ABSTRACT
The superior growth of maize hybrids and some inbreds may be linked to key leaf growth features and critical components in carbon and nitrogen metabolism. Here, we performed simultaneous growth, physiological and heterosis analyses of morpho-physiological traits in four maize inbreds (B73, Mo17, Sids7, Sids63) and two hybrids (B73 × Mo17&Sids7 × Sids63). B73 and Sids7 exhibited alternate superiority over Mo17 and Sids63 in most growth traits. Both hybrids gained an early advantage over their parental inbreds in growth. They showed relatively similar behavior in many growth traits but differed significantly in biomass accumulation. Physiologically, B73 and Sids7 predominated in total pigments. Further, B73 contained the highest levels of sucrose, glucose, amino-N, and total N among inbreds. Both hybrids did not significantly differ in sucrose and total soluble sugars. However, Sids7 × Sids63 had consistently higher N fractions than B73 × Mo17. Both hybrids showed common and specific heterotic patterns. The superior growth of B73, Sids7, and hybrids is positively correlated with a set of leaf morphological (leaf area, leaf breadth, and LAI) and physiological (total chlorophyll, total pigments, and total N) traits. Advantages in these traits and efficient resource utilization support the superior growth of maize genotypes.
Introduction
Maize (Zea mays L.) is an important economic crop worldwide [Citation1]. It is also an excellent C4 experimental system to combine physiological and agronomic studies for many reasons; it has one of the highest photosynthetic rates with a very efficient ability to fix CO2 into carbohydrates, biomass, and yield. It also has a high capacity to uptake, assimilate and remobilize nitrogenous and carbon intermediates, traits that significantly impact growth and yield [Citation2]. Further, a wide genetic diversity in growth, development and grain yield has been reported in maize germplasm and such variability has been the driving force for searching and developing new genotypes with favorable traits [Citation3,Citation4].
Maize seeds contain proteins and starch as main food reserves [Citation5,Citation6]. Once germination signal (s) is perceived, critical physiological changes are initiated to mobilize seed reserves into metabolically active intermediates to support germination, seedling and early vegetative growth until the development of photosynthetically active leaves. During these stages, seed storage proteins provide the necessary nitrogen (N) for germination whereas starch is converted into metabolically active sugars. These seed-derived N and carbon resources gradually diminish as the germination progresses. Post-germination, roots actively absorbs N from soil either as inorganic or organic forms with nitrate being the most preferable N form for most plants [Citation7,Citation8]. The absorbed nitrate undergoes reduction then assimilation into various amino acids that support plant growth and development [Citation9]. Within plants, N presents in different forms such as ammonia, nitrate, nitrite, proteins and many other nitrogenous compounds; however, amino acids represent the major form of cellular N [Citation10]. More than 50% of the organic carbon can be shuttled into amino acid biosynthesis in some parts of plants [Citation11]. Therefore, plants have to have a strict coordination between amino acid biosynthesis and carbon metabolism to maintain C/N balance in various tissues/organs at different growth stages under diverse environmental conditions [Citation12,Citation13].
Maize growth, development, and productivity are shaped by genetic constitution of maize genotypes and their consequences on gene expression. In addition, critical environmental conditions such as soil fertility and climatic changes-related parameters such as alterations in temperature, CO2 emission, rainfalls, and drought stress can significantly affect maize growth and productivity [Citation14]. Previous studies revealed significant differences in many growth- and physiology-related traits among parental maize inbreds and their hybrids. Hybrids usually exhibit better plant growth vigor, physiological adaptation and overall field performance than their parental inbreds and such improved performance of hybrids is attributed to heterosis (hybrid vigor) [Citation15,Citation16]. The impact of heterosis on plant growth and productivity can be equivalent to that of adequate fertilization [Citation17]. The extent of heterosis is greatly influenced by the genetic diversity between their parental inbreds where greater genetic difference induces stronger heterosis [Citation3,Citation18,Citation19]. In maize, heterosis is usually discussed in terms of grain yield. However, other phenotypic traits such as root growth and development, plant height, leaf features, leaf/stem biomass, flowering time and ear height can also exhibit heterosis [Citation20–Citation23].
