ABSTRACT
Doxorubicin (DXR) is a broad-spectrum anti-cancer. Doxorubicin irreversible toxicity resulting from oxidative damage limits its therapeutic use. Boswellic acids (BAs) are inhibitors of 5-lipoxygenase and have been used in traditional medicine for their powerful anti-inflammatory effects and cellular protective effects. This study tested the protecting mechanisms of BAs against nephrotoxicity induced by DXR in mice and explored their antioxidant and antiapoptotic activities in the form of immunohistochemical expression of Bcl2 in the tissue of the kidney. DXR (6 mg/kg) was injected intraperitoneally weekly to mice along with BAs (125, 250 and 500 mg/kg) daily. The experiment continued for three weeks. It was found that the elevated serum urea and creatinine in DXR-treated mice were ameliorated by BAs. Furthermore, BAs decreased malondialdehyde and increased glutathione levels in renal tissues of DXR-treated mice. The immunostained kidney tissue showed an anti-apoptotic effect for BAs as it increased expression of Bcl2 in mice co-treated with BAs with DXR compared to the DXR control mice. Western blot analysis demonstrated that DXR control group showed greater expression for renal caspase 3 and mice administered BAs (125–500 mg/kg) along with DXR showed significant downregulations. These findings were supported by the DNA laddering assay and histopathological examination of renal tissues stained with haematoxylin+eosin or periodic acid Schiff. Results suggest BAs for nephroprotection against toxicity induced by DXR.
Introduction
Antineoplastic therapy usually devastates the physiological homoeostatic mechanisms and influences many organs during treatment [Citation1]. Through the past five decades, DXR has been used as an effective anti-cancer treatment. It is an anthracycline antibiotic with anti-neoplastic function mediated mainly by affecting the DNA synthesis machinery of the cell [Citation2]. It has been found to be effective against a broad range of tumours such as cancers of the lung, breast, ovary, uterus, uterine cervix in addition to haematological malignancies [Citation3–Citation5]. Though the clinical use of DXR is restricted owing to its serious adverse effects including hepatotoxicity, cardiotoxicity, pulmonary toxicity, myelosuppression, and nephrotoxicity. Doxorubicin-induced nephrotoxicity comprises a state of progressive glomerular damage associated with atrophic tubular lesion [Citation1].
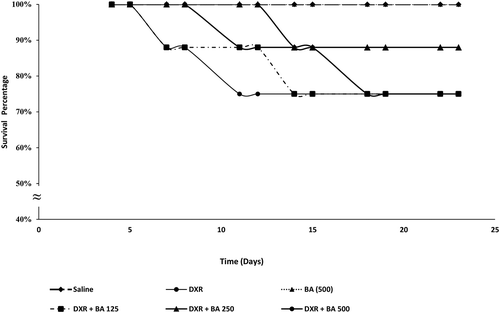
Figure 7. Effects of BAs 125, 250 & 500 mg/kg on the survival rates. Values were analyzed by Kaplan–Meier analysis, using Log-Rank test; n = 8 mice/group. The mean difference was statistically significant at p < 0.05.
The mechanism of DXR nephrotoxicity was reported to be correlated with its cytotoxicity, manifested by the destruction of cell membranes and other cellular components as a result of DNA intercalation [Citation6]. This cytotoxicity is assumed to be facilitated by oxidative stress induced by excess reactive oxygen species (ROS). ROS activity alters specific intracellular structures such as proteins, lipids and nuclear DNA [Citation2]. Mitochondrial DNA is another target for ROS activity, resulting in mutations, rearrangements, and transcriptional errors. These alterations affect not only mitochondrial functions but overall cellular activity, threatening its survival and ending with cell death [Citation2]. Furthermore, DXR toxicity was found to disrupt kidney function and could have a profound effect on total body metabolism [Citation7]. Biochemical and physiological alterations of kidney tissue could affect the extracellular space due to the intense relationship between the cell environment and the extracellular space [Citation8].
Cells are naturally provided with antioxidant mechanisms protecting them from ROS activity in order to keep a balanced internal milieu. However, in critical situations such as oxidative stress, these mechanisms are not sufficient and fail to keep the balance [Citation9]. Thus, exogenous antioxidants are suggested to have a protective and/or curative role against ROS-induced cytotoxicity.
Another mechanism involved in DXR cytotoxicity is cellular apoptosis [Citation10–Citation12]. The process of apoptosis involves several apoptotic key elements released in the cytosol. The combined action of these factors activates a hierarchy of caspases, the latter are found inside the cell as procaspases which are inactive precursors. The activation of downstream caspases eventually leads to apoptotic cell death.
Boswellic acids (BAs) constitute a promising natural remedy for several diseases due to their antioxidant, anti-inflammatory and anti-neoplastic potentials. They are the extracts of Boswellia serrata (B. serrata) tree, Frankincense, which has a lengthy history of usage in folk medicine in India for their powerful healing effects [Citation13].
Bertocchi et al. [Citation14], described the content of the pure resin of B. Serrata, BAs represent 65–85% of bioactive molecules. In their study, HPLC curves of different preparations of B. Serrata showed the highest peaks to be BAs. Meins et al., Gerbeth et al. and Pozharitskaya et al. have stated that the major pharmacological active principles of the boswellia serrata extract are the boswellic acids and the BAs are responsible for the underlying mechanism of action of B. Serrata [Citation15–Citation17]
There is a growing evidence on the anti-oxidant properties that further encouraged studying the effect of BAs against oxidant-induced injuries; including the multiple organ toxicities induced by DXR. B. serrata extract was reported to protect against DXR-induced cardiotoxicity [Citation18] and hepatotoxicity in rodents [Citation19] -due to the antioxidant and antiapoptotic activities- and to show renoprotective activity against gentamicin-induced nephrotoxicity in rats [Citation20]. BAs were also reported to produce antioxidant and hepatoprotective effect against non-alcoholic fatty liver diseases produced after feeding rats with a high-fat diet [Citation21]. One recent study demonstrated the neuroprotective effect of BAs in experimentally induced Parkinsonism in rats [Citation22].
Up to our knowledge, the effectiveness of BAs in opposition to DXR-triggered nephrotoxicity was not examined previously. Thus, this work endeavoured to study the protective effect of BAs in antagonizing nephrotoxicity of DXR in mice focusing on its antioxidant and antiapoptotic activities.
