reported to carry a sensitivity of 63% and specificity of 78% for detecting NAFLD [Citation33]. In our study, plasma ALAT levels were associated with increasing severity of metabolic syndrome, demonstrating the link between metabolic syndrome and liver steatosis also in patients with T2DM in Greenland. Although median plasma ALAT levels were similar between Greenlanders and Danes, a larger proportion of the Greenlanders presented with elevated plasma ALAT levels. Furthermore, Greenlanders had higher plasma ASAT than Danes. These observations suggest a higher frequency of liver steatosis in Greenlanders. Since ultrasonography, Fibroscan® and histopathology were unavailable, we used FIB-4 and APRI scores as surrogate markers of liver fibrosis. A large study with 541 NAFLD patients demonstrated a diagnostic performance (area under the receiver operator curve) of 0.80 for the FIB-4 score and 0.73 for the APRI score. Moreover, the cut-off for advanced fibrosis used in the present work (FIB-4 score >2.67) carried a specificity of 98% [Citation15]. Notably, the FIB-4 score was slightly lower in the Greenlandic population than in Danes, suggesting that Greenlanders are less likely to develop liver fibrosis regardless of modest transaminasemia. However, the proportion of patients with advanced fibrosis (FIB-4 score >2.67) was similar and low (1.7–2.6%) in both populations. In order to affirm the hypothesis that Greenlanders are less likely to develop liver fibrosis than Danes, future studies will benefit from a longitudinal design and require patient cohorts with a larger control group, a larger proportion suffering from moderate and advanced liver fibrosis, and inclusion of Fibroscan® or biopsy specimens for comparison. Also, other promising non-invasive biomarkers of liver steatosis and fibrosis may be relevant to evaluate in Arctic populations in future studies [Citation34].
Furthermore, we investigated the correlations between the FIB-4 score and T2DM and metabolic syndrome risk factors in Greenlanders and Danes. However, we were unable to identify any strong or clinically meaningful associations. This may be explained by the metabolic differences between the two groups. Yet, we cannot exclude whether statistical uncertainties may play a role in the small Danish population. Also, to establish a true association between the FIB-4 score and fibrosis risk factors, validation studies in the Inuit population, preferably using biopsies previously obtained for clinical purposes, are warranted.
Studies in other parts of the world generally report a high prevalence of liver disease in diabetes populations. A recent systematic review with meta-analysis estimated the global prevalence of NAFLD in patients with T2DM to be 30–68% [Citation35]. In general populations, a higher prevalence of liver disease than the Inuit T2DM population has been described. Among adults in the USA, the prevalence of NALFD was 21.9% [Citation36]. In Mexican adults, 8.1% showed a high probability of fibrosis [Citation37]. Advanced fibrosis has been observed in 2.8% of the general French population aged over 40 years [Citation38]. However, caution must be taken in comparing results since the methods of estimating liver disease (FibroTest and NAFLD fibrosis score) in these studies vary from ours. The Greenland Inuit are related to the Alaska-Native people, in particular, the ethnic groups Aleut and Yupik. For the Alaska-Native people, NAFLD has been reported as the second most common cause of chronic liver disease with a prevalence of approximately 2% among patients with an encounter at the Alaska Native Medical Center [Citation39]. In another study of a selected Alaska-Native population with chronic hepatitis B virus (HBV) infection, aminotransferase elevations were attributed to NAFLD in 24.7% [Citation40]. A similar proportion of Greenland Inuit with T2DM had aminotransferase elevations in the present work.
A major strength of this study is the inclusion of all patients diagnosed with T2DM with permanent residence in Greenland. In particular, we included a substantial number of Greenlanders, which provided robust data for this population group. However, a limitation in the study is the small control group of Danes and with a different gender distribution compared with the Greenlanders although the gender difference among the Danes, with fewer females than males, resembled the background Danish population in Greenland [Citation6]. Despite these limitations of a small and demographically different control group, the inclusion of Danes for comparison remains relevant since Greenland is an autonomous territory in the Danish Realm and thus the healthcare system structure from Denmark is applied in Greenland. Additionally, the most recent study that examined diabetic complications in Greenlanders used Danes/non-Greenlanders for comparison [Citation12].