The genetic, molecular, and physiological mechanisms of heterosis are not fully understood [Citation24,Citation25]. Genetically, two main hypotheses have been proposed to explain the genetic basis of heterosis. The dominance hypothesis attributes heterosis to the accumulation of growth and yield-favoring alleles and complementation of harmful recessive ones in maize hybrids. Over-dominance hypothesis refers heterosis to the superior action of heterozygosity of alleles at individual loci over homozygosity at the same loci [Citation26,Citation27]. Epistasis and other molecular mechanisms including epigenetic, differential gene expression in hybrids and inbreds, and siRNA have also been implicated in controlling heterosis [Citation27–Citation30]. Physiologically, heterosis has been attributed to alterations in enzyme activity, protein metabolism, energy use, mitochondrial metabolism, metabolic flux/balance, circadian clock functions, carbon fixation, phytohormonal level (particularly GA) and cell cycle progression in inbreds and hybrids [Citation18,Citation23,Citation31].
Various physiological and metabolic traits have been used to phenotypically express heterosis [Citation18,Citation32]. Relatively little information is available about the mutual impact of heterosis on physiological processes during early vegetative growth in maize inbreds and many commercial maize hybrids [Citation16,Citation23,Citation25]. In addition, the relationships among the genetic constitution, phenotypes and physiological processes underlying growth in inbreds and their hybrids remain largely unknown. A better understanding of such relationships and linking key physiological processes to plant growth and grain yield is critical for the production of new hybrids with desirable traits [Citation33]. Therefore, the current study aimed at testing whether the improved growth of superior maize genotypes is correlated with a particular combination of leaf morphological and physiological features. This was carried out via simultaneous analysis of growth, leaf features, efficiency of biomass accumulation, photosynthetic pigments, various components of carbon and N metabolism in four famous maize pure inbred lines (B73, Mo17, Sids7, and Sids63) and two of their single-cross hybrids (B73 × Mo17 and Sids7 × Sids63) during their early vegetative growth. The impact of different types of interactions of parental inbreds’ genomes in the hybrids’ background on hybrids phenotypes was also investigated.
Materials and methods
Genetic stocks
Six maize genotypes were used in the current study. These genotypes included the two famous USA inbred lines (B73 and Mo17) and two Egyptian inbred lines (Sids7 and Sids63) along with two of their corresponding single cross hybrids (B73 × Mo17 and Sids7 × Sids63). These inbred lines were selected because of their importance in breeding and genome sequencing programs. B73 and Mo17 are elite US maize inbreds that have been used to develop high yielding B73 × Mo17 hybrid. In addition, they were used as parents of the famous IBMRIL (Intermate B73 × Mo17 Recombinant Inbred Lines) population which is one of the principal biological materials for gene discovery via quantitative trait loci (QTL) approach [Citation34,Citation35]. Further, B73 was selected for the maize genome sequencing project [Citation36]. Moreover, B73 and Mo17 are the ancestors of many modern commercial inbreds [Citation37]. On the other hand, Sids7 and Sids63 are Egyptian inbred lines that were used to develop Sids7 × Sids63 which is one of the high-yielding local maize hybrids and known locally as SC10 [Citation38–Citation40]. The two hybrids were selected because of their high heterotic responses and their intensive commercial use in the USA and in Egypt, respectively. Enough inbred and hybrid seeds were produced by selfing and crossing the selected inbreds respectively.
Field site and experimental design
Field evaluation was carried out in the nursery of the Department of Botany, Faculty of Science, Mansoura University, Mansoura, Egypt. The experimental design was split plot design. The experimental field was divided into four main plots: two for inbreds and two for hybrids. Plots contained rows of 3 m long and spaced 50 cm apart. Each plot was subdivided into subplots of four rows for each inbred and eight rows for each hybrid. Seeds were hand-sewn with a 25 cm between plants within a row. Plants were thinned to one plant per hill. Plots were kept weed-free manually and irrigated every 7 days. Plant samples were collected at the end of the early vegetative stage at 25 days after sowing (DAS) and processed for downstream analyses.
Analysis of seeds main components
Fifty grams seeds from each genotype were ground in a porcelain mortar into a fine powder. For determination of total proteins, total N in aliquots of the powdered grains was determined using microkjeldahl method and the crude protein was calculated by multiplying the total nitrogen by 6.25 [Citation41]. For lipids, ten grams of the powdered samples were extracted in petroleum ether (b.p.60–80ºC) in a Soxhlet apparatus for sixteen hours. Extracts were then dried over anhydrous sodium sulfate and evaporated to dryness. The residues were dried at 80ºC for ten minutes, cooled, weighed and expressed as percent lipids [Citation41]. For total carbohydrates determination, two grams of the powdered grains were ground in 80% ethanol and processed for determination of total carbohydrates by anthrone [Citation42].
