Abstract
Daptomycin is currently registered for use in treatment of complicated skin and skin structure infections (cSSSI) and Staphylococcus aureus bacteraemia. The role of daptomycin in treatment of severe Gram-positive infections needs to be considered outside of these specific and rigid indications. Based on surveillance data, supporting literature and within the context of antimicrobial stewardship, the South African Society for Clinical Microbiology (SASCM) provides recommendations for appropriate use of daptomycin. These recommendations were formulated by members of the National Antimicrobial Committee (NAC), a sub-committee of SASCM, following consultation and review of the relevant literature.
Introduction
Daptomycin belongs to the class of antimicrobial agents known as cyclic lipopeptides. It exhibits bactericidal activity against most Gram-positive bacteria through a unique mechanism of action that involves cationic binding to the cytoplasmic cell membrane causing rapid depolarisation of membrane potential.
Daptomycin has a very low frequency of spontaneous development of resistance in vitro, although resistance development in vivo has been described in a methicillin-resistant Staphylococcus aureus (MRSA) endocarditis patient on daptomycin treatment.Citation1 Resistance to daptomycin in S. aureus is heterogenous, similar to that observed for ß-lactams and glycopeptides; and, at present, there is no single mutation or mechanism of action that confers resistance.Citation2 The extremely low occurrence of resistant strains has precluded the determination of a resistant clinical breakpoint by the Clinical Laboratory Standards Institute (CLSI), although the European Committee on Antimicrobial Susceptibility Testing (EUCAST) has defined a resistant clinical breakpoint of > 1 μg/ml.Citation3,4
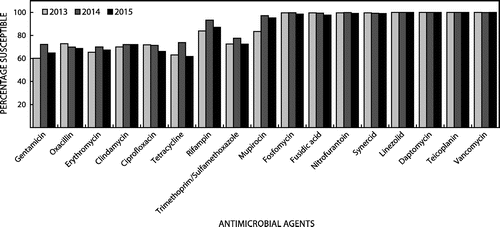
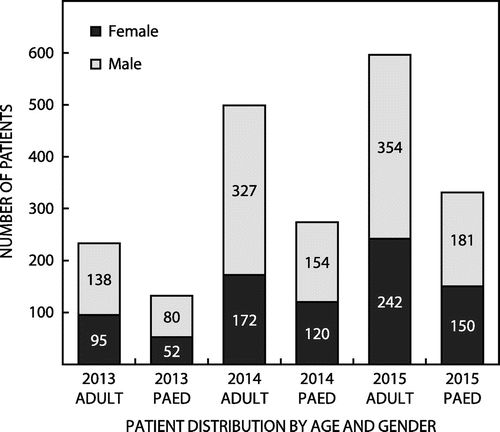
The Centre for Opportunistic, Tropical and Hospital Infections (COTHI) at the National Institute for Communicable Diseases (NICD) is performing national public sector surveillance for S. aureus bacteraemia (SAB), with enhanced surveillance in Gauteng (three sentinel sites) and the Western Cape (two sentinel sites). Figure illustrates the national demographics of 2065 patients with SAB over a three-year period with distribution by age group and gender. Selected antimicrobial susceptibility testing (AST) results for isolates from the enhanced surveillance sites is presented in Figure . These data highlight the uniform susceptibility over a three-year period to daptomycin, linezolid and the glycopeptides. There has been a slight increase in the MRSA rate over the same period.
Figure 1: Age and gender distributions of the 2065 patients with S. aureus bacteraemia over a three-year period.
These daptomycin surveillance data is concordant with international surveillance data, where Sader et al. have demonstrated no increasing resistance to daptomycin over an eight-year period.Citation5 In this surveillance program, including 164 457 Gram-positive isolates, non-susceptibility rates are less than 0.2%, with S. aureus non-susceptibility recorded in only 0.05% of isolates (total of 97 542 S. aureus isolates). These data indicate that, despite prolonged availability and use of daptomycin, current non-susceptibility rates are very low and periodic surveillance seems appropriate for monitoring susceptibility rates.
