ABSTRACT
We conducted a scoping review to determine incidence and risk factors for postpartum haemorrhage (PPH) in rural Indigenous women. We systematically searched PubMed (Medline), EMBASE, and CINAHL for all peer-reviewed articles and grey literature regarding Indigenous ethnicity, rural settings, and PPH incidence, risk factors, or maternal outcomes published from inception to 11 January 2021. Eleven articles were deemed relevant after screening and quality assessment using the National Institutes of Health scoring system for mixed study reviews. Of these, 3 articles were good quality, 1 was fair, and 7 were poor. Nine possible risk factors were recorded. The outcomes studied were transfusion, hysterectomy and mortality. PPH research in rural Indigenous women is scarce, mostly low quality and fails to represent most Indigenous cultures and countries. Women from Indigenous groups in rural Canada, Australia and the USA are at higher risk for PPH but specific risk factors are unknown. While widely differing populations made the data difficult to synthesise, this inaugural scoping review highlights a need for further research and increased obstetrical resources in areas where rural Indigenous women reside.
Introduction
Discriminatory policy and colonisation have created entrenched health disparities among Indigenous peoples in healthcare, including maternal health and childbirth [Citation1,Citation2]. Maternal health is a vital component of acceptable primary care [Citation3], yet both developing and developed nations still struggle with the obstetrical emergency postpartum haemorrhage (PPH; haemorrhage of at least 500 mL from the maternal reproductive tract within 24 hours of delivery) [Citation4]. PPH is a life-threatening obstetrical emergency with significant maternal morbidity and mortality [Citation4,Citation5]: a quarter of global maternal deaths [Citation4]. It is also becoming increasingly frequent in the developed world [Citation6–11]. The incidence of PPH in Canada, for example, increased by 22% from 2003 to 2010 [Citation9]. Nunavut experienced a 209% increase in PPH during the same period [Citation9]. Although the authors acknowledged challenges that affect the diagnosis of PPH and estimation of blood loss, they concluded that rising rates of PPH were not artefactual. Nunavut reported a 17.1% incidence of PPH which was much higher than the Canadian average of 5.6%. As Nunavut’s population is 85% Inuit, these findings suggest there may be a higher risk of PPH that is increasing overtime in Inuit and other remote Indigenous women.
Furthermore, many Indigenous women live in rural areas [Citation12] where delivery of maternity care can be challenging [Citation12]. Although Indigenous women can go to urban centres to give birth, many prefer to deliver locally for cultural and traditional reasons [Citation12]. Understanding rural Indigenous women’s risk of obstetrical complications such as PPH can guide health care to support birthing close to home [Citation12]. However, the literature on PPH in rural Indigenous women is scant and there has been no synthesis of the evidence in this area. Therefore, we have conducted the first scoping review to identify what is known about the incidence, risk factors and outcomes of PPH in this population.
Methods
Search strategy & selection criteria
To identify peer-reviewed studies, we searched PubMed (Medline), EMBASE, and CINAHL from inception to 11 January 2021. No date or language limiters were applied. For grey literature, we searched ProQuest Dissertations & Theses, Australian Indigenous Health Info Net Bibliography, Lowitja Institute (Australia), Circumpolar Heath Bibliographic Database, British Columbia Network Environments for Indigenous Health Research (Canada), Native Health Database (USA), NZ Ministry of Health Maori Health Publications (New Zealand), LILACS, Google and Google Scholar (including custom searches for government documents and university repositories). We hand searched the references from each included study for relevant citations and added these to the pool of included articles. We also conducted forward citation tracing for each included study.
Search
We conducted a comprehensive literature search for peer-reviewed studies and grey literature following recommendations by Arksey and O’Malley [Citation13]. A combination of keywords and controlled vocabulary terms were used to capture the articles and reports related to PPH and rural Indigenous women. Search strategy terms were identified in consultation with content experts and a librarian. See for database search strategies. We used Endnote X8 (Clarivate Analytics) to manage the citations of all articles identified through the database searches.
Table 1. Search strategies.
Study selection
Titles and abstracts of all captured articles were screened for their relevance by a single reviewer and checked by another when necessary. We included articles in any language regarding PPH incidence, risk factors, or maternal outcomes in rural and remote Indigenous women, and excluded non-empirical articles (e.g. opinion papers). See for operational definitions of inclusion criteria. Two authors reviewed the full text of the eligible articles independently.