Materials and methods
Medications
Adricin vials (doxorubicin HCl) were obtained from EIMC United Pharmaceuticals (Cairo, Egypt) in the form of 2 mg/mL vial. Vials were diluted with sterile saline. B. serrata standardized extract was procured from Advance Physician Formulas, Inc. (USA) in a tablet form containing 65% BAs. BAs-containing tablets were powdered and then suspended in distilled water.
Animals
Adult male Swiss albino mice with body weight between 18 to 26 g each were utilized. The mice were purchased from the modern veterinary office for laboratory animals in Cairo and housed in plastic cages at room temperature (26 ± 3°C) while food and water provided ad libitum.
Ethics statement
This study was carried out in strict accordance with the international and national guidelines for animal research [Citation23]. The protocol was approved by the Research Ethics Committee of the Faculty of Medicine, Suez Canal University (Ismailia, Egypt). (Protocol Number: 3130) on 16/8/2017. Animals were sacrificed under ketamine anaesthesia, and all efforts were made to minimize suffering.
Experimental groups
Mice were assigned into groups (8 mice each) in a random way:
(1) saline group: mice received normal saline by intraperitoneal (i.p., 16 mL/kg) injection weekly for 3 consequent weeks (vehicle for doxorubicin) and received daily oral distilled water (10 mL/kg) for 21 days (vehicle for BAs).
(2) DXR control group: mice received the cumulative dose of DXR (18 mg/kg i.p.) divided on three injections (6 mg/kg) every week for 3 consequent weeks. This was according to a pilot study for the dose modified from [Citation1,Citation18,Citation24,Citation25], i.e. DXR was given to the animals at the 1st, 8th and 15th day of the study.
(3) BAs control group: the animals of which received the largest dose of BAs (500 mg/kg) every day from day 1–21.
(4–6) DXR and BAs combination groups: the mice in these groups received daily doses of BAs (125, 250, or 500 mg/kg, p.o.) [Citation18], respectively, for 21 days starting from day 1 to day 21 and were injected with the dose of DXR as mentioned in DXR control group.
Doxorubicin nephrotoxicity assay
At the 22nd day of the study, blood was withdrawn from the mice by cardiac puncture under anaesthesia and serum samples were obtained after centrifugation for 10 min at 1500 × g. Animals were then killed by cervical dislocation. Then, an incision was made to the abdomen and the two kidneys were dissected. The left kidney was stored at −80ºC for later homogenization or DNA extraction. The right kidney was perfused in 10% phosphate-buffered formalin for histopathological studies.
Measurement of serum creatinine and urea
Serum creatinine (Cr) and urea were measured in the serum of experimental animals using commercially available kits from Diamond Diagnostics (Cairo, Egypt). In brief, the assay of creatinine depends on alkaline-picric acid method while urea assay was done by an enzymatic method applying a urease enzyme kit.
DNA extraction and laddering using agarose gel electrophoresis
DNA laddering was made to detect DNA fragmentation. Bio Basic EZ-10 spin column genomic DNA kit from Markham (Canada) was used to extract DNA from renal tissue following the kit protocol. The extracted DNA was diluted, loaded on the agarose gel with 100 bp DNA ladder as a DNA molecular weight marker (Solis Biodyn, Tartu, Estonia), electrophoresed and exposed to UV light. Photos of the gel with the DNA bands were imaged by a gel-documenting system and then evaluated using advanced version 2 Gel Doc EZ Imaging System from Bio-Rad.
Tissue malondialdehyde level and reduced glutathione
Malondialdehyde (MDA) is an indicator for oxidative stress affecting the cell constituents such as lipids. Frozen tissue samples were homogenized using a Teflon tissue homogenizer and then centrifuged to obtain the clear supernatant which divided into aliquots for later use in the assay of renal MDA and reduced glutathione (GSH) using enzymatic kits according to the instructions listed by the company (Biodiagnostics, Giza, Egypt). MDA reacts with thiobarbituric acid in an acid media in a boiling water bath (95°C, for 1 h). This reaction product is pink in colour and was quantitatively measured at 534 nm as mentioned previously [Citation26,Citation27]. Moreover, reduction of 5,5~ dithiobis (2-nitrobenzoic acid) (DTNB) by GSH produces a yellowish compound [Citation28]. The colour of the product was measured at 405 nm.
Measuring renal caspase 3 protein expression by western blot analysis
Caspase 3 protein was estimated by immunoprobing of Western blots [Citation29]. A tissue homogenate was prepared in the STE buffer as 10% (w/v) [sucrose (0.32 mol/L), Tris (5 mmol/L), ethylenediaminetetraacetic acid (2 mmol/L), phenylmethylsulfonyl fluoride (1 mmol/L), benzamidine (5 mmol/L) and leupeptin (20 μg/mL)]. After that, the homogenate was centrifuged at 4°C at 1400 × g for 10 min. After estimation of protein in tissue samples using the Bradford method, proteins were denaturated using sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS–PAGE) and transferred onto nitrocellulose membranes by Western blot. These membranes were incubated overnight at 4°C in 3% (w/v) bovine serum albumin in 0.1% (v/v) Tween 20 TBS, then probed with primary rabbit polyclonal antibodies (caspase 3 antibodies, Thermo Scientific, USA) at 4°C overnight. This was followed by adding β-actin monoclonal antibody and incubation on a roller shaker for 1 h at 4°C. Then, membranes were incubated at room temperature for 1 h with the suitable secondary antibody linked to the horseradish peroxidase reporter enzyme [Citation18,Citation30]. A ChemiDoc scanner was used to scan the adjusted bands and then the densitometrical analysis was done for determination of the intensity of each band.
Histopathology of the stained kidney specimens
The excised formalin-fixed kidneys were used to prepare sections of maximum of 5 μm thickness. Further, sections were stained with Haematoxylin & Eosin (H + E) for histopathological examination. Periodic acid Schiff (PAS) was used to stain other tissue sections to assess the integrity of the basement membranes and the brush border of the proximal convoluted tubules epithelium. The histomorphological evaluation of the kidney sections was performed blindly. To assess the histopathological changes, 10 fields of each section were randomly selected and examined under 400 x magnification.