Daptomycin is currently registered for use in treatment of complicated skin and skin structure infections (cSSSI) and S. aureus bacteraemia including right-sided S. aureus endocarditis. The role of daptomycin in treatment of severe Gram-positive infections needs to be considered outside of these specific and rigid indications. Based on surveillance data, supporting literature and within the context of antimicrobial stewardship SASCM provides recommendations for appropriate use of daptomycin. These recommendations were formulated by members of the National Antimicrobial Committee (NAC), a sub-committee of SASCM, following consultation and review of the relevant literature.
Recommendations for clinical use of daptomycin
Empiric therapy
Daptomycin can be considered for empirical antimicrobial therapy in severe infections where broad-spectrum Gram-positive cover is necessary. This is supported by current literature where Bassetti et al. demonstrated a 10-fold higher risk of mortality in patients receiving empiric vancomycin therapy, as compared to high-dose (8 – 10 mg/kg) daptomycin therapy.Citation6
The following principles and guidelines apply to daptomycin’s use as an empiric agent:
| 1. | Daptomycin is an alternative/substitute to glycopeptides (vancomycin or teicoplanin) and linezolid, where these agents may be considered as part of an empiric regimen. | ||||
| 2. | The lower respiratory tract (LRT) must be excluded as a potential source of sepsis. If there is any concern or possibility that the LRT is the source then daptomycin should not be prescribed. This is due to the inactivation of daptomycin by pulmonary surfactant.Citation7 Diagnostic stewardship should be incorporated as a component of the antimicrobial stewardship program to assist in this determination. | ||||
| 3. | Antimicrobial stewardship principles should be adhered to in the use of this agent. There should be a valid reason and necessity for use of a broad-spectrum Gram-positive agent. Daptomycin should not be used in place of narrower-spectrum agents where these are appropriate. e.g. if S. aureus cover is necessary but the risk of MRSA is low, daptomycin should not be prescribed in place of cloxacillin/ß-lactams. Similarly de-escalation to a narrower-spectrum agent is mandatory following susceptibility results. | ||||
Directed therapy
| 1. | Although daptomycin has very specific clinical and microbiological indications, there is wide experience and supporting literature for its use outside of these indications. From a clinical perspective, other than lower respiratory tract infections, it can be considered equivalent to its counterparts, viz. glycopeptides and linezolid. Daptomycin broad-spectrum activity is suitable as directed therapy against a number of Gram-positive pathogens including MRSA but also streptococci and enterococci based on rational recommendations from the antimicrobial stewardship team. However, it is not considered a substitute for ß-lactam agents as they remain the preferred treatment option when susceptibility to these agents is retained. In certain clinical instances, e.g. hypersensitivity reaction to ß-lactam agents, daptomycin may be a suitable alternative, although it is recommended that these be discussed with a clinical microbiologist. | ||||
| 2. | For enterococci, especially vancomycin-resistant enterococci (VRE), daptomycin has a distinct role in directed therapy. It is an acceptable alternative to linezolid for directed therapy of VRE-associated infections. Dosing-modification (see below) is necessary when used as directed therapy for enterococcal infections. | ||||
| 3. | Daptomycin can also be used as part of a combination-directed therapy regime for specific difficult-to-treat infections, e.g. infective endocarditis; osteomyelitis; prosthesis-related infections, etc. The rationale here is similar to that for high-dose therapy, where pharmacodynamic drug exposures may be suboptimal due to patient-related factors (augmented renal clearance, poor penetration to target site) or microbe-related factors (elevated MIC, biofilm formation, host defense protein resistance). Additionally, there may be situations where combination therapy mitigates against resistance. Daptomycin activity is highly dependent on binding to the cell membrane, which is influenced by the net surface charge. Concomitant antimicrobial therapy that inhibits modification of the net surface charge facilitates binding of daptomycin, thus countering resistance mechanisms. There are reported synergistic interactions between daptomycin and a variety of antimicrobial agents including: ß-lactams (ampicillin, cloxacillin, ceftaroline); fosfomycin; gentamicin; trimethoprim-sulfmethoxazole; and, rifampicin.Citation8 | ||||
Dosing of daptomycin
The registered doses for daptomycin are 4 mg/kg daily for cSSSI and 6 mg/kg daily for bacteraemia and endocarditis. Daptomycin is a concentration-dependent agent and demonstrates a prolonged post-antibiotic effect (~6.8 hours). It is 90% protein bound and has a long half-life (~8 hours), allowing for once-daily dosing.Citation2 In patients with renal failure (GFR of <30 mls/min), the dosing interval is adjusted from 24 to 48 hours.
