ABSTRACT
Sugars from sugar-sweetened beverages (SSBs) are an important risk factor for tooth decay. The study goal was to determine if there was variation in added sugar intake across communities and between and within households. In this cross-sectional study, intakes of total sugar, added sugar, and sugar-sweetened beverages (SSBs) were estimated for 282 Alaska Native children ages 0–10 years from 131 households in three Yukon-Kuskokwim (YK) Delta communities using biomarker equations based on hair carbon and nitrogen isotope ratios previously developed for the Yup’ik population. ANOVA was used to assess associations between each predictor (community and household) and outcome (estimated total sugars, added sugars, and SSB intake). Between- and within-household variation was estimated using a linear mixed-effects model with a random intercept for households with three or more children. There was no significant difference in mean estimated total sugar (p = 0.29), added sugar (p = 0.24), or SSB intake (p = 0.40) across communities. Significant variations were observed between and within households, with within-household variation amounting to 59% of the between-household variation. Added sugar intake in Alaska Native children from the three study communities is higher than the recommended maximum, and the variation is greater within households than between households.
Introduction
Tooth decay (dental caries) is the most prevalent childhood disease in the U.S., with low-income and minority children disproportionately affected [Citation1,Citation2]. The relationship between sugar intake and tooth decay is well established. Data from the U.S. National Health and Nutrition Examination Survey have demonstrated positive associations between added sugar intake (grams/day) and tooth decay for children [Citation3–6]. The diet of children is heavily influenced by their environment, including the household dietary milieu, but few studies have examined variation in sugar intake between and within households [Citation7,Citation8].
The prevalence of paediatric tooth decay is particularly high in Alaska Native (AN) communities. A study from 1991 by Jones et al. based on survey data reported that nearly two-thirds of AN preschoolers had untreated tooth decay, compared to about one-fifth of non-AN preschoolers [Citation9]. More recent data show that the high tooth decay prevalence persists for AN children [Citation10]. Results of the 2018–2019 Indian Health Services Oral Health Survey indicate that slightly more than 43% of American Indian and AN children between 3 and 5 years of age have untreated decay, compared to 10% for non-Hispanic white children [Citation11]. AN children are also almost 3 times as likely to have early childhood caries (ECC) than their white counterparts, a particularly severe form of tooth decay affecting children under age 6 years, with more than 71% of AN children experiencing ECC compared to about 25% for non-Hispanic white children [Citation11].
There are multiple explanations that contribute to these oral health inequities, including geographic remoteness, lack of access to fluoridated water, fluoride hesitancy, and sugar-sweetened beverage (SSB) consumption. SSBs are one of the most commonly reported dietary items in Yup’ik AN communities and are a sigificant source of sugar that leads to tooth decay [Citation12]. Interventions to address SSB intake depend on methods that can accurately measure added sugar intake. Self-reported measures (e.g. 24-hour dietary recalls, food frequency questionnaires) are susceptible to error and bias and can be time-consuming to collect [Citation13–15]. An alternative method of dietary assessment is the use of objective biomarkers measured in tissues such as red blood cells, plasma, hair, and nails. Past work demonstrates that a hair-based biomarker, using naturally-occurring variations in stable isotope ratios, is a valid way to measure total sugar, added sugar, and SSB intake in Yup’ik communities, and that it is feasible to collect hair samples from children as young as age 6 years [Citation16,Citation17]. The main advantage of using hair is that collection is non-invasive, quick, and acceptable to participants [Citation15]. Furthermore, sample storage and transport are easy and not temperature sensitive.
One notable gap in the scientific literature is the dearth of studies on community-level and household-level variation in added sugar intake, with no such studies in the YK Delta region. Area-level variation across different communities within discreet geographic areas has not been studied. Understanding the degree of heterogeneity in added sugar intake at the community and household levels would provide insight into developing relevant behavioural interventions that target SSB intake. More importantly, heterogeneity may be useful for interpreting the results of such interventions and indicate a need for different approaches based on baseline added sugar intake, with more intensive efforts aimed at communities and households with higher levels of added sugar intake. This study is part of larger efforts to develop community-based interventions aimed at helping households to reduce added sugar intake, preventing tooth decay, promoting oral health, and addressing oral health inequities. The purpose of this study was to investigate variation in added sugar intake at the household and community levels for Yup’ik AN children.
Methods
Study setting
The study was conducted in three AN communities in the Yukon-Kuskokwim (YK) Delta. The YK Delta is about 50,000 square miles in size and is one of the largest deltas in the world [Citation18]. The region has approximately 25,000 residents and 85% are of AN descent, most of whom are Yup’ik. The main service hub of the YK Delta is the city of Bethel, with 58 smaller surrounding communities. Foods such as fish and fish roe, seal, seabirds and akutaq (a traditional Alaska Native side dish or dessert) contribute about 20% of energy, and many residents participate to some degree in a traditional subsistence lifestyle of hunting, fishing, and gathering [Citation19,Citation20].
