ABSTRACT
Invasive Haemophilus influenzae type a (iHia) disease was detected in Alaska and Northern Canada in 2002 and 2000, respectively. From 2006 to 2017, 164 iHia cases (Alaska=53, Northern Canada=111) were reported. Rates of iHia disease per 100,000 persons were higher in Northern Canada compared to Alaska and were significantly higher in Indigenous (Alaska 2.8, Northern Canada 9.5) compared to non-Indigenous populations (Alaska 0.1, Northern Canada=0.4). Disease rates were highest in Indigenous children <2 years of age (Alaska 56.2, Northern Canada=144.1) and significantly higher than in non-Indigenous children <2 (Alaska 0.1, Northern Canada 0.4). The most common clinical presentation in children <5 years was meningitis of age and pneumonia in persons ≥5 years old. Most patients were hospitalised (Alaska=87%, Northern Canada=89%) and fatality was similar (Alaska=11%, Northern Canada=10%). MLST testing showed sequence types ST23 and ST576 in Northern Canada and ST576, ST23 and ST56 in Alaska. Alaska and Northern Canada have high rates of iHia disease. A vaccine is needed in these regions to protect young children.
Introduction
Haemophilus influenzae is a bacterial pathogen that can cause a range of illnesses from respiratory infections to severe invasive diseases such as meningitis, pneumonia and septic arthritis [Citation1]. The organism can be encapsulated or non-encapsulated (non-typeable); the encapsulated strains are labelled as serotypes a–f, with serotype b (Hib) historically identified as the leading cause of bacterial meningitis in children in the USA and Canada [Citation2]. Indigenous people in Alaska and Northern Canada have an increased risk for Hib disease and, prior to the introduction of the Hib vaccine, had some of the highest rates of Hib disease in the world [Citation3–5]. Incidence of invasive disease caused by this pathogen has declined dramatically since vaccine introduction, however, increases in disease caused by other serotypes, in particular, serotype a (Hia), have been noted [Citation6,Citation7]. Conjugate vaccines for Hib do not provide protection for Hia [Citation8]. Collaborative data sharing through the International Circumpolar Surveillance (ICS) project has highlighted the emergence of this serotype in Indigenous populations in Alaska and Northern Canada [Citation9,Citation10]. Here, we report on the continuing occurrence of invasive Hia disease in Arctic populations.
Methods
ICS is an international network of Arctic regions that share public health surveillance data on invasive bacterial diseases including Streptococcus pneumoniae, Haemophilus influenzae, Neisseria meningitidis, and groups A and B streptococci [Citation11–13]. Participants include northern provinces and territories in Canada (northern Labrador, northern Québec, Northwest Territories, Nunavut, and Yukon), Finland, Greenland, Iceland, Norway, Sweden (Norrbotten and Vasterbotten) and the US Arctic (Alaska). Network members collect cases through their respective surveillance systems and submit data to ICS headquarters at the US Centers for Disease Control and Prevention’s Arctic Investigations Program in Anchorage, Alaska, for compilation, analysis, reporting and publication. The ICS network was established in 1999; collection of H. influenzae data began in 2000. Participants in Canada, Greenland, Finland, Norway, Sweden and the US Arctic submitted data on H. influenzae. This analysis used only data from Canada and the US Arctic; the other participating regions either had no or very few Hia cases (Greenland, n=0, Norway, n=5) or did not provide serotype data (Finland, Sweden). A previous publication described Hia in the North American Arctic during the years 2000–2005 [Citation9]; this manuscript reports on cases identified during the years 2006–2017.
Annualised incidence rates were calculated using population estimates for Northern Canada and Alaska obtained from Statistics Canada [Citation14], and the Alaska Department of Labor and Workforce Development [Citation15], respectively. The Indigenous population denominator for Northern Canada was estimated using the Census of Population estimates and the 2011 National Household Survey from Statistics Canada [Citation14]. The estimated populations for Northern Canada and Alaska were 154,823 and 714,394, respectively; Indigenous people comprised 60% of the population in Northern Canada and 19% in Alaska.