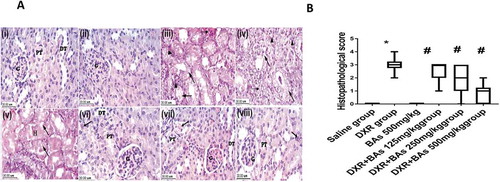
The score given to assess renal damage was categorized as 0–5, according to the strength of lesions observed in renal tubules (vacuolization, hyaline casting, flattening and tubular degeneration, dilated tubular lumen and necrosis) as 0: is given to normal renal structure, 1: for mild deviations from normal (25% of cortical tubules), 2: for moderate deviations (involving from 25%-50% of cortical tubules), 3: for marked deviations including more than 50%, but less than 75% of cortical tubules) and 4: for deviations involving 75% of cortical tubules [Citation31].
Immunohistochemistry for bcl2
For immunohistochemical expression of Bcl2 in the kidney tissue, 5 μm thick paraffin sections were deparaffinized, followed by antigen retrieval and incubation at 4°C with the primary antibody against Bcl2 from Abcam (ab7973) (Cambridge, UK) for an overnight. Then, sections were incubated with peroxidase-conjugated secondary antibody. Diaminobenzidine (DAB) was employed to visualize the peroxidase. Sections were then counterstained with Mayer’s haematoxylin, washed in running water, dehydrated in graded ethanol and mounted. The cytoplasmic immunoreaction for Bcl2, which was the criterion of cellular activity, was visualized using the light microscope. At least three sections per animal were examined. In each section, the number of Bcl2 positive cells/500 epithelial cells were counted. The average percentage of stained cells was calculated for each animal and averaged for each group.
Morphometric analysis
Area of proximal (PCT) and distal-convoluted tubules (DCT) (μm2), glomerular tuft area (GA) (μm2) and the epithelial thickness of proximal and distal convoluted tubule (μm) were measured using ‘Image J 1.49 v/Java 1.6.0_244 (64-bit)’ (National Institutes of Health, USA). Magnified PAS-stained slides (X400) were used for this purpose. Twenty-five glomeruli, proximal and distal tubules in the cortex of each animal were Randomly selected. Using calibration system that transforms the image pixels into micrometre units was performed before each analysis [Citation32,Citation33]
Analysis of statistical differences
Numeric data with normal distribution were presented as mean ± standard error of mean (SEM). Means were compared using the one-way ANOVA test. The significance between the study groups was tested by employing the Tukey–Kramer post-hoc test. P values <0.05 were considered as a level of statistical significance. Kidney scores were analyzed by Kruskal–Wallis test then Dunnett’s test for comparisons between groups and the presented as box plots which was drawn by R Studio Version 1.0.136 – © 2009–2016, Inc.
Results
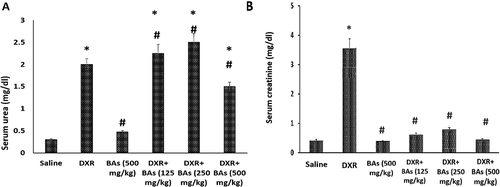
The results of serum assays indicated that both serum urea and creatinine increased significantly in mice treated for three weeks with DXR (6 mg/kg/week) in comparison to the saline group. In mice treated with BAs (500 mg/kg) per se, no significant differences were detected in these parameters in comparison with mice treated with saline.
Mice received BAs (125 or 250 mg/kg) did not show improvements in serum urea, in contrast, mice protected with BAs (500 mg/kg) showed lower serum urea versus DXR control group (p < 0.05) (). Moving to the serum creatinine level, mice treated with 125, 250 or 500 mg/kg of BAs along with DXR showed reduced serum level of creatinine in comparison to the DXR control mice ().
Figure 1. Effect of boswellic acids on serum creatinine and urea in mice treated with doxorubicin. Serum urea (c) and creatinine (b) in mice treated with doxorubicin (18 mg/kg, i.p.) in combination with boswellic acids (125, 250 or 500 mg/kg). Data are expressed as mean±SEM and analysis was done by one-way ANOVA followed by Tukey–Kramer’s post-hoc test. *Compared to saline group, #Compared to DXR group, P-value < 0.05, CI = 95%, n = 8.
Moreover, estimation of MDA in the kidney homogenate demonstrated greater concentration (9.31-fold) in DXR control group versus the saline control. Cotreatment by the 125, 250 or 500 mg/kg of BAs significantly reduced the MDA level in comparison to DXR group (). A significant difference was observed in the MDA level between BAs (125 mg/kg and 500 mg/kg) groups and the saline group. On the other hand, cotreatment with BAs 250 mg/kg had no significant difference when compared to the saline group ().
Figure 2. Effect of boswellic acids on renal malondialdehyde and reduced glutathione levels in doxorubicin-treated mice. Data are represented as mean±SEM and analysis was done by one-way ANOVA followed by Tukey–Kramer’s post-hoc test. *Compared to saline group, #Compared to DXR group, P-value < 0.05, CI = 95%, n = 8.
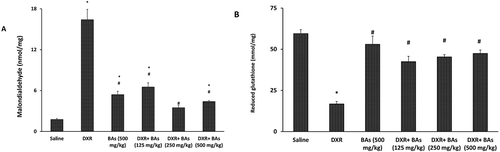
In addition, reduced glutathione was downregulated in DXR control group versus the saline group (28.3% of the value recorded in the saline control group). Cotreatment with 125, 250 or 500 mg/kg of BAs significantly increased reduced glutathione when compared to DXR group ().
Western blot assay for caspase 3 demonstrated a 9.8-fold increase in its expression in DXR control group compared to the saline group (). Treatment with BAs (125, 250 or 500 mg/kg) significantly reduced the caspase 3 expression. The effect of the high dose of BAs (500 mg/kg) was different from that observed with the lowest dose (125 mg/kg) ().
Figure 3. Effect of boswellic acids on renal expression of caspase 3 effect on DNA fragmentation in mice treated with doxorubicin. (a) Image for the Western blot assay for caspase 3 and beta actin as a housekeeping genes. (b) Columns for the fold changes increase in expression relative to the saline group. Data are expressed as mean±SEM and analysis were done by one-way ANOVA followed by Tukey–Kramer’s post-hoc test. *Compared to saline group, #Compared to DXR group and ¶ compared to DXR +BAs (125 mg/kg group), P value < 0.05, CI = 95%, n = 8. C. Agarose gel electrophoresis shows DNA fragmentation, Lane M:DNA marker with 100 bp, Lane 1,3,4&5 show intact DNA [saline, DXR+BAs (125 mg/g), DXR+BAs (250 mg/g) and DXR+BAs (500 mg/g)] while Lane 2 shows DNA streakes of DNA fragmentaion in DXR group.