Research team and study personnel
This study was conducted by a research team from the University of Washington (UW) and University of Alaska Fairbanks (UAF). The study personnel consisted of faculty, postdoctoral trainees, professional research coordinators, and trained student research assistants. Study personnel recruited participants, obtained written informed consent from caregivers, and were trained on the hair collection protocol by UAF staff. The processing and analysis of collected hair was performed by UAF staff.
Study participants and recruitment procedures
The inclusion criteria to participate in this study were as follows: 1) Yup’ik AN child; 2) ages 0 to 10 years; 3) currently residing in the study community; and 4) written consent from the child’s primary caregiver and written assent from the child if age 8 years or older. There were no exclusion criteria. The Yup’ik communities were selected based on convenience sampling and past participation in oral health studies conducted by the team, but previous analyses indicate similar sociodemographic characteristics in these communities [Citation21]. Households with children in the target age range were contacted by study personnel using phone numbers made available through contact lists provided by the Yukon-Kuskokwim Health Corporation (YKHC) and from the local health clinic patient rosters. All potential participants met with study staff at the local health clinic to learn about the purpose of the study and to ask questions. Caregivers provided informed written consent for their child to participate in the study. Children ages 8 years and older provided written assent to participate in the study. The YKHC Human Studies Committee, YKHC Executive Board, and the Tribal Councils in the three communities reviewed and approved the study protocol as well as the final manuscript, and the study was approved by the University of Washington (UW) institutional review board.
Study procedures
Data were collected over a two-week period in October 2019. A 14-item paper questionnaire was administered by trained study personnel to each child’s caregiver that included questions about the child (age, sex, whether the child is Yup’ik, whether the child is a picky eater), caregiver (relationship to child, age, sex), and household (number of children and corresponding ages, extent to which Yup’ik food customs and traditions are followed) (Appendix). Questions were also asked regarding whether the child’s hair is coloured, the degree of colouring (partial vs. total), and whether the caregiver would be interested in a future intervention study. After the survey, the child’s weight was obtained through a digital scale (Digital Glass Bathroom Scale with Spa Blue, TaylorUSA) and recorded. The same digital scale was used in each study community, and the scale was zeroed before each data collection session. About 20 strands of hair were cut from the back of the child’s head as previously described, secured with a metal clip, labelled to indicate the cut end, and stored in a plastic bag [Citation17]. For very young children, the same procedure was followed with assistance from the caregiver. A sufficient hair sample was collected from all study participants. Participants were given $10 gift cards as a thank you for participation.
Assessment of sugars intake
Intakes of total sugar, added sugar, and sugar-sweetened beverages (SSB) were assessed using objective biomarkers measured in hair samples and model coefficients for each type of intake from a prior validation study [Citation22]. The approach uses natural variations in hair stable isotope ratios, specifically the carbon isotope ratio (CIR) and the nitrogen isotope ratio (NIR). Corn and sugar cane have elevated CIRs relative to other foods in the US food supply and are the source of most US added sugars [Citation23,Citation24]. In contrast, NIRs are associated primarily with marine food intake (fish and marine mammals) and are strongly associated with total consumption of traditional foods in the study population [Citation17,Citation25,Citation26]. In a validation study of 68 Yup’ik people ranging in age from 14 to 79 years, a model including both the CIR and NIR was associated with intakes of total sugar, added sugar, and sugar-sweetened beverages, as assessed using four 24-hour recalls [Citation22]. Those model coefficients were used in this study to estimate total sugar, added sugar, and SSB intake. In the validation study, sugar intakes, presented as geometric means (95% CI), were 89 (76, 103) g/d of total sugars, 74 (62, 88) g/d of added sugars, and 1.4 (1.1, 1.8) servings of SSB. For the youngest participants (ages 14–20 years, N = 11), intakes were 136 (109, 171) g/d total sugars, 115 (87, 151) g/d added sugars, and 2.3 (1.4, 3.4) servings of SSB. Total sugar was defined as the sum of all mono and disaccharides consumed and included primarily fructose, glucose, and sucrose. Added sugar was defined as the sum of sugars and syrups added to foods during food preparation and commercial food processing. SSB was the sum of servings of sweetened soft drinks and sweetened fruit drinks.
Laboratory procedures
Hair samples were hand-transported to UW and then mailed to UAF via FedEx for processing. This was to allow for batch mailing and tracking. Prior to analyses, a 1 cm portion of the hair sample most proximal to the scalp was cleaned, prepared, and analysed for CIR and NIR using continuous flow isotope ratio mass spectrometry at the Alaska Stable Isotope Facility, as previously described [Citation27]. Isotope ratios were measured as delta values, calculated as (Rsample/Rstandard − 1)∙1000‰, where Rsample is the ratio of heavy to light isotope in the sample and Rstandard is the ratio of heavy to light isotope in international standards (V-PDB for carbon and atmospheric N for nitrogen). Each 1 cm of hair captures dietary intake from approximately the previous 1–2 months [Citation18]. The remaining hair sample was destroyed and the 1 cm portion was destroyed during processing. No hair samples were banked.