The pharmacokinetic/pharmacodynamic (Pk/Pd) parameter for significant bactericidal activity is the AUCfree/MIC ratio, and is organism-dependent ranging from 16.5 to 189.Citation9 The higher exposures are MIC-driven. Experimental in vivo data indicate that for a standard 4 mg/kg daily dose there is a 95% probability of target attainment for S. aureus and ß-haemolytic streptococci at an MIC ≤ 2 μg/ml; and, a 96.2% probability of target attainment for Enterococcus faecalis and Enterococcus faecium at an MIC ≤ 8 μg/ml.Citation2,10 Monte Carlo simulations using pharmacokinetic data from clinical studies indicate that for S. aureus at an MIC ≤ 1 μg/ml, the probability of achieving a bacteriostatic effect is: (i) 65.7 – 97.4% for a dose of 6 mg/kg daily; (ii) 96.3 – 99.9% for a dose of 8 mg/kg daily; and, (iii) 99.8 – 100% for a dose of 10 mg/kg daily.Citation11 In the Clinical Practice Guideline by the Infectious Diseases Society of America (IDSA), higher daily doses of 8 – 10 mg/kg of daptomycin are recommended by some experts for treatment of bacteraemia, as well as endocarditis, with a level B-III recommendation.Citation12 EUCAST recommends a high dose (defined as >8 mg/kg daily) for treatment of enterococcal infections.Citation13
The Pk/Pd analysis of daptomycin supports use of higher doses, although this is largely based on MIC data; however, standard doses are sufficient for the majority of indications. This is supported by global surveillance data where the MIC90 for almost 100 000 S. aureus isolates remains at 0.5 μg/ml.Citation5 However, there are reasons and specific indications for high-dose therapy, and these need to be considered to avoid clinical failure and possible development of resistance. The rationale and specific indications for high-dose daptomycin are detailed below.
| 1. | Rationale for higher doses of daptomycin
| ||||||||||||||||||||||||||||||||||||||||
| 2. | Recommendations for higher doses of daptomycin
| ||||||||||||||||||||||||||||||||||||||||
Adverse events in the context of high-dose daptomycin
Side effects associated with use of daptomycin, like many antimicrobial agents, are minimal but may include the following: hypersensitivity reactions; elevation in creatinine phosphokinase (CPK) levels and associated myopathy; eosinophilic pneumonia; and, renal toxicity. The majority of studies evaluating side effects associated with its use have been in the context of registered doses of 4 – 6 mg/kg. There is obvious concern that at higher doses these side effects may become more prominent and a limiting factor in its use.
The majority of studies conducted to date have been retrospective analyses of case studies where high-dose daptomycin has been used. These studies generally indicate that high-dose daptomycin therapy is safe, and there are very few instances where discontinuation of therapy is necessitated by an adverse event.Citation8 A limitation to these analyses includes the varied dosing regimens and the actual definition of high-dose therapy. The two largest studies include a retrospective review of 102 patients treated with high-dose daptomycin for infective endocarditis, and an outcomes registry database established by the pharmaceutical company, including 1097 patients who received high-dose therapy.Citation21,22
The infective endocarditis study by Durante-Mangoni et al. is a single-centre study evaluating their experience in treating this clinical entity with daptomycin over a seven-year period.Citation21 The authors defined a high dose as >6 mg/kg/day, and had 94 microbiologically confirmed cases. The median dose of daptomycin received was 8.2 mg/kg (range 6.1 – 11.4 mg/kg), with a mean duration of treatment of 21.7 ±12.3 days (range 1 – 60 days, median 20 days). In total, 15 patients experienced a CPK increase (13 mild, CPK ≤ 5 X ULN; 2 moderate, CPK > 5 X ULN), of which four discontinued therapy. Renal toxicity, as defined by the Risk Failure Injury Loss End stage renal disease criteria, occurred in nine patients with none of the patients requiring discontinuation of therapy. Eosinophilia was observed in 16 patients, of which two had respiratory symptoms compatible with interstitial lung disease. Six of these patients were also receiving concurrent ß-lactam therapy. Four patients developed an allergic or idiosyncratic reaction. The authors of this study conclude that high-dose daptomycin therapy was associated with good clinical outcomes and a favourable safety profile. In the context of their study, there were no treatment-related deaths; and, prolongation of hospital stay attributable to the high-dose daptomycin occurred in two patients.