Growth analysis
Growth parameters were determined on the aboveground parts of ten individual randomly selected plants per genotype. Plant heights were measured from soil surface to the tip of the most recent extending leaf from the whorl using a measuring tape. Numbers of leaves with visible collars in each of the selected plants were recorded. Leaf features were monitored on the uppermost fully expanded leaf with a visible collar. Leaf length and breadth were measured using a tape and the leaf area was determined according to [Citation43], [Leaf Area = Leaf length x Leaf width x (0.75)]. Specific leaf area (SLA, cm2g−1 leaf weight) for each plant was calculated as described by [Citation44] [SLA = (total leaf area/total leaf weight)]. Leaf area index (LAI) was calculated using total leaf area per plant and the total ground area occupied by each plant according to [Citation45] [LAI = (total leaf area/ground area)].
Physiology-related growth traits such as fresh and dry weights of leaves and total aboveground plant parts were also monitored. Six representative plants were harvested and the fresh weights of the total aboveground plant parts were recorded. The laminas of all leaves from each plant were then separated and their collective fresh weights were determined. The lamina of the uppermost fully extended leaf from each plant was labeled and the plant materials were then dried in an electric oven at 80°C for 72 hours and dry weights were determined using sensitive digital balance.
Physiological analysis
To correlate the obtained morphological data to the physiological status of the leaf, the same set of plants that were used in dry biomass accumulation were also used in the physiological analysis.
Extraction and estimation of photosynthetic pigments
A weighted quantity of leaf tissues were extracted in 80% acetone. The levels of total chlorophyll and carotenoids (Cars) in acetone extracts were determined spectrophoto-metrically as described by [Citation46] for chlorophyll and by [Citation47] for carotenoids. The levels of photosynthetic pigments were expressed as mg/g fresh weight.
Extraction and estimation of carbohydrates
A weighted quantity of dry leaf tissues were extracted in ethanol with periodic shaking. Glucose in the ethanol extracts was estimated by O-toluidine [Citation48] whereas sucrose and TSS were determined using anthrone reagent as described by [Citation49] and [Citation50] respectively.
Extraction and estimation of nitrogenous constituents
N fractions were extracted from a weighted quantity in distilled water [Citation51]. The ammonia-N was determined spectrophotometrically at 450 nm using Nessler’s reagent [Citation52]. The level of amino-N was estimated according to [Citation53]. Total nitrogen was determined directly in a weighted quantity of leaf powdered tissues using the Kjeldahl method [Citation54].
Heterosis analysis
Better- Parent (BP) heterosis was determined for each of the studied traits for both hybrids as the percentage deviation of F1 means from BP according to [Citation55] [Heterosis over the BP % = [(F1−BP)/BP × 100], where, F1 = mean performance of F1 hybrid, BP = mean performance of BP.
Statistical analysis
Data were statistically analyzed using COHORT/COSTAT program (798 Lighthouse Ave. PMB 329, Monterey, CA, 93940, USA). Duncan test was chosen to compare means with significance level at P ≤ 0.05. Correlation analysis between biomass accumulation and the tested traits was carried out using Microsoft Excel software.
Results
Changes in seed main components
shows the natural variations in the chemical composition of seeds of the tested inbred and hybrids. B73 and Sids7 did not significantly differ in their crude protein. On the other hand, Mo17 and Sids63 exhibited significantly higher levels than Sids7, however, only Mo17 surpassed the records of B73. The seeds of both hybrids contained a relatively similar level of crude protein which was significantly lower than those of their corresponding parental inbreds. For carbohydrates, both inbreds and hybrids showed a non-significant difference in total carbohydrates in their seeds. Regarding total lipids, Mo17 had the lowest records among inbreds. Sids63 and B73 had relatively similar levels of total lipids but only Sids63 surpassed that of Sids7. Both hybrids did not significantly differ in total lipids of their seeds. B73 × Mo17 exhibited intermediate levels of total lipids compared to its BP (B73) and low parent (LP) (Mo17). Sids7 × Sids63 exhibited significantly lower total lipids in its seeds than its parents.
Table 1. Genotypic differences in seed main components. Means with different letters indicate significant statistical difference between genotypes at (P ≤ 0.05) whereas means with similar letters indicate non-significant difference between genotypes at (P ≤ 0.05).