Study variables
The outcome variables were as follows: (1) estimated total sugar intake (grams/day); (2) estimated added sugar intake (grams/day); and (3) estimated number of SSB servings. These were generated from δ13C and δ15N using previously estimated coefficients for a Yup’ik AN population as described above [Citation17,Citation27]. Child’s community and household were the main independent variables. Additional independent variables were as follows: (1) child’s age (continuous); (2) child’s sex (male/female); (3) caregiver-reported level of adherence to Yup’ik food customs and traditions (a lot/sometimes/never); (4) whether the child is a picky eater (no/yes); and (5) child’s weight (kg). Adherence to Yup’ik food customs and traditions was defined as participating in the traditional subsistence lifestyle such as hunting, fishing, and gathering supplies like berry picking as well as consuming traditional AN foods.
Statistical analysis
Demographic characteristics were summarised for all children in the study as well as for each study community. Differences between the three communities in child’s age and weight were assessed using one-way ANOVA. Differences between the three communities in child’s sex, whether the child is a picky eater, and consumption of traditional foods were assessed using a two-way chi-square test. Associations between each predictor and outcome variable were assessed using one-way ANOVA. The means of the outcome variables between communities were also compared. Between- and within-household variation in the three outcome variables was estimated using a linear mixed-effects model with a random intercept for household based on households with three or more children. All data analyses were conducted in R Version 4.0.3 [Citation28].
Results
Demographic characteristics
A total of 428 children were on the contact lists. A primary caregiver for 408 children were contacted by phone. Of the 408 children screened, 155 did not participate. Forty-six children of those who did not participate were not eligible because of age or because they had moved away from the community (N = 46); the remaining 109 children were eligible but were not interested (N = 22), were out of town (N = 21), or did not show up for their scheduled study visit (N = 66). An additional 29 child participants were recruited by very high frequency radio announcements, postings on Facebook, word of mouth, and posted advertisements in local clinics and other community spaces. A total of 282 children provided a hair sample for the study. The overall mean (SD) age was 5.3 (±3.0) years, and 51% of children were female. Most of the participating caregivers were mothers (65%), followed by fathers (14%), grandmothers (8.9%), and aunts (6.7%). When asked if they followed the traditional Yup’ik way of life, 57% of caregivers reported they followed these customs “a lot” and 43% reported following these customs “sometimes”. When asked if their child was a “picky eater”, 43% of caregivers reported yes. The mean weight of children was 51lbs (SD: 20lbs; range: 7–119 lbs).
A summary of the demographic characteristics of the children in each study community is presented in , where the community names are not reported to maintain privacy. There was no significant difference in demographic characteristics across the three communities.
Table 1. Demographic characteristics of the children in each of the study communities A, B, and C. Abbreviations: SD = standard deviation.
Outcome variables
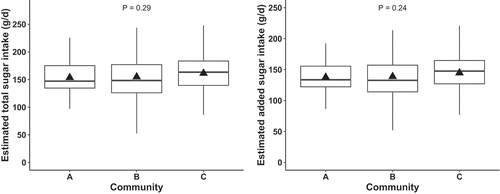
The overall daily mean estimated total sugar intake was 156 g (SD: 37 g), daily mean estimated added sugar intake was 140 g (SD: 31 g), and daily mean estimated intake of SSB was 2.7 servings (SD: 0.6 servings). There was no significant difference in mean estimated total sugar (p = 0.29) or added sugar (p = 0.24) intake across the three study communities (). Mean estimated SSB intake per day also did not vary significantly (p = 0.40) (data not shown). We note that total sugar, added sugar, and SSB intake estimates are highly correlated, because they are estimated from the same hair CIR and NIR measurements using different coefficients.
Figure 1. Boxplot of estimated total sugar and added sugar intake (g/d) in study communities A (n = 85), B (n = 125), and C (n = 72) as measured by hair biomarker for Yup’ik Alaska Native children ages 0 to 10 years in the Yukon-Kuskokwim Delta (N = 282). The triangle is the mean and the horizontal box plot lines correspond from bottom of box to top: 25th percentile (Q1), median percentile, and 75th percentile (Q3).