A case of invasive H. influenzae disease was defined as illness in a resident of the surveillance area from whom H. influenzae was isolated or identified by polymerase chain reaction (PCR) from a normally sterile site sample including blood, cerebrospinal fluid, pleural fluid, peritoneal fluid, or joint fluid. Clinical manifestations of H. influenzae were determined by a review of the patient’s medical record.
Isolates from cases of invasive H. influenzae were forwarded to reference laboratories in Alaska and Northern Canada for species confirmation, serotyping and multilocus sequence typing (MLST). In Alaska, H. influenzae was confirmed by using Gram stain and factor X and V requirements (Differentiation Disks; Difco Laboratories, Detroit, MI, USA); a similar method is used in Canada (Oxoid, ON, Canada). Capsular serotyping was performed by slide agglutination with Difco antisera (Difco, Detroit, MI, USA) in ALASKA and (Difco, Oakville, ON, Canada) in Northern Canada (except the Laboratoire de Sante Publique in Quebec which used PCR). Serotype results in Alaska and Northern Canada were confirmed by PCR; PCR amplification of serotype-specific genes was done by using primers and probes as reported by Maaroufi, et al. [Citation16] (Alaska) or Falla, et al. [Citation17] (Northern Canada). MLST of seven housekeeping gene loci was done on all invasive Hia isolates in Alaska and Northern Canada according to a previously described method [Citation18]. The sequence type (ST) assignments were made by using the H. influenzae MLST website (http://haemophilus.mlst.net/).
Results
Overall Hi
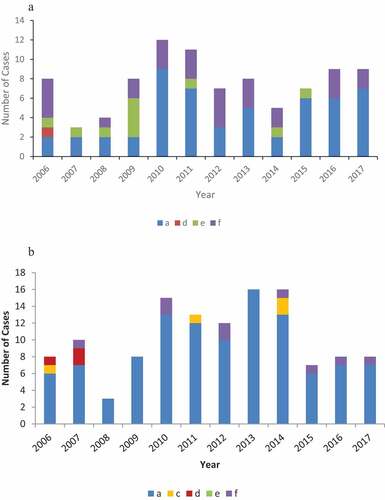
A total of 430 cases (Alaska n=253, Northern Canada n=177) of invasive Hi were identified during 2006–2017; 412 isolates from these cases (Alaska n=241, Northern Canada n=171) were available for serotyping. Of these, 151 isolates (37%) were non-typeable (Alaska n=127, Northern Canada n=24); the remaining 261 isolates were 164 (63%) Hia (Alaska n=53, Northern Canada n=111); 43 (17%) Hib (Alaska n=23, Northern Canada n=20), 4 (2%) Hic (Alaska n=0, Northern Canada n=4), 4 (2%) Hid (Alaska n=1, Northern Canada n=3), 10 (4%) Hie (Alaska n=10, Northern Canada n=0) and 36 (14%) Hif (Alaska n=27, Northern Canada n=9) ()).
Descriptive epidemiology
Among the 53 Alaska invasive Hia cases, 44 (83%) occurred in children <2 years of age, 5 (9%) occurred in children 2–4 years of age and the remaining 4 cases occurred in persons ranging from 8 to 77 years of age (). Median age was 0.9 years (10.8 months), and 62% of cases were male. In children <5 years old, 94% (n=45) were age appropriately vaccinated for Hib. Forty-eight (91%) cases occurred in Indigenous people. Six cases (11%) were fatal; three occurred in children <1 year old, two in children 2–4 years of age and one was an adult. In Northern Canada, 66% (n=73) of the 111 invasive Hia cases occurred in children <2 years of age, 9 (8%) occurred in children 2–4 years of age and the remaining 29 (26%) cases occurred in persons ranging from 5 to 80 years of age (). Median age was 1.4 years, and 52% of cases were male. In children <5 years old, 95% (n=69) were age appropriately vaccinated for Hib; 96% (n=107) of cases occurred in Indigenous people. Ten (10%) of the 100 cases with known outcome data were fatal; five cases occurred in children <1 year of age, one case was 2–4 years of age and four in persons >5 years old ().