Table 2. Inclusion criteria and their operational definitions.
Data collection processes & quality appraisal
Data was extracted from each article independently by two reviewers (SA and SD) using a data extraction tool in Microsoft Excel. The results were cross-checked and any disagreement was resolved in biweekly meetings. A calibration exercise was undertaken before data extraction and the tool was modified where agreement between SA and SD was poor (K < 0 · 5). Data extracted included year of publication, place of publication, type of publication, study design, country, geographical setting of study, setting of delivery, type of service provider, site of study, duration of study, data collection method, population (ethnicity, age range, percentage in rural and remote areas), number of PPH cases, sample size (grand total, total Indigenous, total non-Indigenous), PPH-related outcomes, PPH-related risk factors (before and during index pregnancy) and, incidence of PPH (for Indigenous and non-Indigenous populations). PPH-related outcomes and PPH-related risk factors were selected to be as inclusive as possible.
A quality appraisal was conducted using National Institute of Health (NIH) scoring system for mixed study reviews [Citation14]. This includes 14 items regarding the internal validity of the study. Each item is rated as yes, no, or cannot determine. An overall judgment of good, fair or poor quality was made based on the majority of items: an article was rated “good” if 9 or more items were rated yes, “fair” if 7 or 8 items were rated yes and “poor” if 6 or fewer items were rated as yes. SD and TH independently appraised the quality of all included studies, then met via teleconference to discuss their decisions and rationale for those decisions in an attempt to reach a consensus on the quality of all 11 articles. If consensus could not be reached a third author (SA) was consulted to make a final decision. We did not use quality appraisal to exclude low-quality studies. Risk of bias was also assessed within and across studies using the same questions from the NIH scoring system for mixed study reviews. We then compared quality across studies by entering their responses into an organised table, colour coding the answers and verifying that the aforementioned quality appraisal rules were applied uniformly.
Analysis
The number of identified articles and their characteristics were described. A narrative synthesis was conducted to summarise the incidence of PPH and proportion of risk.
Results
Studies included
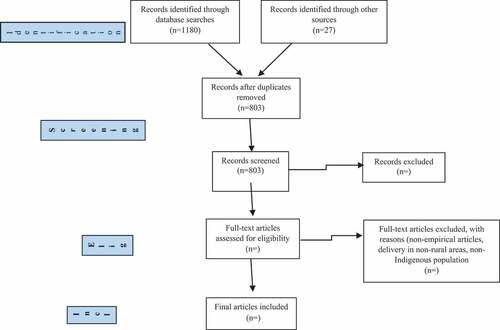
presents the PRISMA flow chart outlining details of the study identification and selection process. Data were extracted without disagreement. An overview of the included studies is found in . All studies were observational with the majority using a retrospective cohort design and data collected from medical records contained in hospital databases. The studies varied considerably by population studied and date of publication, ranging from 1965 to 2016. Four studies were over 40 years old. Two of the 11 articles examined PPH in rural Indigenous women as a primary outcome; 1 compared PPH incidence in Native American women to other ethnicities [Citation15], and the other examined a single PPH risk factor in Native American women [Citation16]. In the remaining nine studies PPH was examined only as a secondary outcome.
Table 3. Characteristics of included studies.
Study quality
Using the 14-item NIH scale to assess study quality, 3 articles were rated as good quality, 1 as fair, and 7 as poor (see ). Most studies reported clear research questions, objectives and study populations; there appeared to be little susceptibility to participant selection bias. However, only half of the studies clearly defined the outcome measures and none reported using assessor blinding. Lastly, few studies reported sufficient information on missing data to determine if this was an issue. Importantly, most of the studies that were judged as poor quality (n = 4) were because they lacked transparent reporting on many of the NIH items, which may be due to the fact that they were published over 40 years ago before the reporting guidelines were published.
Table 4. Results of quality appraisal.
Definition and incidence of PPH
Three different definitions of PPH were used among the 8 articles that defined PPH. Four articles used estimated blood loss (EBL) ≥ 500 mL while 3 used provider diagnosis in medical records to define a PPH case. One study from the USA defined PPH as 3 g/dl postpartum haemoglobin drop.
Eight of 11 articles reported incidence of PPH: 4 in Inuit populations, 3 in North American non-Inuit Indigenous populations, and 1 in Australian Indigenous populations. PPH incidence ranged from 5.8% to 15.4% in Inuit populations, 7.9% to 18.2% in non-Inuit North American populations, and 29.9% in Indigenous Australian populations. One Australian study reported a 2.0% incidence of severe PPH, defined as EBL ≥ 1500 mL. Severe PPH was neither defined nor recorded in the other 10 studies.