Within- vs. between-household variation
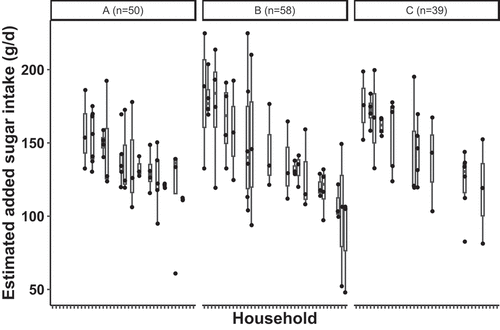
When considering within- and between-household variation for households with three or more children (40 households and 147 children), greater variation was observed within versus between households, with a standard deviation of ±32 g/d for total sugar intake, ±27 g/d for added sugar intake, and ± 0.55 for SSB servings per day (; total sugar intake and SSB not shown). However, between-household variation was also substantial. The standard deviation for the between-household total sugar intake of ±18 g/d, ±16 g/d for added sugar intake, and ± 0.32 for SSB servings per day (; total sugar intake and SSB not shown).
Figure 2. Boxplot of mean estimated added sugar intake (g/d) by household and community for households with 3 or more children in study communities A (n = 50), B (n = 58), and C (n = 39) as measured by hair biomarker for Yup’ik Alaska Native children ages 0 to 10 years in the Yukon-Kuskokwim Delta. The horizontal box plot lines correspond from bottom of box to top: 25th percentile (Q1), median percentile, and 75th percentile (Q3).
Factors associated with sugar intake
Children reported to be picky eaters had significantly higher mean estimated total sugar, added sugar, and SSB intake compared to non-picky eaters (165 versus 150 g total sugar/day, 148 versus 134 g added sugar/day, and 2.8 versus 2.6 servings SSB/day; all p < .001; ). Male children had slightly higher mean estimated total sugar, added sugar and SSB intake than female children; although only the differences in total and added sugar were significant (161 versus 152 g total sugar/day; p = 0.031; 144 for males versus 136 g added sugar/day for females; p = 0.029; ). Child’s age, adherence to Yup’ik customs and traditions, and child’s weight were not significantly associated with daily sugar intake, added sugar intake, or SSB servings.
Figure 3. Boxplot of mean estimated added sugar intake (g/d) by picky eater status (no [N = 160] and yes [N = 122]) as measured by hair biomarker for Yup’ik Alaska Native children ages 0 to 10 years in the Yukon-Kuskokwim Delta. The triangle is the mean and the horizontal box plot lines correspond from bottom of box to top: 25th percentile (Q1), median percentile, and 75th percentile (Q3).
![Figure 3. Boxplot of mean estimated added sugar intake (g/d) by picky eater status (no [N = 160] and yes [N = 122]) as measured by hair biomarker for Yup’ik Alaska Native children ages 0 to 10 years in the Yukon-Kuskokwim Delta. The triangle is the mean and the horizontal box plot lines correspond from bottom of box to top: 25th percentile (Q1), median percentile, and 75th percentile (Q3).](/cms/asset/fd92f1c4-c0b8-4d65-833f-08de745d13ad/zich_a_2336286_f0003_b.gif)
Figure 4. Boxplot mean estimated added sugar intake (g/d) by sex (female [N = 144] and male [N = 138]) as measured by hair biomarker for Yup’ik Alaska Native children ages 0 to 10 years in the Yukon-Kuskokwim Delta. The triangle is the mean and the horizontal box plot lines correspond from bottom of box to top: 25th percentile (Q1), median percentile, and 75th percentile (Q3).
![Figure 4. Boxplot mean estimated added sugar intake (g/d) by sex (female [N = 144] and male [N = 138]) as measured by hair biomarker for Yup’ik Alaska Native children ages 0 to 10 years in the Yukon-Kuskokwim Delta. The triangle is the mean and the horizontal box plot lines correspond from bottom of box to top: 25th percentile (Q1), median percentile, and 75th percentile (Q3).](/cms/asset/0d8cd87e-8b76-49fa-8f80-7e3ec9d0d991/zich_a_2336286_f0004_b.gif)
Discussion
In this study, hair samples were collected from children in three Yup’ik AN communities to test the association of added sugar intake between communities, between households within communities, and within households with multiple children. This study is part of efforts to develop a community-based intervention aimed at helping households improve their diets by reducing added sugar intake, which is expected to prevent tooth decay, promote oral health, and address oral health inequities. There were no significant differences in added sugar intake across the three study communities, but there were significant differences in between- and within-household variation in added sugar intake, with greater variation observed within households. Other significant variation in child-level factors between each of the study communities included being a picky eater and male sex.