![Figure 3. Effect of boswellic acids on renal expression of caspase 3 effect on DNA fragmentation in mice treated with doxorubicin. (a) Image for the Western blot assay for caspase 3 and beta actin as a housekeeping genes. (b) Columns for the fold changes increase in expression relative to the saline group. Data are expressed as mean±SEM and analysis were done by one-way ANOVA followed by Tukey–Kramer’s post-hoc test. *Compared to saline group, #Compared to DXR group and ¶ compared to DXR +BAs (125 mg/kg group), P value < 0.05, CI = 95%, n = 8. C. Agarose gel electrophoresis shows DNA fragmentation, Lane M:DNA marker with 100 bp, Lane 1,3,4&5 show intact DNA [saline, DXR+BAs (125 mg/g), DXR+BAs (250 mg/g) and DXR+BAs (500 mg/g)] while Lane 2 shows DNA streakes of DNA fragmentaion in DXR group.](/cms/asset/a0778aee-e620-4176-9932-daa3a33f612b/teba_a_1586359_f0003_b.gif)
Laddering assay showed a normal intact band of DNA for samples taken from the saline group; meanwhile, fragmented apoptotic DNA was found in DXR group. Concurrent therapy with BAs (125, 250 or 500 mg/kg) with DXR protected the DNA against the harmful effect of DXR and showed intact DNA bands ().
Histopathological examination of the kidney specimens stained with H&E from the experimental groups highlighted significant tubular changes in the DXR group and showed the highest KTDS versus the saline group (p < 0.05). These alterations included swelling and vacuolization of the tubular epithelial cells, tubular dilatation, focal hyalinization, degeneration, and necrosis. In addition, tubulo-interstitial inflammatory infiltration and focal haemorrhages were detected. Apoptotic bodies were also spotted. Although there were atrophic changes within the epithelium of the distal-convoluted tubules, the pathological changes were more distinct within the proximal ones. Significant regression of the histopathological changes was detected in the groups treated with BAs (125, 250 or 500 mg/kg) concurrently with DXR, and the damage score was significantly reduced approaching the control value in mice treated with 500 mg/kg of BAs ().
Figure 4. Effect of boswellic acids on histopathology and scores of mice treated with doxorubicin using haematoxylin and eosin staining. (a) Images for sections from the kidney represent control groups stained with H&E (x 40): (i) Normal control group and (ii) BAs control group showing sections of normal kidney. (iii, iv& v): sections from the kidney of the DXR group stained with H + E (x 40) showing KTDS. Interstitial changes (arrowhead) including focal haemorrhage and tubulointerstitial inflammatory infiltrate. Tubules (arrow) include degenerations, vacuolation, disorganized arrangement and dilatation of tubules. Pyknotic cells and necrosis (dashed arrow) and the presence of hyaline casts in cortical tubules (H). vi, vii & viii) Images for sections from the kidney of the groups treated with a combination of DXR and different doses of BAs stained with H + E (x 40): vi) DXR + BAs (125 mg/kg) group showing moderate tubular changes (KTDS II & focal III). vii) DXR + BAs (250 mg/kg) group showing mild tubular changes (KTDS I & focal II). viii) DXR + BAs (500 mg/kg) group showing normal kidney tissue with occasional mild changes in some sections (KTDS 0 & focal I). B) Boxplot graph for the median histologic score in renal sections. Ten fields of each section were randomly selected and examined under x 400 magnification, and the median of 8 animals was calculated. DXR; doxorubicin, BAs: boswellic acids, KTDS: kidney tubular damage score. Box plot was done by R-Studio Version 1.0.136 – © 2009–2016, Inc. * Compared to saline group, #Compared to DXR group, P-value < 0.05, CI = 95%, n = 8.
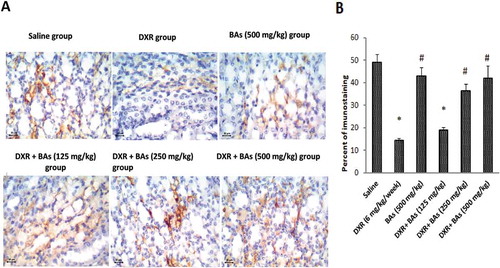
Interestingly, a decrease in Bcl2 immunostaining was illustrated in DXR group versus saline group. Cotreatment by BAs (250 or 500 mg/kg) significantly increased the percent of Bcl2 immunostaining compared to DXR control group ().
Figure 5. Effect of boswellic acids on renal immunohistochemical expression of Bcl2 in mice treated with doxorubicin. (a) Images for sections from the kidney of experimental groups stained with antibodies against Bcl2 (x). (b) Column chart for the mean percentage of stained cells in renal sections. Data are expressed as mean± SEM and its analysis was done by one-way ANOVA followed by Tukey’s post-hoc test. *Compared to saline group, #Compared to DXR group, n = 8, CI = .95% & P-value < 0.05.
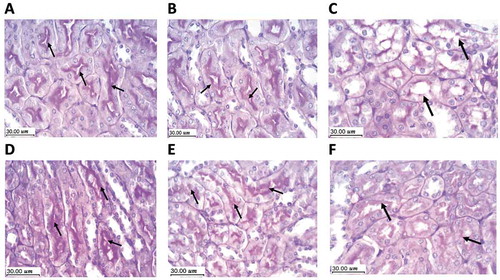
Examination of the kidney specimens stained with PAS from the experimental groups revealed marked thickening of the glomerular mesangial matrix, the disintegration of the tubular basement membranes, as well as the destruction of the cilia of the proximal convoluted tubular epithelium in the DXR group versus the saline control or BAs (500 mg/kg) control group. Restoration of the normal tubular and glomerular architecture gradually took place with the increase of BAs dose in the groups treated with BAs (125, 250 or 500 mg/kg) concurrently with DXR ().