Changes in morphological traits
Plant height
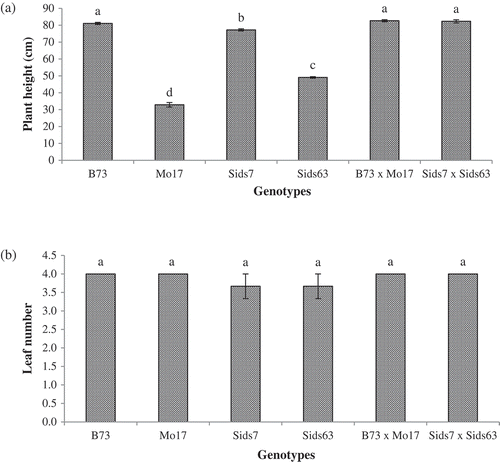
Our results revealed significant genotypic differences in plant height among the tested genotypes (). B73 and Mo17 had the tallest and the shortest plant height among inbreds respectively. Sids7 and Sids63 showed intermediate plant heights with Sids7 being significantly taller than Sids63. The two hybrids did not differ in their plant heights and both were significantly taller than all inbreds except B73 at this growth stage.
Figure 1. Natural variations in plant height (a) and number of leaves per plant (b) of the tested parental inbreds and their single cross hybrids during their early vegetative growth (25 DAS). Shown are the means of the tested traits ± standard error. Means with different letters indicate significant statistical difference among genotypes at (P ≤ 0.05).
Leaves number per plant
shows the mean values of the number of leaves per plant in the tested inbreds and hybrids. Our results revealed non-significant differences in leaves number per plant among all genotypes (inbreds and hybrids).
Leaf features
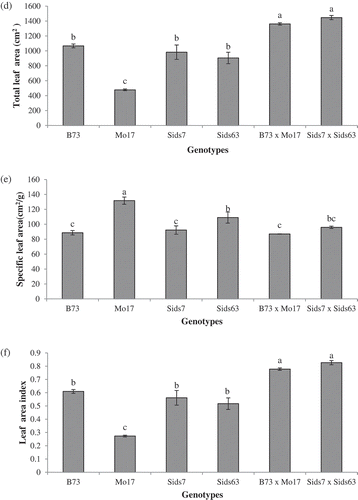
The natural variability in leaf features (area, length, breadth, total area/plant, SLA and LAI) among the tested genotypes are given in (panels A, B, C, D, E, and F). The results revealed significant difference (P < 0.05) in leaf area among most of the tested genotypes (). In inbreds, Sids7 and B73 had the largest leaf area without significant difference in-between. Sids63 attained the 3rd order and was significantly higher than Mo17. In hybrids, Sids7 × Sids63 showed a slightly larger leaf area than B73 × Mo17 and both hybrids had a significantly greater leaf area than all inbreds.
Figure 2. Natural variations in plant height (a) and number of leaves per plant (b) of the tested parental inbreds and their single cross hybrids during their early vegetative growth (25 DAS). Shown are the means of the tested traits ± standard error. Means with different letters indicate significant statistical difference among genotypes at (P ≤ 0.05).
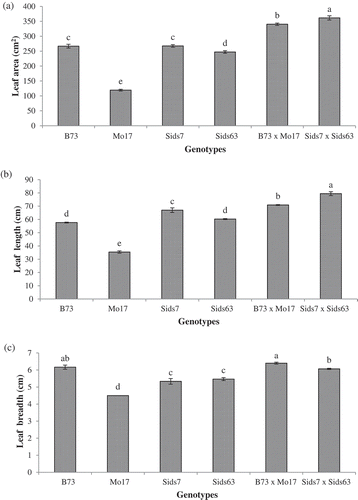
revealed significant differences in leaf length among most of the tested inbred lines. Sids7 had the longest leaf (67 cm) among inbreds whereas Mo17 had the shortest leaf length (35.40 cm). B73 and Sids63 had intermediate values of leaf length without significant difference between them (~59 cm). Both hybrids had significantly longer leaves than all inbreds with Sids7 × Sids63 having significantly longer leaves than B73 × Mo17.
Significant differences in leaf breadth were also observed among most of the tested inbreds (). However, the results revealed a pattern that differed significantly from that of leaf length. B73 had the widest leaf breadth (6.16 cm) whereas Mo17 maintained the narrowest leaf (4.50 cm) among inbreds. Sids7 (5.33 cm) and Sids63 (5.46 cm) had intermediate mean values of leaf breadth without significant difference between them. Both hybrids had wider leaf than all inbreds except B73 which had relatively similar leaf breadth to that of both hybrids. B73 × Mo17 had slightly wider leaves than Sids7 × Sids63.
indicated a significant difference (P < 0.05) in the total leaf area per plant among most of the tested genotypes. Because of the reported non-significant differences in the number of leaves among genotypes (), the total leaf area per plant exhibited a pattern that mirrored that of single leaf area ().
revealed significant differences in SLA among most of the tested inbreds. Mo17 followed Sids63, had significantly higher SLA than B73 and Sids7 which exhibited relatively similar SLA. Both hybrids had relatively similar SLA which was similar to their corresponding LPs, B73 and Sids7 respectively.
indicated that the inbred lines B73, Sids7, and Sids63 had relatively similar LAI (~ 0.56) which was significantly higher than that of Mo17 (0.27). Both hybrids exhibited relatively similar LAI that was significantly surpassed that of all inbreds.