Humphrey defined PPH as EBL > 500 mL in a Australian population that was 29.4% indigenous, but did not provide the case number or the incidence of PPH in indigenous women. See for a breakdown of PPH incidence by study location and article.
Table 5. Definition and incidence of PPH derived from the identified studies.
Risk factors for PPH
See for an overview of PPH risk factors identified by included articles. Five of 11 studies collectively reported on 9 possible PPH risk factors and one protective factor [Citation15–19]. The nine risk factors were indigenous ethnicity, maternal age, gravida, parity, labour induction or augmentation, birth weight, retained placenta, magnesium sulphate usage and chorioamnionitis. The protective factor was active management of the third stage of labour (AMTSL).
Table 6. Summary of PPH risk factors derived from the identified studies.
Indigenous ethnicity was studied in 4 of the 5 studies reporting on risk factors. Two studies reported a disproportionately high incidence of maternal death due to PPH among indigenous women in British Colombia [Citation18,Citation19], but did not provide data on incidence or mortality of PPH among indigenous women. The remaining two studies found a significantly increased risk of PPH in indigenous Australian women [Citation17] and Native American women [Citation15].
Humphrey examined various risk factors and outcomes within a population of 15908 women that was 29.4% indigenous in Northern Queensland, Australia. The indigenous subgroup showed an increased risk of PPH and transfusion compared to the non-indigenous subgroup [Citation17].
Chalouhi et al. examined indigenous ethnicity as a risk factor and was also the sole study to examine the remaining eight risk factors. The study investigated whether Native American women had a higher risk of PPH compared to non-native and white women with singleton, term deliveries that included caesarean sections. Native American women made up 70.7% of the study population and had a higher risk of PPH compared to both the combined non-native subgroup (11.6% vs. 7.0%, p = 0.02) and the white subgroup (11.6% vs. 6.7%, p = 0.01). Native American women also had a higher rate of uterine atony, higher EBL and postpartum drop in haemoglobin in Native American women vs. non-native women [Citation15]. Decreased gravidity (g < 5), retained placenta and use of magnesium sulphate were significant predictors of PPH, while maternal age <35, parity <5, labour induction and augmentation, birth weight and chorioamnionitis were not significant predictors of PPH. There was no risk factor analysis among the Indigenous subgroup. Chalouhi et al. concluded that Native American ethnicity was a risk factor for both uterine atony and PPH. Active management of the third stage of labour (AMTSL) was studied as a protective factor against PPH. Fenton et al. [Citation16] was the sole study to examine a single variable for PPH in an entirely rural and Indigenous population. The study examined whether AMTSL, defined as 10 units of oxytocin given either intravenously or intramuscularly immediately after the delivery of the anterior shoulder, was effective in reducing maternal blood loss. Providers chose to either give routine care or AMTSL in the retrospective cohort study. The cohorts were of unequal size with fewer women receiving AMTSL than routine care, defined as 20–40 units of oxytocin IV over 1–2 hours after placental delivery.
The study demonstrated that AMTSL significantly reduced PPH as defined as >3 g/dl decrease in Hb. AMTSL also significantly reduced the decrease in Hb (p = 0.001) and reduced EBL (p = 0.02). There was no significant effect on EBL ≥500 ml (p = 0.28). Wan Vagner noted that AMTSL was provided in 49.8% of deliveries during their study of Inuit from Nunavik from 2000 to 2007, but did not investigate its effect on PPH. AMTSL was not reported in any other studies.
PPH-related outcomes
shows PPH-related outcomes derived from the 11 included studies. Transfusion, hysterectomy and mortality were the three PPH-related outcomes that were reported. Overall, six studies examined transfusion as an outcome of PPH. Chalouhi et al. reported a 34.4% rate of transfusion among cases of PPH in Native American women with no statistically significant difference between the transfusion rate of the non-native subgroup (36.6%). Fenton and al. reported a transfusion rate of 11.1% (n = 3) in indigenous women with PPH and no statistical difference in rates between the AMTSL (n = 0) and routine care groups (n = 3). Three studies from the Canadian arctic reported transfusion rates of 7.1%, 21.1% and 36.5% among Inuit women with PPH [Citation20–22]. In Australia, Humphrey did not provide numbers or incidence of transfusion among PPH cases, but did find a significant associated between Australian indigenous ethnicity and transfusion.