Figure 6. Protective effect of boswellic acids for renal tissues in mice treated with doxorubicin using Periodic acid-Schiff staining (PAS). Scale bar = 30 µm. A (saline group) and B (BAs 500 mg/kg control group) show the normal architecture of renal parenchyma with an expression of PAS positive material in the basement membrane and the cilia of the epithelium lining the proximal convoluted tubules (arrow). C: DXR control group shows tubular disorganization and lack of PAS positive material expression within the tubular epithelium. D, E & F: DXR+BAs (125, 250 or 500 mg/kg), respectively. Restoration of the tubular epithelium took place in the 3 groups but clearly seen the highest dose of BAs indicated by the PAS positively stained brush border (arrow). DXR: doxorubicin, BAs: boswellic acids, PAS: periodic acid-Schiff.
Morphometric assessment of PCT and DCT area in the DXR group showed a significant increase in comparison to the control (saline) and BAs group (p < 0.001) and they almost retained their normal size in the (DXR+BAs 500 mg/Kg) group (p < 0.05). there was no significant difference between the DXR+ BAs groups regarding this parameter. The epithelial height in both PCT and DCT in the DXR group showed a significant decrease in comparison to the control and BAs groups (p< 0.001) and regained its thickness in the (DXR+BAs 500 mg/Kg) group. This was statistically significant when compared to the DXR group and the groups treated with a lower dose of BAs (p< 0.05) Morphometric assessment of glomerular area showed no significant difference between the study groups. Also, there was no significant difference between the saline and BAs groups regarding all the parameters ().
Table 1. Morphometric analysis of mice kidneys of the study groups showing mean ± SD of Glomerular area (GA) (μm2), Area of proximal-convoluted tubules (PCT area) (μm2), Area of distal-convoluted tubules (DCT area) (μm2), height of the PCT Epithelium (PCT height) (μm) and height of the DCT Epithelium (DCT height) (μm) in the different studied groups.
Effects of BAs 125, 250 and 500 mg/kg on the survival rates
Study groups and the normal control group (100%; p > 0.05; Data not shown). Moreover, the DXR-induced nephrotoxicity group of mice showed a survival percentage (80%) which was insignificantly lower than that of the normal control group (p > 0.05). Treatment with Bas 250 mg/kg along with DXR showed higher statistically non-significant survival percentages (88%) ().
Discussion
This study elucidated the protecting activity of BAs against nephrotoxicity induced by DXR in mice. The utilized doses of DXR in the current study were tested previously in mice [Citation18]. A cumulative dose of DXR (18 mg/kg) was used to produce renal damage that was evident by higher serum urea and creatinine and by renal histopathological changes shown by two different stains. Renal damage induced by DXR, in rats and other strains of mice, was documented in previous studies. A single injection DXR (10 mg/kg) to female BALB/c mice was reported to produce proteinuria and glomerulosclerosis after 20 days [Citation34]. In the current study design, DXR was sufficient to induce glomerular sclerosis and other histopathological changes indicating the nephrotoxic effect for the utilized dose.
The morphometric results of the current study showed significant differences of PCT and DCT area between DXR-treated and control groups. Also, the height of both the PCT and DCT revealed a significant decrease in DXR-treated group, indicating a widening of the tubules and loss of their cilia in the DXR group. The most convenient restoration of these parameters was achieved in the DXR+BAs 500 mg/KG group. With respect to these findings, morphometric results are adapted with histopathologic findings. Although Periyasamy et al.,2014 [Citation35] stated that administration of DXR in rats alters the biochemical parameter rather than the histopathological picture of the kidney, species and dose difference may cause the result variation.
The kidney changes induced by DXR in experimental animals involve increased permeability of glomerular capillaries and atrophy of tubules [Citation8]. Our results demonstrated histopathological alterations in the DXR-treated mice which exhibited the highest damage score, in the form of tubular widening, vacuolation of their lining epithelium and accumulation of casts within their lumen. Apoptotic bodies were also detected along the renal tissue specimens. Moreover, marked glomerular mesangial matrix expansion and focal tubular basement membranes disintegration and damage of the epithelial brush border of the proximal-convoluted tubules were detected in the DXR control group. The tubular damage and inflammatory changes were more prominent in mice treated with DXR compared to the other study groups.
The current study highlighted increases in serum markers of kidney damage, creatinine and urea. DXR induced renal injury was ameliorated in mice treated with BAs as indicated by the partial recovery in kidney function and histological examination. Significant reductions were observed in the elevated serum creatinine level among the BAs treated groups compared to DXR group. Meanwhile, there was a significant improvement in serum urea only in mice protected with 500 mg/kg of BAs. Creatinine level reflects the glomerular filtration rate (GFR) and is based on its steady production from muscle creatine and its relatively constant rates of renal excretion. Creatine is extracted from plasma by glomerular filtration and then excreted in the urine without considerable tubular reabsorption [Citation36]. Early in the course of acute kidney injury, for example, the GFR is markedly reduced; however, there has not yet been time for Cr or urea to accumulate and, therefore, levels of serum Cr or urea do not always precisely reflect the extent of renal dysfunction. Hence, moderate changes in GFR may not be revealed by serum Cr levels or urea [Citation37].
It is evident that the high production of ROS in renal tissue contributes to DXR toxicity [Citation38–Citation40]. Many disease processes are triggered by an unevenness between the pro- and the antioxidant elements [Citation41,Citation42]. In the current study, administration of DXR increased MDA level and decreased GSH. This agrees with other previous reports [Citation43,Citation44]. It was suggested that the high concentration of reduced glutathione in the kidney cortices advocates the conception of involvement of free radicals in renal toxicity due to DXR [Citation34]. Glutathione, a non-protein thiol, has a basic role in detoxification of xenobiotics, in defending cells through ROS scavenging [Citation45,Citation46] and is a powerful suppressor of neoplasm. It has also a pivotal effect as an endogenous antioxidant group and is believed to possess an important action in the protection mechanisms [Citation47].
Isolated tissues mitochondria were found to produce high amounts of oxygen radicals when subjected to DXR [Citation10,Citation48]. Mitochondria are believed to be the cornerstone in this toxicity and superoxide anions produced by particular dehydrogenase systems. In addition, singular electrons are shuttled to DXR, producing oxygen radicals by the auto-oxidation of DXR and semiquinones [Citation48,Citation49].