The European Cubicin® Outcomes Registry and Experience (EU-CORE) is a non-interventional, multicenter, retrospective patient registry of all patients treated with daptomycin. An analysis of the database identified 452 patients who received daptomycin doses of >6 to <8 mg/kg/day, and 645 patients who received daptomycin doses of ≥8 mg/kg/day. In these two sub-groups, a serious adverse event was reported in 62 (13.7%) and 57 (8.8%) patients, respectively. Discontinuation of therapy prompted by an adverse event occurred in 23 (5.1%) and 30 (4.7%) patients, which compared favourably with the 195 (4.0%) patients on a dose of < 6 mg/kg/day.Citation22
| 1. | Recommendations for monitoring for adverse events when using high-dose daptomycin therapy
| ||||||||||||||||||||||||||||||||||||||||||||||
It is important that we utilise our limited armamentarium of antimicrobial agents in a responsible and appropriate manner. The importance of choosing the most appropriate drug for the isolated bug, in the context of a specific infection and with appropriate dosing so as to optimise the pharmacodynamic exposure cannot be over-emphasised. Patient outcome is dependent on dose optimisation, thus utilisation of antimicrobials must be carefully evaluated from a clinico-microbiological perspective.Citation23,24 Daptomycin is no exception and prudent use is required, yet its value in treating serious Gram-positive infections should not be underestimated, and daptomycin should be considered as a useful alternative where applicable.
Acknowledgements
NAC members: Norma Bosman (National Health Laboratory Services), Ruth Lekalakala (National Health Laboratory Services), Krishnee Moodley (Lancet Laboratories), Madeleine Olivier (Pathcare), Khine Swe Swe Han (National Health Laboratory Services), Juno Thomas (Consultant Clinical Microbiologist) and Andrew Whitelaw (National Health Laboratory Services).
References
- Dortet L, Anguel N, Fortineau N, et al. In vivo acquired daptomycin resistance during treatment of methicillin-resistant Staphylococcus aureus endocarditis. Int J Infect Dis: IJID: Official Publication of the International Society for Infectious Diseases. 2013;17(11):e1076–7.10.1016/j.ijid.2013.02.019
- Humphries RM, Pollett S, Sakoulas G. A current perspective on daptomycin for the clinical microbiologist. Clin Microbiol Rev. 2013;26(4):759–80.10.1128/CMR.00030-13
- CLSI. Performace standards for antimicrobial susceptibility testing: CLSI supplement M100S. 26th ed. Wayne: Clinical and Laboratory Standards Institute; 2016.
- EUCAST. The European committee on antimicrobial susceptibility testing. Breakpoint tables for interpretation of MICs and zone diameters. Available from: http://www.eucast.org2016
- Sader HS, Farrell DJ, Flamm RK, et al. Daptomycin activity tested against 164457 bacterial isolates from hospitalised patients: summary of 8 years of a Worldwide Surveillance Programme (2005–2012). Int J Antimicrob Agents. 2014;43(5):465–9.10.1016/j.ijantimicag.2014.01.018
- Bassetti M, Ansaldi F, De Florentiis D, et al. Is empiric daptomycin effective in reducing mortality in Staphylococcus aureus bacteraemia? A real-life experience Intensive Care Med. 2015;41(11):2026–8.10.1007/s00134-015-4021-9
- Silverman JA, Mortin LI, VanPraagh AD, et al. Inhibition of daptomycin by pulmonary surfactant: in vitro modeling and clinical impact. J Infect Dis. 2005;191(12):2149–52.10.1086/jid.2005.191.issue-12
- Gould IM, Miró JM, Rybak MJ. Daptomycin: the role of high-dose and combination therapy for Gram-positive infections. Int J Antimicrob Agents. 2013;42(3):202–10.10.1016/j.ijantimicag.2013.05.005