In the present study, there is a risk of underestimating the prevalence of diabetes, steatosis and fibrosis since not everyone in the population of Greenland has easy access to medical facilities. Diabetes diagnostic has high awareness in the Greenlandic healthcare system, especially after 2010, when HbA1c was introduced as a standard test [Citation10]. However, T2DM might be underdiagnosed in certain areas in Greenland although the proportion of Greenlanders with undiagnosed T2DM has decreased substantially during the past decade [Citation41]. Missing variables for the FIB-4 score was more prevalent in Greenlanders living outside Nuuk. Furthermore, 597 patients (37.5%) lacked measurements of either ASAT or platelets; thus, we were unable to estimate liver fibrosis in these patients. The patients without an available FIB-4 score had slightly higher median HbA1c (53 vs. 51 mmol/mol), higher median BMI (32.2 vs. 31.6 kg/m2) and higher median total cholesterol (4.7 vs. 4.4 mmol/l) and, thus, might have a higher risk of more advanced NAFLD. Albumin levels are not routinely measured in the study population, and we were therefore unable to calculate the NAFLD fibrosis score. Furthermore, we were unable to pull data from the EMR concerning the duration of T2DM, which affects the risk of developing liver disease [Citation42].
Another limitation is the ability to control for other risk factors, mainly alcohol intake and chronic viral hepatitis. Alcohol consumption patterns among Greenlanders and Danes differ. Binge drinkers and abstainers are more frequent in Greenland, whereas more Danes report a regular alcohol intake [Citation43]. A study assessing the association between alcohol consumption and liver disease in Greenland in a cohort of patients attending alcohol treatment centres reported lower ALAT levels and a lower prevalence of biopsy-verified liver fibrosis or cirrhosis in Greenlanders than Danes [Citation44]. Therefore, alcohol as a confounder is of minor concern, especially for our large group of Greenlanders. Moreover, patients with T2DM in Greenland are offered free regular controls and lifestyle interventions where alcohol habits are discussed and, if indicated, alcohol treatments are offered [Citation12]. As such, we may expect a higher awareness on the harmful effects of alcohol intake for this selected group of T2DM patients than in the general Greenlandic population. In our study population, only few patients were tested for chronic viral hepatitis even though the prevalence of chronic HBV infection is high, with approximately 8% of all Inuit being chronically infected. Furthermore, HBV is endemic in local populations, where up to 29% have HBV antibodies [Citation45]. In contrast, HCV is rare in Greenland [Citation46]. Due to the high prevalence of especially HBV, it is recommended that NAFLD patients with elevated ALAT and/or a high fibrosis score be tested for chronic viral hepatitis. This is in accordance with recommendations from other Arctic and sub-Arctic native populations [Citation47].
Conclusion
Greenlanders with T2DM have better glycaemic control despite higher plasma lipid levels and higher BMI than Danes living in Greenland. Furthermore, more Greenlanders with T2DM have elevated plasma ALAT levels but lower FIB-4 score compared with Danes. Thus, the presence and severity of NAFLD in Greenlanders and Danes with T2DM differ, which indicates that Greenlanders may carry protective factors that decrease their likelihood of developing liver disease. Importantly, advanced liver disease was low (<3%) in both populations. Our findings are highly relevant for patients with T2DM residing in Greenland and may be transferable to other Arctic populations. Furthermore, the results may guide clinicians in the Arctic, and especially the Greenlandic, healthcare systems in their assessment and monitoring of patients with T2DM.
Author contributions
All authors contributed to the conceptualisation of this study. AGM and FOH drafted the first manuscript version. RHG performed the statistical analyses. All authors contributed to the interpretation of data. All authors critically reviewed and approved the final manuscript version. As part of the Steno Diabetes Center Greenland, this project was financially supported by the Novo Nordisk Foundation under grant NNF20SA0064190.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Asrani SK, Devarbhavi H, Eaton J, et al. Burden of liver diseases in the world. J Hepatol. 2019;70(1):151–9.
- Eslam M, Sanyal AJ, George J. MAFLD: a consensus-driven proposed nomenclature for metabolic associated fatty liver disease. Gastroenterology. 2020;158(7):1999–2014.e1.
- Adams LA, Anstee QM, Tilg H, et al. Non-alcoholic fatty liver disease and its relationship with cardiovascular disease and other extrahepatic diseases. Gut. 2017;66(6):1138–1153.
- Hashimoto E, Taniai M, Tokushige K. Characteristics and diagnosis of NAFLD/NASH. J Gastroenterol Hepatol. 2013;28(4):64–70. Suppl.
- Younossi ZM, Stepanova M, Younossi Y, et al. Epidemiology of chronic liver diseases in the USA in the past three decades. Gut. 2020;69(3):564–568.