As reported before in other studies, the inflammatory process involves leukocytes and mast cells which can be found in the damaged tissues and are a target of the ‘respiratory burst’ due to oxidative injury and ROS release. The excessive lipid peroxidation is the starting point of this link between the inflammation and oxidative injury [Citation50] it was believed that BAs prevent the oxidant damage by two mechanisms, mainly its antioxidant property and its anti-inflammatory actions [Citation18]. In this study, BAs reduced the increased MDA levels and significantly enhanced GSH level in a dose-dependent manner versus DXR group. The enhanced GSH level in BAs-treated mice may partially explain its nephroprotective effect as GSH provides a thiol group triggering detoxification processes of glutathione peroxidase [Citation51]. Moreover, co-treatment with BAs diminished peroxidation of lipids in the kidney tissue with significant protection of tubular structures against DXR-induced injury.
In the present study, quantification concerning apoptotic nuclei revealed that DXR-induced toxicity caused high significant enhanced apoptosis (fragmented apoptotic DNA). These fragments generated a DNA ladder when analyzed via agarose gel electrophoresis. The generation of district fragments of DNA of nucleosomal bulk (about 180 bp) is a biochemical apoptosis lineament in most cells [Citation52,Citation53].
Apoptosis is a basic mechanism for eliminating undesirable cells during developmental and homeostatic processes [Citation52,Citation54]. Apoptotic cell death is evoked by various pathologic stimuli as well [Citation55].
It is believed that the DXR-triggered oxidative stress leads to cell death by apoptosis [Citation56]. Apoptosis caused by DXR is mediated via different pathway signals like activation of p53 [Citation57], caspase-12 [Citation58] and caspase 3 [Citation54,Citation59]. Advances in molecular mechanisms involved in DXR-induced nephrotoxicity considered apoptosis to be endogenously induced active cell processes by which external stimuli activate metabolic pathways resulting in cellular death [Citation52,Citation54]. The DNA damage and mitochondrial apoptosis encouraged by oxidation in the tubular cells were also diminished in BAs-treated mice.
This observed DNA damage is consistent with a previous study [Citation60]. The impact of this DNA damage was significantly reversed with BAs cotreatments in the three dose levels (intact DNA bands); this suggests that BAs have protecting function against DXR nephrotoxicity in mice. In this context, it is proved that Bcl2, the anti-apoptotic protein linked to the outer mitochondrial membrane, prevents apoptosis by averting Bax, the pro-apoptotic protein, from perforating the outer mitochondrial membrane [Citation1]. The current study demonstrated a greater expression of Bcl2 in mice co-treated with BAs along with DXR compared to the DXR control mice which implies an anti-apoptotic effect for BAs.
This work further explored the contribution of the antiapoptotic effect of BAs in the nephroprotective effect. Western blot assay for caspase 3 demonstrated high expression in DXR group than in the control group reflecting renal apoptosis. This finding is consistent with other experimental studies reporting increase in caspase 3 in kidney tissues following DXR administration [Citation61]. BAs treatment in various doses showed significant reduction of caspase 3 expression, especially with the highest dose of BAs (500 mg/kg). Hence, in consistence with previous results, our hypothesis proposed that the main underlying mechanism in DXR-induced nephrotoxicity is the production of free radicals and the outcome is cell apoptosis. Nevertheless, there are many pathways may lead to activation of caspase 3 through the receptor-mediated pathway that may also play a role in cell death. DXR has also been found to activate cytokines [Citation62] and may have affected caspases by receptor-mediated pathways [Citation10]. Thus, other research can further investigate the usage of specified antioxidants and caspase inhibitors to prohibit apoptosis.
Conclusions
In conclusion, our results revealed that an 18 mg/kg cumulative dose of DXR caused renal injury in mice which was manifested as glomerular and tubular damages. Our research hypothesis was to re-establish the oxidant/antioxidant homeostasis to provide a nephroprotective effect. The current study revealed that co-treatment with BAs along with DXR conserved kidney tissues and partly opposed its nephrotoxic effect as evidenced by reducing markers of renal injury and improving the histopathological alterations. Antioxidant and anti-apoptotic properties of BAs inhibited this renal damage. Although the more detailed mechanisms still need to be elaborated, BAs may be considered as a promising therapy to minimize DXR-triggered nephrotoxicity.
Research Highlights
This study indicated that Boswellic acids (BAs) ameliorated doxorubicin (DXR)-induced nephrotoxicity in mice.
This effect is at least in part mediated by antiapoptotic and antioxidant effects.
BAs decreased malondialdehyde and increased glutathione levels in renal tissues of DXR-treated mice.
Caspase-3 showed greater expression in DXR group and significant downregulation in mice treated with BAs.
BCl2 showed higher expression in groups co-treated with BAs along with DXR than the DXR-treated mice thus implies an anti-apoptotic effect for BAs.
Acknowledgments
Researchers wish to acknowledge the Central Lab. of the “Center of Excellence in Molecular and Cellular Medicine (CEMCM)” for using the labs in capturing the histopathology and immunostaining pictures.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Ayla S, Seckin I, Tanriverdi G, et al. Doxorubicin induced nephrotoxicity: protective effect of nicotinamide. Internet. Int J Cell Biol. 2011. cited 2018 Jan 21. Available from: https://www.hindawi.com/journals/ijcb/2011/390238/abs/
- Deavall DG, Martin EA, Horner JM, et al. Drug-induced oxidative stress and toxicity. Internet. J Toxicol. 2012 cited 2018 Jan 21;2012:1–13. Available from: https://www.hindawi.com/journals/jt/2012/645460/abs/
- Moroney JW, Schlumbrecht MP, Helgason T, et al. a phase i trial of liposomal doxorubicin, bevacizumab, and temsirolimus in patients with advanced gynecologic and breast malignancies. Clin Cancer Res. 2011 Nov 1;17(21):6840–6846.
- Slingerland M, Guchelaar H-J, Gelderblom H. Liposomal drug formulations in cancer therapy: 15 years along the road. Drug Discov Today. 2012 Feb 1;17(3):160–166.
- Zhong Y-J, Shao L-H, Li Y. Cathepsin B-cleavable doxorubicin prodrugs for targeted cancer therapy (review). Int J Oncol. 2013 Feb 1;42(2):373–383.
- Balli E, Mete UO, Tuli A, et al. Effect of melatonin on the cardiotoxicity of doxorubicin. Histol Histopathol. 2004;19(4):1101–1108.