Fresh weight of leaves and total aboveground parts
The tested parental inbreds and hybrids accumulated significantly different fresh weight in their leaves (). The highest leaves fresh weight was recorded by Sids7 (72.26 g) followed by B73 (60.47 g). Significantly lower leaves fresh weights were found in both Sids63 (48.69 g) and Mo17 (26.07 g). Both hybrids accumulated significantly higher fresh weights in leaves than their corresponding parental inbreds. B73 × Mo17 accumulated slightly higher leaves fresh weight than Sids7 × Sids63.
Figure 3. Variability in fresh weight of leaves (a) and total aboveground parts (b) in the tested parental inbreds and their single cross hybrids during early vegetative growth. Shown are the means of the tested traits ± standard error. Means with different letters indicate significant statistical difference among genotypes at (P ≤ 0.05).
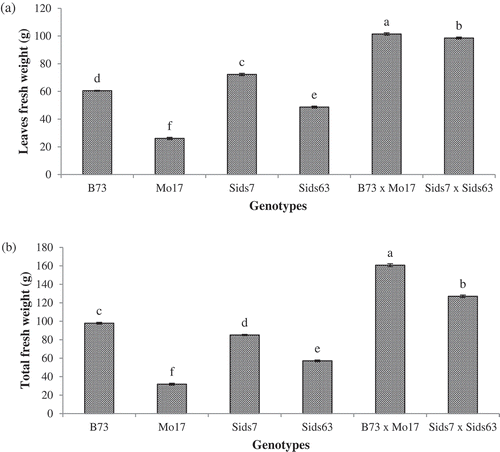
revealed that the tested genotypes accumulated significantly different amounts of fresh matter in their aboveground parts. B73 accumulated the highest total shoot fresh weight (97.85g) whereas Mo17 accumulated the lowest (31.82g) among inbreds. Both Sids7 and Sids63 accumulated intermediate levels of total aboveground fresh weight with Sids7 (85.10g) being more efficient than Sids63 (57.10g). Both hybrids accumulated significantly higher total fresh weight than their corresponding parents with B73 × Mo17 accumulating more fresh matter than Sids7 × Sids63.
Biomass accumulation in leaves and total aboveground parts
The tested genotypes varied significantly in dry weight of their leaves (). In inbreds, the highest leaves dry weights accumulation were found in B73 (12.08 g) and Sids7 (10.6 g). Significantly lower amounts of leaves dry weights were observed in Sids63 (8.28 g) and Mo17 (3.64 g). Unlike inbreds, both hybrids accumulated relatively similar leaf dry weight which was significantly higher than their parental inbreds.
Figure 4. Genotypic differences in biomass accumulation in leaves (a) and total aboveground parts (b) in the tested parental inbreds and their single cross hybrids during early vegetative growth. Shown are the means of the tested traits ± standard error. Means with different letters indicate significant statistical difference among genotypes at (P ≤ 0.05).
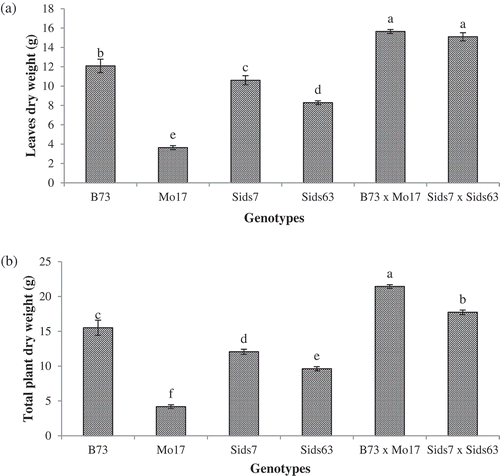
demonstrated that dry weights were significantly affected by genotypic differences among genotypes. B73 and Sids7 predominated over the other two lines in their biomass accumulation with B73 (15.51 g) being significantly higher than Sids7 (12.06 g). Sid63 had the 3rd position and accumulated higher biomass (6.62 g) than Mo17 (4.18 g). Both hybrids attained higher aboveground biomass than their corresponding parental inbreds with B73 × Mo17 (21.43 g) being more efficient in biomass accumulation than Sids7 × Sids63 (17.75 g).