Table 7. Summary of PPH-related outcomes derived from the identified studies.
The only study to mention hysterectomy as an outcome of PPH reported a single incidence of hysterectomy but in a very low number of PPH cases (n = 5) as part of an ethnographic study [Citation23].
Three studies recorded maternal mortality and reported no deaths from PPH or other causes. Thomas [Citation18] reported disproportionately high PPH-related maternal deaths in indigenous women in British Colombia, Canada who comprised 2.4% of the population but made up 16.1% of the maternal deaths and over half of all deaths due to PPH. This over-representation of indigenous women in maternal deaths due to PPH suggested an increased risk of death due to PPH, but the study did not provide statistics on the mortality rate of indigenous women with PPH. There were no data on other maternal outcomes.
Circumpolar region
Four of the 11 included studies explored PPH in rural Inuit women in the Canadian circumpolar regions; 2 in Nunavik and 2 in Nunavut. These studies were retrospective cohort studies of poor quality designed to describe maternal outcomes or characterise maternal risks. PPH was included as one of several outcomes with no analysis of PPH risk factors. Three of the four studies are over 20 years old. PPH incidence in the three studies that defined PPH by provider diagnosis were 15.4%, 14.2% and 10.1%. The fourth study that defined PPH as EBL > 500 mL reported a lower incidence of PPH of 5.8%. Three studies reported on maternal outcomes of PPH. Transfusion rates in PPH were 7.1% and 21.1% in Nunavik [Citation20,Citation21]. One study out of Nunavut reported that 32% of all 3rd stage accidents which included both PPH and retained placenta without PPH received transfusions [Citation22]. The number and percentage of PPH cases with transfusion was not defined. One study from Nunavik reported a mortality rate of 0.0% [Citation21]. There were no included studies from any other circumpolar regions such as Greenland, Scandinavia, Russia or USA.
Discussion
This is the first scoping review of PPH in rural Indigenous women. PPH incidence in rural indigenous women ranged from significantly between studies. Incidence of PPH in Inuit and non-Inuit North American indigenous populations ranged from similar to the national incidence of PPH in Canada [Citation9] and USA [Citation24] to markedly higher. A single study from Australia reported a markedly higher incidence of PPH than the general incidence of Australia [Citation25].
PPH risk factors and maternal outcomes in rural Indigenous women were rarely examined. Two studies demonstrated that indigenous ethnicity is a significant PPH risk factor in Native American women and indigenous Australian women. Possible risk factors for PPH in indigenous women are low gravity (<5), retained placenta and magnesium sulphate use, although these were not analysed for significance within the indigenous subgroup. Similarly, risk factors that were not statistically significant in a 70.7% indigenous population may be significant if analysed within the indigenous subgroup. AMTSL appears to be a protective factor against PPH that significantly reduced maternal blood loss and postpartum Hb drop of 3 g/dl but not when using the definition of EBL > 500. The study sample size was quite small and cohorts were unequal, but this finding is in keeping with the current recommendations of AMTSL as the standard of care to prevent PPH [Citation26].
With regard to PPH-related outcomes, blood transfusion was the most examined; there may be a high rate of transfusion resulting from PPH in rural Indigenous women but this could also be due to differences in transfusion practices. One study from 1979, for example, reported a 32% transfusion rate but this was for Inuit women living in remote communities hours away from a hospital with surgical services. This high transfusion rate could be related to lack of surgical backup rather than severity of PPH. There were insufficient data to make any generalisations about hysterectomy or mortality due to PPH.
In summary, the increased PPH risk is the only agreement between existing studies, yet they do not give any insight about which risk factors correlate with increased risk within rural Indigenous women. Similar themes emerge when examining studies of rural indigenous women from circumpolar regions. Only four studies of poor quality from two regions of Canada that include Inuit population were identified. As three of these studies are over 20 years old, their findings may no longer be relevant. Still, 3 of the 4 studies reported higher PPH incidences than the Canadian incidence suggesting that Inuit women may be at higher risk for PPH. There was no research on risk factors for PPH. Transfusion rates reported in three of the studies varied considerably. More research from other circumpolar regions is required, including management of PPH across the circumpolar regions.