Table 1. Characteristics of Alaskan and Northern Canadian persons with invasive Hia disease, 2006–2017.
Table 2. Cases and annualised incidence rate of invasive Hia disease by age and ethnicity, 2006–2017.
Incidence rates
In Alaska, the overall annualised crude invasive Hia incidence rate was 0.6 cases per 100,000 population (). In Alaska children less than 5 years old, the rate was 7.5 per 100,000 population and in children less than 2 years old, the rate was 16.9 per 100,000 population. In Indigenous children less than 2 years old, the rate was 51 times higher than that in non-Indigenous children (p<0.001). The overall annualised crude invasive Hia incidence rate in Northern Canada was six cases per 100,000 population. In Northern Canada children less than 5 years old, the rate was 50.1 per 100,000 population and in children less than 2 years old, the rate was 109.8 cases per 100,000 population. There was one case in Northern Canada non-Indigenous children less than 2 years old (6.1 per 100,000) compared to 73 cases (144.1 cases per 100,000) in Indigenous children in this age category. When stratified by race, rates were consistently significantly higher in the Alaska and Northern Canada Indigenous population than in non-Indigenous population.
Clinical illness
In children under the age of 5 years, the most common clinical illnesses due to invasive Hia infection were meningitis (45% Alaska, 27% Northern Canada), bacteraemia (26% Northern Canada) and pneumonia (25% Alaska). In individuals over the age of 5 years, pneumonia (25% Alaska, 46% Northern Canada) and bacteraemia (50% Alaska, 28% Northern Canada) were the most common clinical illnesses (). Eighty-seven per cent of all cases were hospitalised in Alaska and 90% in Northern Canada ().
Table 3. Clinical illnesses1 in Alaska and Northern Canada persons with invasive Hia by age, 2006–2017.
Subtyping data
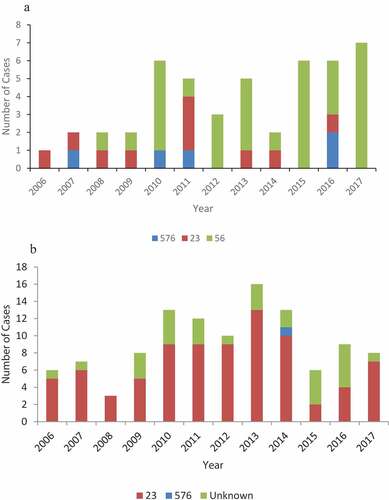
Subtyping of the Hia isolates by MLST showed that the Alaska isolates were comprised of three STs: ST576 (n=5, 11%), ST23 (n=10, 21%) and ST56 (n=32, 68%) () and of the 83 Northern Canada isolates with MLST data, 82 isolates were sequence type ST23 and 1 isolate was sequence type ST576 ().
Discussion
Hia was described in the literature as early as 1931 [Citation19], however, increases in incidence were not noted until the post-Hib vaccine era [Citation7,Citation9,Citation10,Citation20,Citation21]. Cases of invasive Hia have been detected worldwide, and the severity of disease caused by this infection, similar to Hib, warrants consideration of a vaccine for control [Citation21–25]. In the USA alone, the incidence of invasive Hia disease has increased more rapidly since 2002 (13% annually) than disease caused by other non-b serotypes or nontypeable strains [Citation20,Citation26]. Hib vaccine uptake among Hia cases in Alaska and Northern Canada suggests that if an Hia vaccine is developed, a high level of coverage could be achieved.