- Adamis A, Miller J, Bernal M, et al. INCREASED VASCULAR ENDOTHELIAL GROWTH-FACTOR LEVELS IN THE VITREOUS OF EYES WITH PROLIFERATIVE DIABETIC-RETINOPATHY. Am J Ophthalmol. 1994 Oct;118(4):445–450.
- Yagmurca M, Erdogan H, Iraz M, et al. Caffeic acid phenethyl ester as a protective agent against doxorubicin nephrotoxicity in rats. Clin Chim Acta. 2004 Oct 1;348(1–2):27–34.
- Beaulieu WT, Bressler NM, Gross JG. Panretinal photocoagulation versus ranibizumab for proliferative diabetic retinopathy: patient-centered outcomes from a randomized clinical trial reply. Am J Ophthalmol. 2017 May;177:233.
- Green PS, Leeuwenburgh C. Mitochondrial dysfunction is an early indicator of doxorubicin-induced apoptosis. Biochim Biophys Acta (BBA) - Mol Basis Dis. 2002 Oct 9;1588(1):94–101.
- Fisher PW, Salloum F, Das A, et al. Phosphodiesterase-5 inhibition with sildenafil attenuates cardiomyocyte apoptosis and left ventricular dysfunction in a chronic model of doxorubicin cardiotoxicity. Circulation. 2005 Apr 5;111(13):1601–1610.
- Das J, Ghosh J, Manna P, et al. Taurine suppresses doxorubicin-triggered oxidative stress and cardiac apoptosis in rat via up-regulation of PI3-K/Akt and inhibition of p53, p38-JNK. Biochem Pharmacol. 2011 Apr 1;81(7):891–909.
- Khosravi MS, Mahmoodian H, Moghadamnia A, et al. The effect of frankincense in the treatment of moderate plaque-induced gingivitis: a double blinded randomized clinical trial. Clinical Trial Daru. 2011;19(4):288.
- Bertocchi M, Isani G, Medici F, et al. Anti-inflammatory activity of boswellia serrata extracts: an in vitro study on porcine aortic endothelial cells. Oxid Med Cell Longev. 2018;2018:1–9.
- Meins J, Behnam D, Abdel-Tawab M. Enhanced absorption of boswellic acids by a micellar solubilized delivery form of boswellia extract. Nfs J. 2018Jun;11:12–16.
- Gerbeth K, Meins J, Kirste S, et al. Determination of major boswellic acids in plasma by high-pressure liquid chromatography/mass spectrometry. J Pharm Biomed Anal. 2011 Dec;56(5):998–1005.
- Pozharitskaya ON, Ivanova SA, Shikov AN, et al. Separation and quantification of terpenoids of boswellia serrata roxb. extract by planar chromatography techniques (TLC and AMD). J Sep Sci. 2006 Sep;29(14):2245–2250.
- Ali SA, Zaitone SA, Moustafa YM. Boswellic acids synergize antitumor activity and protect against the cardiotoxicity of doxorubicin in mice bearing Ehrlich’s carcinoma. Can J Physiol Pharmacol. 2015 Apr 13;93(8):695–708.
- Khan MA, Singh M, Khan MS, et al. Caspase mediated synergistic effect of boswellia serrata extract in combination with doxorubicin against human hepatocellular carcinoma. [Internet]. Biomed Res Int. 2014 cited 2018 Jan 21;2014:1–11. Available from: https://www.hindawi.com/journals/bmri/2014/294143/abs/
- Alam M, Javed K, Jafri MA Effect of oleo-gum-resin of boswellia serrata (kundur) on renal functions in albino rats. IJTK Vol10(4) [ Internet]. 2011 Oct [cited 2018 Jan 21]; Available from: http://nopr.niscair.res.in/handle/123456789/12837
- Zaitone SA, Barakat BM, Bilasy SE, et al. Protective effect of boswellic acids versus pioglitazone in a rat model of diet-induced non-alcoholic fatty liver disease: influence on insulin resistance and energy expenditure. Naunyn-Schmiedeberg’s Arch Pharmacol. 2015 Jun 1;388(6):587–600.
- Ameen AM, Elkazaz AY, Mohammad HMF, et al. Anti-inflammatory and neuroprotective activity of boswellic acids in rotenone parkinsonian rats. Can J Physiol Pharmacol. 2017 Mar 1;95(7):819–829.
- Naderi MM, Sarvari A, Milanifar A, et al. Regulations and ethical considerations in animal experiments: international laws and islamic perspectives. Avicenna J Med Biotechnol. 2012 Jul;4(3):114–120.
- Khames A, Khalaf MM, Gad AM, et al. Ameliorative effects of sildenafil and/or febuxostat on doxorubicin-induced nephrotoxicity in rats. Eur J Pharmacol. 2017 Jun;805:118–124.
- Elsherbiny NM, El-Sherbiny M. Thymoquinone attenuates doxorubicin-induced nephrotoxicity in rats: role of Nrf2 and NOX4. Chem Biol Interact. 2014 Nov;223:102–108.
- Ohkawa H, Ohishi N, Yagi K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem. 1979 Jun 1;95(2):351–358.
- Kei S. Serum lipid peroxide in cerebrovascular disorders determined by a new colorimetric method. Clin Chim Acta. 1978 Nov 15;90(1):37–43.
- Beutler E, Duron O, Kelly BM. Improved method for the determination of blood glutathione. J Lab Clin Med. 1963 May;61:882–888.
- Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72(1):248–254.
- Xia L, Chen D, Han R, et al. Boswellic acid acetate induces apoptosis through caspase-mediated pathways in myeloid leukemia cells. Mol Cancer Ther. 2005 Mar 1;4(3):381–388.
- Al Kahtani E, Xu Z, Al Rashaed S, et al. Vitreous levels of placental growth factor correlate with activity of proliferative diabetic retinopathy and are not influenced by bevacizumab treatment. Eye. 2017 Apr;31(4):529–536.
- Ilic S, Stojiljkovic N, Veljkovic S, et al. Morphometric study of structural kidney damages caused by cisplatin in rats. effects of quercetin. Acta Microscopica. 2016; 25(3): 121–130.