Estimated sugar intake for children in all three study communities was high. The estimated total sugars per day (mean 156.1 g), estimated added sugars per day (mean 139.9 g) and estimated servings of SSB per day (mean 2.7) exceed the American Heart Association’s recommended maximum of 12 grams per day for children [Citation29]. These estimated values also exceed the average sugar intake for children in the U.S. by more than two-fold, with the American Heart Association reporting that American children consume 66 grams of added sugar per day [Citation30]. However, these results should be interpreted with caution because the biomarker was calibrated in the original study with adolescents and adults and could be overestimating total and added sugar and SSB intake by infants and young children in the current study [Citation27]. Nevertheless, added sugar intake in Yup’ik children has previously been found to contribute as much as 90% of the estimated total sugar intake, most of which was from SSBs [Citation27]. The results from this study are consistent with previous findings of high amounts of added sugars and SSB intake in Yup’ik communities [Citation21,Citation31]. Several reasons can contribute to these findings. First, the geographical remoteness of these communities makes it difficult for community members to access preventive care or healthy foods like fresh vegetables or fruits that are mainly delivered by plane [Citation32]. Community grocery stores are mostly stocked with processed foods, many of which contain high added sugars. Second, most AN communities do not have piped water because of permafrost [Citation33–35]. Lack of piped potable water makes it more likely for children to consume sugary beverages. In fact, sugar-sweetened beverages (SSBs), one of the most common dietary items in Yup’ik AN communities, comprise a disproportionately larger amount of added sugars to the diet [Citation12]. One recent study reported that three-year-old AN children were significantly more likely to consume a sugary drink (53% vs. 19%) and that children in Alaska’s rural Southwest region, which include Yup’ik AN communities, were also significantly more likely to consume sugary drinks compared to those in other regions (70–74% vs. 14–28%) [Citation18,Citation21]. In the United States, over 25% of children consume one or more non-diet sodas/week and 16% consume other sugar-sweetened beverages [Citation36]. Community-based sociobehavioral interventions are needed. One example is promoting healthier items at grocery stores like bottled water and milk – which might include making them more affordable for families – and selling sugar-free beverages to help reduce SSB intake, which could help prevent not only tooth decay, but also other systemic health conditions like obesity, diabetes, hypertension, and cardiovascular disease [Citation37–39].
While there was no significant difference in added sugar intake between communities, substantial between- and within-household differences were observed. Specifically, the finding that within-household variation amounted to 59% of the between-household variation is notable. There is no previous literature on between- or within-household variation in added sugar intake. One potential source of within-household variation may be different individual preferences for added sugars, which may need to be taken into consideration when designing home-based interventions aimed at reducing added sugars [Citation40,Citation41]. There may also be other within-household dynamics contributing to this variation, such as family structure, mealtime practices, and characteristics and activities of the child that may influence added sugar intake [Citation20,Citation42–44]. Further studies should focus on factors that lead to within-household variation in added sugar intake. These findings can be further investigated to inform tailored dietary recommendations or guidelines to individuals within households.
Other significant factors associated with added sugar intake in Yup’ik children included being a picky eater and male sex. The finding that picky eaters have higher added sugar intake than non-picky eaters is inconsistent with recent findings that children who are considered picky eaters are not substantially more likely to have different dietary behaviours and preferences than other children [Citation45,Citation46]. A child’s preference for a narrow range of specific food and beverage items may be relevant in the development of behavioural interventions to reduce sugar intake, especially those that involve replacement of beverages containing added sugars with those containing non-nutritive sweeteners. Caregivers may experience more resistance to dietary behaviour change from picky eaters, which underscores the importance of measuring whether the child is a picky eater, helping caregivers to anticipate resistance, and developing strategies to manage this resistance. Sex differences being significantly associated with added sugar intake is supported by previous studies. This may be attributed to males consuming greater volumes of foods and beverages than females. Another potential explanation is the differing sex roles in the traditional Yup’ik culture, with males having more autonomy (including dietary autonomy) compared to females, which may result in easier access to SSBs for boys [Citation47–49]. Future studies should investigate why male children consume more added sugar than females. It is surprising that no significant differences were observed by child’s age. While it is commonly assumed that sugar intake will generally increase by age, a recent study by Perrar et al. suggested that total sugar intake decreases with age [Citation50]. However, the study by Perrar et al. compared children and adolescents while this study only included participants that were younger. It is also possible that either a higher proportion of calories comes from added sugars among toddlers compared to older children from the study or simply that the toddlers in this study are consuming a high volume of calories. Confirmatory studies should be done to verify this association. These studies should include interventions that are culturally relevant to appropriately address SSB intake within the Yup’ik population.
This is the first known study to examine between- and within-household variation in added sugar intake, which is its main strength because this knowledge is important in understanding how to potentially design household-based interventions aimed at reducing added sugar intake. Still, there were four main study limitations. First, this study was limited to participating households in three communities in the YK Delta, which limits generalisability to Yup’ik communities. This study also focused on children under age 11 years and may not be generalisable to all households or households with older children. Further studies should assess the variation of sugar intake for older children in addition to younger children to improve the validity and generalisability of the study findings. Second, this study had a small sample size, making it difficult to generalise and assess population means. Further studies should confirm these findings with larger sample sizes to ensure consistent results in assessing population means. Third, the findings from this study should be interpreted with caution because the study outcome was measured exclusively using a hair biomarker that has been validated in Yup’ik individuals ages 14 years and older, but not in younger children. There is a need to validate these hair biomarker measurements in Yup’ik children aged 13 years and younger. Nevertheless, it may be that the biomarker functions better at capturing individual-level changes in added sugar intake rather than measuring absolute intake. Fourth, whether the child was breast-fed or formula-fed was not assessed in this study. Previous studies report that children who breastfeed have a lower prevalence of childhood obesity and caries, presumably because of lower added sugar intake. Further studies should assess the effects of breastfeeding on sugar intake among younger children.