While secondary transmission of Hib was well known [Citation27,Citation28], epidemiologically linked Hia cases have only recently been reported in the literature [Citation26,Citation29]. Although there are no formal guidelines for control measures around Hia cases, the American Academy of Pediatrics Committee on Infectious Diseases has recommended that clinicians consider chemoprophylaxis for household contacts of a person with invasive Hia disease, as is recommended for Hib cases [Citation30]. The State of Alaska Department of Health and Social Services, Division of Public Health, has endorsed these recommendations for household contacts of confirmed invasive Hia cases in Alaska following an outbreak of Hia in a remote Alaska village [Citation31]. In Northern Canada, recommendations differ by region; in the Northwest Territories, Nunavik and Yukon, prophylaxis is recommended only for contacts of Hib cases; in Labrador and Nunavut, prophylaxis is recommended for contacts of any invasive Hi case prior to serotype results due to the lag time between case identification and lab testing (personal communication).
Our data show that invasive Hia continues to occur and be a concern in Arctic populations, particularly in Indigenous peoples. When compared to rates published in our previous collaborative analysis [Citation9], overall incidence in Alaska and Northern Canada has increased significantly; in Alaska the overall rate increased from 0.3 cases per 100,000 (2000–2005) to 0.6 cases per 100,000 (2006–2017) (p<0.001) and in Northern Canada the overall rate increased from 3.9 cases per 100,000 (2000–2005) to 6 cases per 100,000 (2006–2017) (p=0.04). Data from this analysis are consistent with previous collaborative work showing a high relatedness among strains [Citation9]; our current data demonstrate that 56 is the predominant sequence type in Alaska and 23 in Northern Canada; these two sequence types are known to be highly related [Citation21]. Indigenous peoples are over-represented in the occurrence of invasive Hia in Alaska and Northern Canada, comprising 91% and 96% of reported cases, respectively. The higher overall rates of invasive Hia in Northern Canada may be attributed to the higher proportion of Indigenous people (60%) in the population than in Alaska (19%). Hia incidence rates in Indigenous peoples are significantly higher overall, particularly in children, than in non-Indigenous persons. Reports published since the late 1990s have shown that Hia disproportionately affects Indigenous populations in North America and Australia [Citation5,Citation7,Citation20,Citation32].
The severity of disease caused by Hia invasive infection is comparable to that seen in invasive Hib cases prior to the introduction of the Hib vaccine. Meningitis is the most common disease in children less than 5 years old with Hia infection in Alaska and Northern Canada, presenting in 45% and 27% of cases, respectively. Studies describing the epidemiology of invasive Hib disease in Alaska and Northern Canada pre-vaccine also report meningitis as the most common disease associated with the bacterial infection as well as case fatality rates that are similar to invasive Hia infections [Citation33,Citation34]. Virulence associated with Hia infections has been previously linked to the bexA deletion [Citation35,Citation36]; however, this genetic mutation has not been found in previously tested Hia isolates from Alaska and Northern Canada [Citation9,Citation10]. Increased risk of respiratory infections in populations that experience household crowding, lack of in-home piped water, poor indoor air quality, remoteness, and poverty may contribute to increased rates and severity of Hia disease [Citation5,Citation23,Citation37,Citation38].
This study is limited by the lack of historical data available for non-b invasive H. influenzae disease prior to the introduction of Hib vaccine in Alaska and Northern Canada. Serotyping efforts at that time were primarily focused on identifying Hib cases; the non-b serotypes were often not specifically detected. This analysis does not include underlying condition data that might be helpful in identifying risk factors for invasive Hia disease. Although underlying condition data was collected during the time of this study, the data was not collected consistently.
Conclusions
This paper is an update to a previously published collaborative analysis of invasive Hia disease in Alaska and Northern Canada. Our data show that invasive Hia continues to cause morbidity and mortality among peoples of the North American Arctic, particularly Indigenous children. The severity of this bacterial infection as well as its increasing prevalence highlights the need for an Hia vaccine for use in Northern Canadian and Alaskan children.
Disclaimer
The findings and conclusions in this report are those of the author(s) and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
Acknowledgments
We thank staff at the Vaccine Preventable Bacterial Diseases Unit of the National Microbiology Laboratory, Winnipeg, MB, Canada, and the microbiology staff at Arctic Investigations Program, Anchorage, ALASKA, USA, for technical assistance. We also acknowledge the staff at clinical laboratories in Northern Canada and Alaska for their participation in this surveillance project.