Changes in physiological traits
Changes in photosynthetic pigments
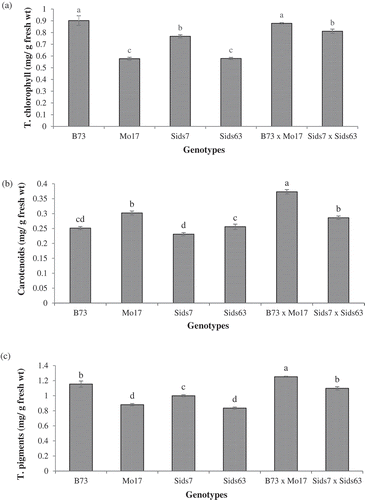
The tested genotypes exhibited significant differences in total chlorophyll, Cars, and total pigments (). B73, followed by Sids7, contained significantly higher levels of total chlorophyll and total pigments than Sids63 and Mo17 without significant difference between the latter two inbreds. The pattern was reversed for Cars where Mo17 contained a higher concentration of Cars than the rest of inbreds which had relatively similar Cars levels. B73 × Mo17 had slightly higher levels of photosynthetic pigments than Sids7 x Sids6. Both hybrids were similar to their better parents in total chlorophyll whereas they surpassed all inbreds in total pigments and Cars.
Figure 5. Comparative levels of photosynthetic pigments in the tested parental inbreds and their single cross hybrids during their early vegetative growth. Panels are total chlorophyll (a), Carotenoids (b), and total pigments (c). Shown are the means of the tested traits ± standard error. Means with different letters indicate significant statistical difference among genotypes at (P ≤ 0.05).
Changes in carbohydrates content
shows the natural variability in glucose, sucrose, and TSS in leaves of both inbreds and hybrids at 25 DAS. B73 and Sids63 had significantly higher levels of glucose than Mo17 and Sids7 without significant difference between members of both groups ((a)). B73 × Mo17 accumulated a significantly lower level of glucose than Sids7 × Sids63. B73 × Mo17 exhibited significantly lower foliar glucose level than its BP (B73) but relatively similar to its LP (Mo17). On the other hand, Sids7 × Sids63 exhibited glucose level within the range of its BP (Sids63) and LP (Sids7).
Figure 6. Comparative levels of various carbohydrates residues in leaves of the tested parental inbreds and their single cross hybrids during their early vegetative growth. Residues include glucose (a), sucrose (b), and total soluble sugars (TSS) (c). Shown are the means of the tested traits ± standard error. Means with different letters indicate significant statistical difference among genotypes at (P ≤ 0.05).
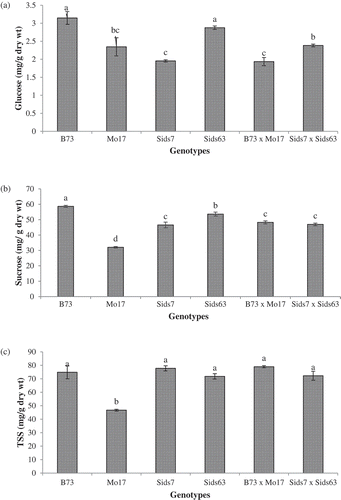
The tested inbreds also varied significantly in their foliar sucrose concentration (). B73 had the highest sucrose level whereas Mo17 had the lowest among inbreds. Sids7 & Sids63 had intermediate levels of sucrose with Sids63 accumulating significantly higher sucrose in its leaves than Sids7. also indicated that both hybrids accumulated relatively similar levels of sucrose in their leaves. B73 × Mo17 had sucrose level within the range of its parental inbreds whereas Sids7 × Sids63 had sucrose level similar to its LP (sids7).
illustrates the levels of TSS in the leaves of the tested inbreds and hybrids. The tested inbreds B73, Sids7, and Sids63 as well as the two hybrids accumulated a relatively similar level of TSS that was significantly higher than that of Mo17.
Changes in N fractions
displays the natural variability in ammonia-N, amino-N and total N in leaves of the studied genotypes at 25 DAS. indicated that leaves of B73, Sids7 and Sids63 had similar levels of ammonia-N (~7.7 mg/g) which was significantly higher than Mo17 (4.46 mg/g). Our data also revealed that Sids7 × Sids63 had a significantly higher level of ammonia-N (6.80 mg/g) than B73 × Mo17 (4.64 mg/g). Compared to their parents, B73 × Mo17 had relatively similar ammonia-N to its LP (Mo17) whereas Sids7 × Sids63 had ammonia-N that was relatively similar to its parents.