- Cha R, Grucz RG Jr, Rybak MJ. Daptomycin dose-effect relationship against resistant gram-positive organisms. Antimicrob Agents Chemother. 2003;47(5):1598–603.10.1128/AAC.47.5.1598-1603.2003
- Safdar N, Andes D, Craig WA. In vivo pharmacodynamic activity of daptomycin. Antimicrob Agents Chemother. 2004;48(1):63–8.10.1128/AAC.48.1.63-68.2004
- Soon RL, Turner SJ, Forrest A, et al. Pharmacokinetic/pharmacodynamic evaluation of the efficacy and safety of daptomycin against Staphylococcus aureus. Int J Antimicrob Agents. 2013;42(1):53–8.10.1016/j.ijantimicag.2013.02.009
- Liu C, Bayer A, Cosgrove SE, et al. Clinical practice guidelines by the infectious diseases society of america for the treatment of methicillin-resistant staphylococcus aureus infections in adults and children: executive summary. Clin Infect Dis. 2011;52(3):285–92.10.1093/cid/cir034
- EUCAST. Guidance document on use of daptomycin to treat enterococcal endocarditis. [cited 2016 Sep 24]. Available from http://www.eucast.org/fileadmin/src/media/PDFs/EUCAST_files/General_documents/EUCAST_daptomycin_guidance_note_20160924.pdf.
- van Hal SJ, Paterson DL, Gosbell IB. Emergence of daptomycin resistance following vancomycin-unresponsive Staphylococcus aureus bacteraemia in a daptomycin-naïve patient—a review of the literature. Eur J Clin Microbiol Infect Dis: Official Publication of the European Society of Clinical Microbiology. 2011;30(5):603–10.10.1007/s10096-010-1128-3
- Fowler VG Jr, Boucher HW, Corey GR, et al. Daptomycin versus standard therapy for bacteremia and endocarditis caused by staphylococcus aureus. N Engl J Med. 2006;355(7):653–65.10.1056/NEJMoa053783
- Falcone M, Russo A, Venditti M, et al. Considerations for higher doses of daptomycin in critically Ill patients with methicillin-resistant staphylococcus aureus bacteremia. Clin Infect Dis. 2013;57(11):1568–76.10.1093/cid/cit582
- Wu G, Abraham T, Rapp J, et al. Daptomycin: evaluation of a high-dose treatment strategy. Int J Antimicrob Agents. 2011;38(3):192–6.10.1016/j.ijantimicag.2011.03.006
- Bassetti M, Nicco E, Ginocchio F, et al. High-dose daptomycin in documented Staphylococcus aureus infections. Int J Antimicrob Agents. 2010;36(5):459–61.10.1016/j.ijantimicag.2010.07.011
- Moise PA, Hershberger E, Amodio-Groton MI, et al. Safety and clinical outcomes when utilizing high-dose (≥8 mg/kg) daptomycin therapy. Ann Pharmacother. 2009;43(7–8):1211–9.10.1345/aph.1M085
- D’Avolio A, Pensi D, Baietto L, et al. Daptomycin pharmacokinetics and pharmacodynamics in septic and critically Ill patients. Drugs. 2016;76(12):1161–74.10.1007/s40265-016-0610-3
- Durante-Mangoni E, Andini R, Parrella A, et al. Safety of treatment with high-dose daptomycin in 102 patients with infective endocarditis. Int J Antimicrob Agents. 2016;48(1):61–8.10.1016/j.ijantimicag.2016.04.022
- Seaton RA, Menichetti F, Dalekos G, et al. Evaluation of effectiveness and safety of high-dose daptomycin: results from patients included in the european cubicin((R)) outcomes registry and experience. Adv Ther. 2015;32(12):1192–205.10.1007/s12325-015-0267-4
- Roberts JA, Paul SK, Akova M, et al. DALI: defining antibiotic levels in intensive care unit patients: are current beta-lactam antibiotic doses sufficient for critically ill patients? Clin Infect Dis. 2014;58(8):1072–83.10.1093/cid/ciu027
- Roberts JA, Abdul-Aziz MH, Lipman J, et al. Individualised antibiotic dosing for patients who are critically ill: challenges and potential solutions. Lancet Infect Dis. 2014;14(6):498–509.10.1016/S1473-3099(14)70036-2