- Greenland Statistics Available online: https://stat.gl/default.asp?lang=en (accessed on 2021 Apr 8).
- Scott JD, Garland N. Chronic liver disease in aboriginal North Americans. World J Gastroenterol. 2008;14(29):4607–4615.
- Andersen S, Rex KF, Noahsen P, et al. Forty-five year trends in overweight and obesity in an indigenous Arctic Inuit Society in transition and spatiotemporal trends. Am J Hum Biol Off J Hum Biol Counc. 2014;26:511–517.
- Jørgensen ME, Bjeregaard P, Borch-Johnsen K. Diabetes and impaired glucose tolerance among the Inuit population of Greenland. Diabetes Care. 2002;25(10):1766–1771.
- Pedersen ML. High awareness of diabetes in the health care system in Greenland measured as a proportion of population tested with glycated haemoglobin within 2 years. Diabetol Metab Syndr. 2017;9(1):30.
- NCD Risk Factor Collaboration (NCD-RisC). Worldwide trends in body-mass index, underweight, overweight, and obesity from 1975 to 2016: a pooled analysis of 2416 population-based measurement studies in 128·9 million children, adolescents, and adults. Lancet. 2017;390(10113):2627–2642.
- Pedersen ML. Microvascular complications in Nuuk, Greenland, among Greenlanders and non-Greenlanders diagnosed with type 2 diabetes. Diabet Res Clin Pract. 2018;136:1–6.
- Okkes IM, Becker HW, Bernstein RM, et al. The March 2002 update of the electronic version of ICPC-2. A step forward to the use of ICD-10 as a nomenclature and a terminology for ICPC-2. Fam Pract. 2002;19(5):543–546.
- Sterling RK, Lissen E, Clumeck N, et al. Development of a simple noninvasive index to predict significant fibrosis in patients with HIV/HCV coinfection. Hepatology. 2006;43(6):1317–1325.
- Shah AG, Lydecker A, Murray K, et al. Comparison of noninvasive markers of fibrosis in patients with nonalcoholic fatty liver disease. Clin Gastroenterol Hepatol Off Clin Pract J Am Gastroenterol Assoc. 2009;7:1104–1112.
- Lin Z-H, Xin Y-N, Dong Q-J, et al. Performance of the aspartate aminotransferase-to-platelet ratio index for the staging of hepatitis C-related fibrosis: an updated meta-analysis. Hepatology. 2011;53(3):726–736.
- Chou R, Wasson N. Blood tests to diagnose fibrosis or cirrhosis in patients with chronic hepatitis C virus infection. Ann Int Med. 2013;159(5):372.
- Huang PL. A comprehensive definition for metabolic syndrome. Dis Model Mech. 2009;2(5–6):231–237.
- Andersen S, Fleischer Rex K, Noahsen P, et al. Raised BMI cut-off for overweight in Greenland Inuit – a review. Int J Circumpolar Health. 2013:72.
- Jørgensen ME, Borch-Johnsen K, Stolk R, et al. Fat distribution and glucose intolerance among Greenland Inuit. Diabetes Care. 2013;36(10):2988–2994.
- Ibrahim MM. Subcutaneous and visceral adipose tissue: structural and functional differences. Obes Rev an off J Int Assoc Study Obes. 2010;11(1):11–18.
- Bjerregaard P, Jørgensen ME, Borch-Johnsen K. Serum lipids of Greenland Inuit in relation to Inuit genetic heritage, westernisation and migration. Atherosclerosis. 2004;174(2):391–398.
- Noahsen P, Andersen S. Ethnicity influences BMI as evaluated from reported serum lipid values in Inuit and non-Inuit: raised upper limit of BMI in Inuit? Ethn Dis. 2013;23(1):77–82.
- Rudkowska I, Dewailly E, Hegele RA, et al. Gene-diet interactions on plasma lipid levels in the Inuit population. Br J Nutr. 2013;109(5):953–961.
- de Knijff P, Johansen LG, Rosseneu M, et al. Lipoprotein profile of a Greenland Inuit population. Influence of anthropometric variables, Apo E and A4 polymorphism, and lifestyle. Arterioscler Thromb a J Vasc Biol. 1992;12(12):1371–1379.
- Larsen TLJ, Jørgensen ME, Pedersen ML, et al. Low prevalence of retinopathy among Greenland Inuit. Int J Circumpolar Health. 2021;80(1):1938420.
- Rokkjaer MS, Jensen MT, Grønbaek H, et al. Discharge diagnoses of liver diseases in Nuuk Greenland compared to a Danish county hospital. Int J Circumpolar Health. 2006;65(2):162–168.