Conclusions
Variation in biomarker-estimated total sugar, added sugar, and SSB intakes within and between households in rural Yup’ik communities were substantial; however, intakes did not vary between communities. Among Yup’ik children under age 11 years, the overall mean estimated total sugar intake was 156 ± 37 g, mean estimated added sugar intake was 140 ± 31 g, and mean estimated intake of SSB was 2.7 ± 0.6 servings. These estimates are substantially higher than dietary recommendations for children, with the caveat that estimates are based on biomarker evaluation and calibration studies in older children and adults. Further studies should explore factors that influence within-household variation in added sugar intake. The current findings can inform the development of community-based interventions aimed at reducing added sugar intake, preventing tooth decay, promoting oral health, and addressing other health problems attributed to added sugars including obesity and diabetes.
IJCH Appendix.docx
Download MS Word (15.1 KB)Acknowledgments
We would like to thank all the participating families in the Yukon-Kuskokwim Delta, without whom the study would not have been possible.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Supplementary material
Supplemental data for this article can be accessed online at https://doi.org/10.1080/22423982.2024.2336286
Additional information
Funding
References
- Marcenes W, Kassebaum NJ, Bernabé E, et al. Global burden of oral conditions in 1990-2010: a systemic analysis. J Dent Res. 2013;92(7):592–11. doi: 10.1177/0022034513490168
- Fleming E, Afful J. Prevalence of total and untreated dental caries among youth: United States, 2015–2016. NCHS Data Brief. 2018;307:1–8.
- Touger-Decker R, van Loveren C. Sugars and dental caries. Am J Clin Nutr. 2003;78(4):881S‐892S. doi: 10.1093/ajcn/78.4.881S
- Palmer CA, Kent R Jr, Loo CY, et al. Diet and caries-associated bacteria in severe early childhood caries. J Dent Res. 2010;89(11):1224‐1229. doi: 10.1177/0022034510376543
- Evans EW, Hayes C, Palmer CA, et al. Dietary intake and severe early childhood caries in low-income, young children. J Acad Nutr Diet. 2013;113(8):1057‐1061. doi: 10.1016/j.jand.2013.03.014
- Chi DL, Scott JM. Added sugar and dental caries in children: a scientific update and future steps. Dent Clin North Am. 2019;63(1):17‐33. doi: 10.1016/j.cden.2018.08.003
- Othman SI, Fertig A, Trofholz A, et al. How time in the US and race/ethnicity shape food parenting practices and child diet quality. Appetite. 2022;171:105870. doi: 10.1016/j.appet.2021.105870
- Valluri S, French SA, Elbel B, et al. Within- and between- household variation in food expenditures among low-income households using a novel simple annotated receipt method. Front Nutr. 2020;7:582999. doi: 10.3389/fnut.2020.582999
- Jones DB, Schlife CM. An oral health survey of head start children in Alaska. Arctic Med Res. 1991;Suppl:659–661.
- Phipps KR, Ricks RL The oral health of American Indian and Alaska Native children aged 1–5 years: results of the 2014 IHS oral health survey. Rockville (MD): Indian Health Services; 2015 [cited 2022 Nov 21]. Available from: https://www.ihs.gov/doh/documents/ihs_data_brief_1-5_year-old.pdf
- Phipps KR, Ricks TL, Mork NP, et al. The oral health of American Indian and Alaska Native children aged 1-5 years: results of the 2018-19 IHS oral health survey. In: Indian health service data brief. Rockville, MD: Indian Health Services; 2019.
- Kolahdooz F, Simeon D, Ferguson G, et al. Development of a quantitative food frequency questionnaire for use among the Yup’ik people of Western Alaska. PloS One. 2014;9(6):e100412. doi: 10.1371/journal.pone.0100412
- Subar AF, Kipnis V, Troiano RP, et al. Using intake biomarkers to evaluate the extent of dietary misreporting in a large sample of adults: the OPEN study. Am J Epidemiol. 2003;158(1):1–13. doi: 10.1093/aje/kwg092
- Davy BM, Jahren AH, Hedrick VE, et al. Association of δ13C in fingerstick blood with added-sugar and sugar-sweetened beverage intake. J Am Diet Assoc. 2011;111(6):874–878. doi: 10.1016/j.jada.2011.03.019
- Prentice RL, Mossavar-Rahmani Y, Huang Y, et al. Evaluation and comparison of food records, recalls, and frequencies for energy and protein assessment by using recovery biomarkers. Am J Epidemiol. 2011;174(5):591–603. doi: 10.1093/aje/kwr140
- Chi DL, Hopkins S, O’Brien D, et al. Association between added sugar intake and dental caries in Yup’ik children using a novel hair biomarker. BMC Oral Health. 2015;15(1):121. doi: 10.1186/s12903-015-0101-z
- Nash SH, Kristal AR, Hopkins SE, et al. Stable isotope models of sugar intake using hair, red blood cells, and plasma, but not fasting plasma glucose, predict sugar intake in a Yup’ik study population. J Nutr. 2014;144(1):75‐80. doi: 10.3945/jn.113.182113
- Jorgenson MT, Roth JE. Landscape classification and mapping for the yukon-kuskokwim delta: final report prepared for: U.S. Fish and wildlife service. Fairbanks, AK, USA: ABR, Inc.-Environmental Research & Service; 2010. 24.