Figure 7. Natural variability in various nitrogen fractions in leaves of the tested parental inbreds and their single cross hybrids during their early vegetative growth. N fractions include ammonia-N (a), amino-N (b), total N concentration (c), and total N content in leaves (d). Shown are the means of the tested traits ± standard error. Means with different letters indicate significant statistical difference among genotypes at (P ≤ 0.05).
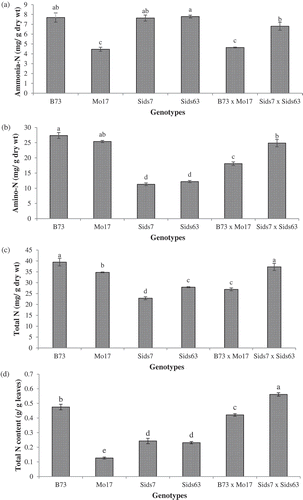
revealed that the American inbred lines (B73 & Mo17) prevailed over (~two folds) the Egyptian inbreds (Sids7 & Sids63) in amino-N in their leaves without significant difference between the two members of each group. In contrast, the Egyptian hybrid, Sids7 × Sids63, had a significantly higher level of amino-N than B73 × Mo17. Compared to their parental inbreds, B73 × Mo17 accumulated a significantly lower level of amino-N than both of its parents, whereas, Sids7 × Sids63 accumulated two folds higher amino-N than Sids7 and Sids63.
indicated that B73 accumulated the highest level of total-N (39.45 mg/g) whereas Sids7 had the lowest (22.86 mg/g) among inbreds. Mo17 and Sids63 accumulated intermediate levels of total N with Mo17 accumulating significantly higher total N (34.74 mg/g) than Sids63 (27.93 mg/g). Sids7 × Sids63 had a significantly higher level of total N (37.27 mg/g) than B73 × Mo17 (26.93 mg/g). Compared to their parents, B73 × Mo17 had significantly lower total N than its LP (Mo17) whereas Sids7 × Sids63 surpassed its BP (Sids63) in this trait.
We used the data of both total N concentration and dry weight of total leaves to assess total N content in the whole leaves as an indirect indicator on N uptake efficiency in the tested genotypes (). B73 exhibited the highest foliar total N content whereas Mo17 had the lowest among inbreds. Sids7 and Sids63 had a similar level of total N content which was within the range of the American lines. Sids7 × Sids63 accumulated significantly higher foliar total N content than B73 × Mo17. The two hybrids exhibited opposite patterns when compared to their parental inbreds. B73 × Mo17 accumulated slightly lower total N content than its BP (B73) whereas Sids7 × Sids63 surpassed its BP.
Changes in BP-heterosis and various inbreds/hybrids phenotypic patterns
The two hybrids exhibited significantly different BP-heterotic estimates in morphological and physiological traits (). B73 × Mo17 showed positive heterotic estimates that varied over a range from 1.9% to 67.7%. Its highest BP-heterotic records were observed in fresh weights of both aboveground parts (67.7%) and leaf (64.2%). Total aboveground biomass, total leaf dry weight, leaf area, total leaf area, LAI and leaf length exhibited moderate heterotic records that ranged from 22.95% to 38.14%. On the other hand, leaf breadth, plant height, and the number of leaves exhibited heterotic estimates less than 4%. For physiological traits, the heterotic records were relatively low. Cars, total pigments and total soluble sugars had positive estimates of 23.5, 8.4, and 5.5% respectively. The rest of the physiological traits showed negative heterotic records. Sids7 × Sids63 showed significantly higher BP-heterotic estimates in about 50% of the tested traits than their corresponding traits in B73 × Mo17 (). Its positive heterotic estimates of the tested morphological and physiology-related growth traits ranged from 6.6% to 49.24%. High heterotic records (35% – 50%) were observed in total fresh and dry weights, LAI, and total leaf area. Leaf length and breadth showed relatively low positive BP-heterotic estimates (~ 11%) whereas the rest of traits showed either no heterosis or negative heterotic records. For physiological traits, total N content and amino N had the highest BP-heterotic records of 130.9% and 104.1% respectively. Like B73 × Mo17, Sids7 × Sids63 showed positive BP-heterotic records for Cars (11.7%) and total pigments (9.9%) and negative BP-heterotic records for total soluble sugars, sucrose, ammonia-N, and glucose. Compared to their parental inbreds, the tested hybrids showed various phenotypic patterns that varied significantly depending on the hybrid as well as the trait under investigation (). These patterns were either below LP (underdominance), equal LP (LP-dominance), within the range of the two parents (additive or non-additive), equal BP (BP-dominance), or above BP (overdominance).