- Pedersen ML, Jacobsen JL, Lynge AR. Micro- and macrovascular complications among Greenlanders and Danes with type 2 diabetes mellitus in Nuuk, Greenland. Int J Circumpolar Health. 2010;69(2):195–207.
- McGarrah RW. Metabolic anthropology: selection pressure shapes fatty acid metabolism in Greenlandic Inuit populations. Circ Cardiovasc Genet. 2017;10(3)).
- Moltke I, Grarup N, Jørgensen ME, et al. A common Greenlandic TBC1D4 variant confers muscle insulin resistance and type 2 diabetes. Nature. 2014;512(7513):190–193.
- Clark JM, Brancati FL, Diehl AM. Nonalcoholic fatty liver disease. Gastroenterology. 2002;122(6):1649–1657.
- Schindhelm RK, Diamant M, Dekker JM, et al. Alanine aminotransferase as a marker of non-alcoholic fatty liver disease in relation to type 2 diabetes mellitus and cardiovascular disease. Diabetes Metab Res Rev. 2006;22(6):437–443.
- Nomura K, Yano E, Shinozaki T, et al. Efficacy and effectiveness of liver screening program to detect fatty liver in the periodic health check-ups. J Occup Health. 2004;46(6):423–428.
- Gantzel RH, Kjær MB, Laursen TL, et al. Macrophage activation markers, soluble CD163 and Mannose receptor, in liver fibrosis. Front Med. 2020;7:615599.
- Younossi ZM, Golabi P, de Avila L, et al. The global epidemiology of NAFLD and NASH in patients with type 2 diabetes: a systematic review and meta-analysis. J Hepatol. 2019;71(4):793–801.
- Wong RJ, Liu B, Bhuket T. Significant burden of nonalcoholic fatty liver disease with advanced fibrosis in the US: a cross-sectional analysis of 2011-2014 National Health and Nutrition Examination Survey. Aliment Pharmacol Ther. 2017;46(10):974–980.
- García-Compeán D, Villarreal-Pérez JZ, Cavazos MEDLO, et al. Prevalence of liver fibrosis in an unselected general population with high prevalence of obesity and diabetes mellitus. Time for screening? Ann Hepatol. 2020;19(3):258–264.
- Poynard T, Lebray P, Ingiliz P, et al. Prevalence of liver fibrosis and risk factors in a general population using non-invasive biomarkers (FibroTest). BMC Gastroenterol. 2010;10(1):40.
- Fischer GE, Bialek SP, Homan CE, et al. Chronic liver disease among Alaska-Native people, 2003-2004. Am J Gastroenterol. 2009;104(2):363–370.
- Spradling PR, Bulkow L, Teshale EH, et al. Prevalence and causes of elevated serum aminotransferase levels in a population-based cohort of persons with chronic hepatitis B virus infection. J Hepatol. 2014;61(4):785–791.
- Pedersen ML. Diabetes care in the dispersed population of Greenland. A new model based on continued monitoring, analysis and adjustment of initiatives taken. Int J Circumpolar Health. 2019;78(sup1):1709257.
- Gupta A, Anoop S, Ansari IA, et al. High prevalence of hepatic steatosis and hepatic fibrosis in patients with type 2 diabetes mellitus. Clin Nutr ESPEN. 2021;46:519–526.
- Bjerregaard P, Larsen CVL, Sørensen IK, et al. Alcohol in Greenland 1950-2018: consumption, drinking patterns, and consequences. Int J Circumpolar Health. 2020;79(1):1814550.
- Lavik B, Holmegaard C, Becker U. Drinking patterns and biochemical signs of alcoholic liver disease in Danish and Greenlandic patients with alcohol addiction. Int J Circumpolar Health. 2006;65(3):219–227.
- Rex KF, Andersen S, Krarup HB. Hepatitis B among Inuit: a review with focus on Greenland Inuit. World J Hepatol. 2015;7(9):1265–1271.
- Rex KF, Krarup HB, Laurberg P, et al. Population-based comparative epidemiological survey of hepatitis B, D, and C among Inuit migrated to Denmark and in high endemic Greenland. Scand J Gastroenterol. 2012;47(6):692–701.
- Ko HH, Chung HV, McMahon BJ, et al. Liver disease in the indigenous populations of the Arctic, sub-Arctic, and Pacific Northwest: an approach to investigations in Alaska and British Columbia. B C Med J. 2006;48:216–221.