- Bersamin A, Zidenberg-Cherr S, Stern JS, et al. Nutrient intakes are associated with adherence to a traditional diet among Yup’ik Eskimos living in remote Alaska native communities: the CANHR study. Intern J Circum Health. 2007;66(1):62–70. doi: 10.3402/ijch.v66i1.18228
- Nash SH, Bersamin A, Kristal AR, et al. Stable nitrogen and carbon isotope ratios indicate traditional and market food intake in an indigenous circumpolar population. J Nutr. 2012;142(1):84–90. doi: 10.3945/jn.111.147595
- Alaska Department of Health and Social Services. Alaska physical activity, nutrition and obesity facts report – 2020 update. [cited 2023 Sep 13]. Available from: https://health.alaska.gov/dph/Chronic/Documents/Obesity/pubs/2020_AKPANFacts.pdf
- Nash SH, Kristal AR, Bersamin A, et al. Carbon and nitrogen stable isotope ratios predict intake of sweeteners in a Yup’ik study population. J Nutr. 2013;143(2):161–165. doi: 10.3945/jn.112.169425
- O’Brien DM. Stable isotope ratios as biomarkers of diet for health research. Annu Rev Nutr. 2015;35(1):565–594. doi: 10.1146/annurev-nutr-071714-034511
- Davy B, Jahren H. New markers of dietary added sugar intake. Curr Opin Clin Nutr Metab Care. 2016;19(4):282–288. doi: 10.1097/MCO.0000000000000287
- Choy K, Nash SH, Hill C, et al. The nitrogen isotope ratio is a biomarker of Yup’ik traditional food intake and reflects dietary seasonality in segmental hair analysis. J Nutr. 2019;149(11):1960–1966. doi: 10.1093/jn/nxz144
- Nash SH, Kristal AR, Boyer BB, et al. Relation between stable isotope ratios in human red blood cells and hair: implications for using the nitrogen isotope ratio of hair as a biomarker of eicosapentaenoic acid and docosahexaenoic acid. Am J Clin Nutr. 2009;90(6):1642–1647. doi: 10.3945/ajcn.2009.28482
- Votruba SB, Shaw PA, Oh EJ, et al. Associations of plasma, RBCs, and hair carbon and nitrogen isotope ratios with fish, meat, and sugar-sweetened beverage intake in a 12-wk inpatient feeding study. Am J Clin Nutr. 2019;110(6):1306–1315. doi: 10.1093/ajcn/nqz208
- R Core Team. R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing; 2022 Version 4.0.3. Available from: https://www.R-project.org/
- Johnson RK, Appel LJ, Brands M, et al. Dietary sugars intake and cardiovascular health: a scientific statement from the American Heart Association. Circulation. 2009;120(11):1011–1020. doi: 10.1161/CIRCULATIONAHA.109.192627
- American Heart Association. How much sugar is too much? 2023. [cited 2023 June 14]. Available from: https://www.heart.org/en/healthy-living/healthy-eating/eat-smart/sugar/how-much-sugar-is-too-much#:~:text=The%20numbers%20are%20even%20worse,this%20added%20sugar%20coming%20from%3F
- Potempa AE, Kelsey LC, Fink KJ, et al. Alaska’s Play every day campaign encourages parents to serve healthy drinks to young children. Health Promot Pract. 2022;23(1):128S~139S. doi: 10.1177/15248399221115763
- America’s Health Rankings. Water fluoridation in Alaska. 2022. [cited 2022 Dec 2]. Available from: https://www.americashealthrankings.org/explore/annual/measure/water_fluoridation/state/AK
- Chi DL. Reducing Alaska Native paediatric oral health disparities: a systematic review of oral health interventions and a case study on multilevel strategies to reduce sugar-sweetened beverage intake. Intern J Circum Health. 2013;72(1):21066. doi: 10.3402/ijch.v72i0.21066
- Atkins CY, Thomas TK, Lenaker D, et al. Cost-effectiveness of preventing dental caries and full mouth dental reconstructions among Alaska Native children in the Yukon-Kuskokwim delta region of Alaska. J Public Health Dent. 2016;76(3):228–240. doi: 10.1111/jphd.12141
- Fuente D, Mosites E, Bressler S, et al. Health-related economic benefits of universal access to piped water in Arctic communities: estimates for the Yukon-Kuskokwim Delta region of Alaska. Int J Hyg Environ Health. 2022;240:113915. doi: 10.1016/j.ijheh.2021.113915