Limitations
This scoping review has some limitations. First, the widely differing populations in the 11 articles made it difficult to summarise the results. Most studies either included PPH as one of many outcomes or included rural Indigenous women as a subgroup in their analyses, making the data hard to synthesise.
Obstetrics, rural medicine, and indigenous healthcare have changed substantially during the 50-year period across which the studies were authored. For example, 5 of the 11 articles were published well before AMTSL, which reduces the risk of PPH [Citation26], became the standard of care. Those five studies may have a higher incidence of PPH due to lack of AMTSL. Most of the available studies also used low-quality observational designs (case series, retrospective cohort, etc.), illustrating a clear need for new, modern research using primary data collection. All studies have occurred in Canada, Australia or the USA. Many countries and their Indigenous groups have no representation in the literature (e.g. Sami of Northern Europe, Māori of New Zealand), and even within the countries studied there are Indigenous groups who have not been included (e.g. Mi’kmaq of Canada). There may be important, unexplored cultural factors to the PPH- rural Indigenous women relationship and it is not clear if increased risk of PPH can be generalised to all rural Indigenous women.
Another potential limitation is the heterogeneity of the definition of PPH used between studies. Three different definitions of PPH were used in the included articles. Three studies recorded EBL ≥ 500cc but used a different measure to define PPH. Van Wagner et al., Carignan and Fenton reported PPH incidences of 15.4%, 14.2% and 18.2% respectively but the incidence of EBL ≥ 500 mL was 37.5%, 39.5% and 24.3% respectively. This highlights the impact of differing definitions of PPH on reported incidence. As well, in 2017 the ACOG in the USA changed the definition to blood loss of ≥1000 mL or blood loss with symptoms of hypovolemia [Citation27]. This differs from the definition used by the WHO and from all of the included articles in the scoping review which preceded 2017.
Some may take issue with collating results from Indigenous populations from different regions of the world; it could be argued that Indigenous populations in Australia have little relation to Inuit or other Indigenous populations in North America. In both cases the reality is that there is a severe paucity in available research on this subject that necessitated the creation of an informative starting point upon which future research can build.
Another limitation is the search strategy which takes a specific approach than a broad one. It is possible that we missed articles of large population studies which adjusted for indigenous ancestry or rural locations. As well, the predominantly English-language databases used many not captured studies in other languages from circumpolar countries.
Conclusion
Our review suggests that there is a lack of research on the risk, incidence and outcomes of PPH in rural indigenous women. Despite this lack of research, the studies identified in this inaugural scoping review of PPH among rural Indigenous women suggest that indigenous ethnicity is a risk factor for PPH and that incidence of PPH and transfusion may be increased in indigenous women. There appears to be even less research of rural indigenous women in the circumpolar region. This subject has been grossly understudied and a clear knowledge gap exists to be addressed by further research. High quality research with a clear definition of PPH is needed to better understand the incidence, outcomes and risk factors of PPH for rural indigenous women in both worldwide and in circumpolar regions to provide better-informed obstetrical care.
Acknowledgments
Thank you to Ms. Rebecca Ford for proofreading this article from an Indigenous perspective.
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- Allan B, Smylie J First peoples, second class treatment: The role of racism in the health and well-being of indigenous peoples in Canada. Toronto: ON: Wellesley Institute; 2015:1–12. Accessed 2018 Mar 20. http://www.wellesleyinstitute.com/wp-content/uploads/2015/02/Summary-First-Peoples-Second-Class-Treatment-Final.pdf
- World Health Organization. Social determinants and indigenous health: the international experience and its policy implications (Adelaide, Australia: The Commission on Social Determinants of Health (CSDH)). 2007. p. 1–41. https://bvsms.saude.gov.br/bvs/publicacoes/indigenous_health_adelaide_report_07.pdf.
- World Health Organization. Alma Ata declaration. Geneva: World Health Organization; 1978. p. 1–3.
- World Health Organization. WHO recommendations for the prevention and treatment of postpartum haemorrhage. Geneva Switzerland: WHO Press; 2012:1–48. Accessed 2017 Mar 9. http://apps.who.int/iris/bitstream/10665/75411/1/9789241548502_eng.pdf
- Schuurmans N, MacKinnon C, Lane C, et al. Prevention and management of postpartum haemorrhage. SOGC Clin Pract Guideline. 2000;22(4):271–281. https://doi.org/10.1016/S0849-5831(16)31530-0.
- Ford JB, Roberts CL, Simpson JM, et al. Increased postpartum hemorrhage rates in Australia. Int J Gynecol Obstet. 2007;98(3):237–243.