- Morsy MM. The effect of formaldehyde on the renal cortex of adult male albino rats and possible protective role of vitamin C. 10. Eur. J. Anat. 2018; 22 (1): 75–84
- Deman A, Ceyssens B, Pauwels M, et al. Altered antioxidant defence in a mouse adriamycin model of glomerulosclerosis. Nephrol Dial Transplant. 2001 Jan 1;16(1):147–150.
- Jambhulkar S, Deshireddy S, Jestadi DB, et al. Quercetin attenuating doxorubicin induced hepatic, cardiac and renal toxicity in male albino wistar rats. AJPCT. 2014; 2 (8): 985–1004
- Rule AD, Bergstralh EJ, Slezak JM, et al. Glomerular filtration rate estimated by cystatin C among different clinical presentations. Kidney Int. 2006 Jan 2;69(2):399–405.
- Rashikh A, Pillai KK, Ahmad SJ, et al. Aliskiren alleviates doxorubicin-induced nephrotoxicity by inhibiting oxidative stress and podocyte injury. J Renin Angiotensin Aldosterone Syst. 2013 Mar 1;14(1):14–22.
- Adamson C, Kanu OO, Mehta AI, et al. Glioblastoma multiforme: a review of where we have been and where we are going. Expert Opin Investig Drugs. 2009 Aug;18(8):1061–1083.
- Carvalho C, Santos RX, Cardoso S, et al. Doxorubicin: the good, the bad and the ugly effect. Curr Med Chem. 2009 Sep 1;16(25):3267–3285.
- El-Sheikh AAK, Morsy MA, Mahmoud MM, et al. Effect of coenzyme-Q10 on doxorubicin-induced nephrotoxicity in rats. [ [Internet]]. Adv Pharmacol Sci. cited 2018 Jan 21; 2012:1–8. Available from: https://www.hindawi.com/journals/aps/2012/981461/abs/
- McCord JM. Human disease, free radicals, and the oxidant/antioxidant balance. Clin Biochem. 1993 Oct 1;26(5):351–357.
- Cigremis Y, Parlakpinar H, Polat A, et al. Beneficial role of aminoguanidine on acute cardiomyopathy related to doxorubicin-treatment. Mol Cell Biochem. 2006 Apr 1;285(1–2):149–154.
- Usta Y, Ismailoglu UB, Bakkaloglu A, et al. Effects of pentoxifylline in adriamycin-induced renal disease in rats. Pediatr Nephrol. 2004 Aug 1;19(8):840–843.
- Abo-Salem OM. The protective effect of aminoguanidine on doxorubicin-induced nephropathy in rats. J Biochem Mol Toxicol. 2012 Jan 1;26(1):1–9.
- Bray TM, Taylor CG. Tissue glutathione, nutrition, and oxidative stress. Can J Physiol Pharmacol. 1993 Sep 1;71(9):746–751.
- Antioxidant enzymes and human diseases. Clinical Biochemistry. 1999 Nov 1;32(8):595–603.
- Sinclair AJ, Barnett AH, Lunec J. Free radicals and antioxidant systems in health and disease. Br J Hosp Med. 1990 May;43(5):334–344.
- Kalivendi SV, Kotamraju S, Zhao H, et al. Doxorubicin-induced apoptosis is associated with increased transcription of endothelial nitric-oxide synthase effect of antiapoptotic antioxidants and calcium. J Biol Chem. 2001 Dec 14;276(50):47266–47276.
- Nohl H, Gille L, Staniek K. The exogenous NADH dehydrogenase of heart mitochondria is the key enzyme responsible for selective cardiotoxicity of anthracyclines. Zeitschrift Für Naturforschung C. 2014;53(3–4):279–285.
- Arulselvan P, Fard MT, Tan WS, et al. Role of antioxidants and natural products in inflammation. Oxid Med Cell Longev. 2016;2016:1–15.
- Oguz F, Beytur A, Sarihan E, et al. Protective effects of molsidomine against doxorubicin-induced renal damage in rats. Clin Invest Med. 2016 Feb 1;39(1):7–14.
- Compton MM. A biochemical hallmark of apoptosis: internucleosomal degradation of the genome. Cancer Metast Rev. 1992 Sep 1;11(2):105–119.
- Wyllie AH. Glucocorticoid-induced thymocyte apoptosis is associated with endogenous endonuclease activation. Nature. 1980 Apr;1(284):555–556.
- Jiang X, Wang X. Cytochrome C-mediated apoptosis. Annu Rev Biochem. 2004;73:87–106.
- Jain M, Kasetty S, Khan S, et al. An insight to apoptosis. J Res Pract Dent. 2014 Feb;3:1–12.
- Mizutani H, Tada-Oikawa S, Hiraku Y, et al. Mechanism of apoptosis induced by doxorubicin through the generation of hydrogen peroxide. Life Sci. 2005 Feb 11;76(13):1439–1453.
- von HR, Li P-F, Dietz R. Signaling pathways in reactive oxygen species–induced cardiomyocyte apoptosis. Circulation. 1999 Jun 8;99(22):2934–2941.
- Jang YM, Kendaiah S, Drew B, et al. Doxorubicin treatment in vivo activates caspase-12 mediated cardiac apoptosis in both male and female rats. FEBS Lett. 2004 Nov 19;577(3):483–490.
- Ueno M, Kakinuma Y, Yuhki K, et al. Doxorubicin induces apoptosis by activation of caspase-3 in cultured cardiomyocytes in vitro and rat cardiac ventricles in vivo. J Pharmacol Sci. 2006 Jun;101(2):151–158.
- Soni H, Pandya G, Patel P, et al. beneficial effects of carbon monoxide-releasing molecule-2 (corm-2) on acute doxorubicin cardiotoxicity in mice: role of oxidative stress and apoptosis. Toxicol Appl Pharmacol. 2011 May 15;253(1):70–80.
- El-Sayed E-SM, Mansour AM, El-Sawy WS. Protective effect of proanthocyanidins against doxorubicin-induced nephrotoxicity in rats. J Biochem Mol Toxicol. 2017 Nov 1;31(11):n/a–n/a.
- Sasaki M, Kobayashi D, Watanabe N. Augmented adriamycin sensitivity in cells transduced with an antisense tumor necrosis factor gene is mediated by caspase-3 downstream from reactive oxygen species. Jpn J Cancer Res. 2001 Sep 1;92(9):983–988.