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- Peltola H. H influenzae in the post-vaccination era. Lancet. 1993;341(8849):864–7.
- Waggoner-Fountain LA, Hendley JO, Cody EJ, et al. The emergence of Haemophilus influenzae types e and f as significant pathogens. Clin Infect Dis. 1995;21(5):1322–1324.
- Bisgard KM, Kao , A, Leake, J , et al. Haemophilus influenzae invasive disease in the USA, 1994-1995: near disappearance of a vaccine-preventable childhood disease. Emerg Infect Dis. 1998;4(2):229–237.
- Scheifele D, Halperin, S Law, B, et al. Invasive Haemophilus influenzae type b infections in vaccinated and unvaccinated children in Canada, 2001-2003. CMAJ. 2005;172(1):53–56.
- Tsang RS, Bruce MG, Lem M, et al. A review of invasive Haemophilus influenzae disease in the Indigenous populations of North America. Epidemiol Infect. 2014;142(7):1344–1354.
- Hammitt LL, Block S, Hennessy TW, et al. Outbreak of invasive Haemophilus influenzae serotype a disease. Pediatr Infect Dis J. 2005;24(5):453–456.
- Millar EV, O’Brien KL, Watt JP, et al. Epidemiology of invasive Haemophilus influenzae type A disease among Navajo and white Mountain Apache children, 1988-2003. Clin Infect Dis. 2005;40(6):823–830.
- Jin Z, Romero-Steiner S, Carlone GM, et al. Haemophilus influenzae type a infection and its prevention. Infect Immun. 2007;75(6):2650–2654.
- Bruce MG, Deeks SL, Zulz T, et al. Epidemiology of Haemophilus influenzae Serotype a, North American Arctic, 2000–2005. Emerg Infect Dis. 2008;14(1):48–55.
- Bruce MG, Zulz T, DeByle C, et al. Haemophilus influenzae serotype a invasive disease, Alaska, USA, 1983-2011. Emerg Infect Dis. 2013;19(6):932–937.
- Parkinson AJ, Bruce MG, Zulz T. International circumpolar surveillance, an Arctic network for the surveillance of infectious diseases. Emerg Infect Dis. 2008;14(1):18–24.
- Desai S, Li YA, Zulz T, et al. Monitoring invasive bacterial diseases in the North American arctic via the international circumpolar surveillance project. Can Commun Dis Rep. 2015;41(3):52–54.
- Bruce M, Zulz T, Koch A. Surveillance of infectious diseases in the Arctic. Public Health. 2016;137:5–12.
- Statistics Canada. 2020; Available from: https://www.statcan.gc.ca/eng/start.
- Department of Labor and Workforce Development. 2020; Available from: https://live.laborstats.Alaska.gov/pop/index.cfm.
- Maaroufi Y, De Bruyne J-M, Heymans C, et al. Real-time PCR for determining capsular serotypes of Haemophilus influenzae. J Clin Microbiol. 2007;45(7):2305–2308.
- Falla TJ, Crook DW, Brophy LN, et al. PCR for capsular typing of Haemophilus influenzae. J Clin Microbiol. 1994;32(10):2382–2386.
- Meats E, Feil EJ, Stringer S, et al. Characterization of Encapsulated and noncapsulated Haemophilus influenzae and determination of phylogenetic relationships by multilocus sequence typing. J Clin Microbiol. 2003;41(4):1623–1636.
- Pittman M. Variation and type specificity in the bacterial species Haemophilus influenzae. J Exp Med. 1931;53(4):471–492.
- Soeters HM, Blain A, Pondo T, et al. Current Epidemiology and trends in invasive Haemophilus influenzae disease-USA, 2009-2015. Clin Infect Dis. 2018;67(6):881–889.
- Tsang RSW, Ulanova M. The changing epidemiology of invasive Haemophilus influenzae disease: emergence and global presence of serotype a strains that may require a new vaccine for control. Vaccine. 2017;35(33):4270–4275.