- Knight M, Callaghan WM, Berg C, et al. Trends in postpartum hemorrhage in high resource countries: a review and recommendations from the international postpartum hemorrhage collaborative group. BMC Pregnancy Childbirth. 2009;9(1):55.
- Joseph KS, Rouleau J, Kramer MS, et al. Investigation of an increase in postpartum haemorrhage in Canada. Bjog. 2007;114(6):751–759.
- Mehrabadi A, Liu S, Bartholomew S, et al. Temporal trends in postpartum hemorrhage and severe postpartum hemorrhage in Canada from 2003 to 2010. Obstet Gynaecol Can. 2014;36(1):21–33.
- Lutomski JE, Byrne BM, Devane D, et al. Increasing trends in atonic postpartum haemorrhage in Ireland: an 11‐year population‐based cohort study. Int J Gynecol Obstet. 2012;119(3):306–314.
- Callaghan WM, Kuklina EV, Berg CJ. Trends in postpartum hemorrhage: USA, 1994–2006. Am J Obstet Gynecol. 2010;202(4):353e1–353e6.
- Society of Obstetricians and Gynaecologists of Canada. SOGC policy statement: returning birth to aboriginal, rural, and remote communities. J Obstet Gynaecol Can. 2010;32(12):1186–1188.
- Arksey H, O’Malley L. Scoping studies: towards a methodological framework. Int J Soc Res Methodol. 2005;8(1):19–32.
- National Institutes of Health. National heart, lung, and blood institute. quality assessment tool for observational cohort and cross-sectional studies. 2019. https://www.nhlbi.nih.gov/health-topics/study-quality-assessment-tools.
- Chalouhi SE, Tarutis J, Barros G, et al. Risk of postpartum hemorrhage among Native American women. Int J Gynecol Obstet. 2015;131(3):269–272.
- Fenton JJ, Baumeister LM, Fogarty J. Active management of the third stage of labor among American Indian women. Fam Med. 2005;37(6):410–414.
- Humphrey MD. Is grand multiparity an independent predictor of pregnancy risk? A retrospective observational study. Med J Aust. 2003;179(6):294–296.
- Thomas WD. Maternal mortality in native British Columbia Indians, a high-risk group. Can Med Assoc J. 1968;99(2):64–67.
- Carpenter CW, Bryans FE. Maternal mortality in British Columbia: a study of 145 deaths from 1955 to 1962. Can Med Assoc J. 1965;92(4):160–170.
- Van Wagner V, Osepchook C, Harney E, et al. Remote midwifery in Nunavik, Québec, Canada: outcomes of perinatal care for the Inuulitsivik health centre, 2000-2007. Birth. 2012Sep;39(3):230–237. Epub 2012 Jun 29. PMID: 23281905.
- Carignan G. Pregnancies and birth among the inuit population of Hudson Bay. 1993. http://www.santecom.qc.ca/Bibliothequevirtuelle/santecom/35567000027323.pdf
- Baskett TF. Obstetric care in the central Canadian Arctic. Br Med J. 1978 Oct 7;2(6143):1001–1004. PMID: 709174; PMCID: PMC1607878.
- Tarlier D, Johnson J, Browne A, et al. Maternal-infant health outcomes and nursing practice in a remote first nations community in Northern Canada. Can J Nurs Res. 2013;45(2):76–100. previously 20.
- Reale SC, Easter SR, Xu X, et al. Trends in postpartum hemorrhage in the USA From 2010 to 2014. Anesth Analg. 2020May;130(5):e119–e122. PMID: 31567319.
- Begley CM, Gyte GM, Devane D, et al. Active versus expectant management for women in the third stage of labour. Cochrane Database Syst Rev. 2011 Nov 9;11:CD007412. Update in: Cochrane Database Syst Rev. 2015;3:CD007412. PMID: 22071837; PMCID: PMC4026059.
- Leduc D, Senikas V, Lalonde A, et al. Active management of the third stage of labour: prevention and treatment of postpartum hemorrhage. J Obstet Gynaecol Can. 2009;31(10):980–993.
- Menard, M. Kathryn MD, MPH; Main, Elliott K. MD: Currigan, Sean M. MPH Executive Summary of the reVITALize Initiative, Obstetrics & Gynecology: July 2014;124(1):150-153. doi:10.1097/AOG.0000000000000322.