- Wang YC, Bleich SN, Gortmaker SL. Increasing caloric contribution from sugar-sweetened beverages and 100 % fruit juices among US children and adolescents, 1988–2004. Pediatrics. 2008;121(6):e1604–14. doi: 10.1542/peds.2007-2834
- Pan L, Li R, Park S, et al. A longitudinal analysis of sugar-sweetened beverage intake in infancy and obesity at 6 years. Pediatrics. 2014;134(1 Suppl):29S–35S. doi: 10.1542/peds.2014-0646F
- Nguyen S, Choi HK, Lustig RH, et al. Sugar-sweetened beverages, serum uric acid, and blood pressure in adolescents. J Pediatr. 2009;154(6):807–813. doi: 10.1016/j.jpeds.2009.01.015
- Kosova EC, Auinger P, Bremer AA. The relationships between sugar-sweetened beverage intake and cardiometabolic markers in young children. J Acad Nutr Diet. 2013;113(2):219–227. doi: 10.1016/j.jand.2012.10.020
- Liem DG, de Graaf C. Sweet and sour preferences in young children and adults: role of repeated exposure. Physiol Behav. 2004;83(3):421–429. doi: 10.1016/j.physbeh.2004.08.028
- Barragán R, Coltell O, Portolés O, et al. Bitter, sweet, salty, sour and umami taste perception decreases with age: sex-specific analysis, modulation by genetic variants and taste-preference associations in 18 to 80 year-old subjects. Nutrients. 2018;10(10):1539. doi: 10.3390/nu10101539
- Mou Y, Jansen PW, Raat H, et al. Association of family feeding and mealtime practices with children’s overall diet quality: results from a prospective population-based cohort. Appetite. 2021;160:105083. doi: 10.1016/j.appet.2020.105083
- Mateos-Agut M, Garcia-Alonso I, De la Gandara-Martin JJ, et al. Family structure and eating behavior disorders. Actas Esp Psiquiatr. 2014;42(6):267–280.
- An R. Diet quality and physical activity in relation to childhood obesity. Int J Adolesc Med Health. 2017;29(2):20150045. doi: 10.1515/ijamh-2015-0045
- Taylor CM, Emmett PM. Picky eating in children: causes and consequences. Proc Nutr Soc. 2019;78(2):161–169. doi: 10.1017/S0029665118002586
- Samuel TM, Musa-Veloso K, Ho M, et al. A narrative review of childhood picky eating and its relationship to food intakes, nutritional status, and growth. Nutrients. 2018;10(12):1992. doi: 10.3390/nu10121992
- Jolles CZ. Yupik Eskimos. In: Ember CR Ember M, editors. Encyclopedia of sex and gender. Boston, MA: Springer; 2003.
- Ayunerak P, Alstrom D, Moses C, et al. Yup’ik culture and context in Southwest Alaska: community member perspectives of tradition, social change, and prevention. Am J Community Psychol. 2014;54(1–2):91–99. doi: 10.1007/s10464-014-9652-4
- Ackerman LA, Ackerman LA. Gender status in Yup’ik society. Etud Inuit. 1990;14(1/2):209–221.
- Perrar I, Schmitting S, Corte KWD, et al. Age and time trends in sugar intake among children and adolescents: results from the DONALD study. Eur J Nutr. 2020;59(3):1043–1054. doi: 10.1007/s00394-019-01965-y
Appendix
Appendix
Added Sugar Intake Measurement Questionnaire
(Administered verbally and in-person)
1. How old are you? _____________
2. What’s your sex? Male/Female
3. How many children in your household and how old are they?
# of children: _____________________
Ages of them: ______________________
Please answer the following questions regarding the child who participates in the study.
4. First, we would like to weigh your child. Please have your child stand on the scale.
Weight: ___________ (lb)
5. What’s your relationship to the child? (please circle one)
6. How old is your child? _____________
7. What’s your child’s sex? Male/Female
8. In general, is your child a picky eater? Yes/No
9. People talk to us about the traditional ways of living. How much do you follow the traditional Yup’ik way of life? A lot/Sometimes/Not at all
10. Is your child Yup’ik? Yes/No
11. Is your child’s hair currently colored? Yes/No
12. If yes to #2, is all of your hair coloured or just sections? All/Just Sections
13. If yes to #2, please circle all of the below types of hair colouring products used
14. Would you be interested in participating in future research? Yes/No