- Ulanova M, Tsang RSW. Haemophilus influenzae serotype a as a cause of serious invasive infections. Lancet Infect Dis. 2014;14(1):70–82.
- Plumb ID, Lecy, KD, Singleton, R, et al. Invasive Haemophilus influenzae serotype a infection in children: clinical description of an emerging Pathogen-Alaska, 2002-2014. Pediatr Infect Dis J. 2018;37(4):298–303.
- Barreto L, Cox AD, Ulanova M, et al. The emerging Haemophilus influenzae serotype a infection and a potential vaccine: implementation science in action. Can Commun Dis Rep. 2017;43(5):85–88.
- Cox AD, Barreto L, Ulanova M, et al. Developing a vaccine for Haemophilus influenzae serotype a: proceedings of a workshop. Can Commun Dis Rep. 2017;43(5):89–95.
- Soeters HM, Oliver, SE, Plumb , ID, et al. Epidemiology of Invasive Haemophilus influenzae Serotype a Disease-USA, 2008-2017. Clin Infect Dis. 2021; 73(2):371–379 .
- MAlaskaintubee S, Istre GR, Ward JI. Transmission of invasive Haemophilus influenzae type b disease in day care settings. J Pediatr. 1987;111(2):180–186.
- Fleming DW, Leibenhaut, MH, Albanes, D, et al. Secondary Haemophilus influenzae type b in day-care facilities. Risk factors and prevention. JAMA. 1985;254(4):509–514.
- Vallejo JG, McNeil JC, Hultén KG, et al. Invasive Haemophilus influenzae disease at Texas Children’s Hospital, 2011 to 2018. Pediatr Infect Dis J. 2019;38(9):900–905.
- Kimberlin D, Lynfield R, Sawyer MH, et al. Red book: 2021-2024 Report of the committee on infectious diseases. 32 ed. Itasca IL: American Academy of Pediatrics; 2021.
- Update on Haemophilus influenzae type a invasive disease - Alaska, 2014-2018. Alaska Epidemiology Bulletin 2018 cited 2020 Jan 28; No. 9:[ Available from: http://epibulletins.dhss.Alaska.gov/Document/Display?DocumentId=1982.
- Menzies RI, Markey, P, Boyd, R, et al. No evidence of increasing Haemophilus influenzae non-b infection in Australian Aboriginal children. Int J Circumpolar Health. 2013; 72. doi:10.3402/ijch.v72i0.20992.
- Scheifele DW. Recent trends in pediatric Haemophilus influenzae type B infections in Canada. Immunization monitoring program, active (IMPACT) of the Canadian Paediatric Society and the Laboratory Centre for Disease Control. CMA2J. 1996;154(7):1041–1047.
- Ward JI, Lum MKW, Hall DB, et al. Invasive Haemophilus influenzae type b disease in Alaska: background epidemiology for a vaccine efficacy trial. J Infect Dis. 1986;153(1):17–26.
- Adderson EE, Byington CL, Spencer L, et al. Invasive Serotype a Haemophilus influenzae infections with a virulence genotype resembling Haemophilus influenzae Type b: emerging pathogen in the vaccine era? Pediatrics. 2001;108(1):E18.
- Lima JB, Ribeiro G, Cordeiro S, et al. Poor clinical outcome for meningitis caused by Haemophilus influenzae serotype A strains containing the IS 1016-bexA deletion. J Infect Dis. 2010;202(10):1577–1584.
- Hennessy TW, Ritter T, Holman RC, et al. The relationship between in-home water service and the risk of respiratory tract, skin, and gastrointestinal tract infections among rural Alaska natives. Am J Public Health. 2008;98(11):2072–2078.
- Wenger JD, Zulz T, Bruden D, et al. Invasive pneumococcal disease in Alaskan children: impact of the seven-valent pneumococcal conjugate vaccine and the role of water supply. Pediatr Infect Dis J2. 2010;29(3):251–256.