Table 2. BP-heterosis and various phenotypic patterns of the growth and physiological traits in the tested hybrids compared to their parental inbreds. F1,hybrid; B, B73; M, Mo17; S7, Sids7; S63, Sids63; BP, Better Parent; LP, Lower Parent; and MD, Mid parent. ≈ indicates that the phenotypic records of the tested traits in both genotypes are non-significantly different.
Discussion
The tested genotypes showed remarkable natural variability in growth
Our growth analysis revealed interesting points (–): (1) the tested inbreds differed significantly in critical growth traits such as plant height, leaf morphological features (area, length, and breadth), fresh and dry weights of both leaves and total aboveground parts. (2) The American inbred B73 and Egyptian inbred Sids7 exhibited alternative superiority over Sids63 and Mo17 in most growth parameters at 25 DAS. (3) Both B73 × Mo17 and Sids7 × Sids63 hybrids generally exhibited more vigorous growth traits than their parental inbreds, and (4) both hybrids showed relatively similar behavior in most of their initial growth parameters such as plant height, leaf number, and total leaf area. These results are in agreement with those of [Citation56] who reported similar growth rates of a number of maize hybrids in the juvenile stage under similar N fertilization conditions. The general superiority of hybrids over their parental inbreds in overall growth is well documented [Citation23,Citation57,Citation58].
In general, plant growth is affected by physiological factors such as the level of seed reserves and the efficiency of their utilization as well as the efficiency of uptake and utilization of new soil-derived resources. In the current study, the observed variations among genotypes suggest physiological heterogeneity in seed reserves and/or efficiencies of resources utilization among the tested genotypes. The importance of food reserves for early growth in maize has been reported [Citation58,Citation59]. However, our analysis of seeds’ total proteins, carbohydrates, and lipids in the tested genotypes showed either non-significant differences or little variances () that do not support a major role for these reserves in explaining the observed growth differences among genotypes. Recent research also reported that seeds size and mass may not have a major role in driving growth superiority [Citation23]. Therefore, the growth superiority of B73 and Sids7 in the current study is largely due to their higher efficiencies of utilization of both existing and newly absorbed resources compared to Mo17 and Sids63. Such enhanced efficiency may also be associated with improved root traits in these two inbreds [Citation60]. Similar enhanced physiological efficiencies of resource utilization may also stand behind the superiority of hybrids over their parental inbreds. Along with that, the superiority of hybrids over their parental inbreds has been attributed to many growth-promoting traits such as greater meristematic activity, more rapidly growing seminal root, longer radical, more active growth after seed germination and early seedling formation, faster rate of develop and larger mature organs in both shoot and root [Citation29,Citation61]. Such improved efficiencies have been partially attributed to artificial selection for better growth traits in the superior genotypes. Finally, the relatively similar growth records of B73 × Mo17 and Sids7 × Sids63 in some growth traits and their comparable seeds composition () suggest that similar initial growth-controlling mechanisms are functional in both hybrids or the two genomes of the parental inbreds of each of the two hybrids may interact in different ways that bring the overall growth to similar levels in both hybrids.
The improved growth of inbreds and hybrids is associated with enhanced leaf area, LAI, photosynthetic pigments and efficient resource utilization
Biomass accumulation is the most accurate parameter for monitoring plant growth in response to genotypic and environmental stimuli. Consistent with that our analysis revealed interesting points: (1) superior growth of B73, Sids7, and both hybrids was associated with significantly high biomass accumulation in their leaves and total aboveground parts ((a,b)). (2) Such improved growth was positively and strongly correlated with leaf morphological features like leaf area (0.85**), leaf breadth (0.91**), LAI (0.90**) and important physiological traits such as total chlorophyll (0.85**) and total pigments (0.82**) (supplementary Table S1). (3) B73 also recorded significantly higher levels of glucose and sucrose than the rest of the tested inbreds (). Similar results have been reported in sorghum and maize during early growth stages [Citation19,Citation23]. The combination of the above leaf features and photosynthetic pigments enhances leave’s photosynthetic efficiency and thus improve growth, biomass accumulation and yield in maize and other species [Citation18,Citation19,Citation62]. This hypothesis is supported by recent reports on higher net photosynthetic rate in B73 than Mo17 seedlings [Citation23]. This may partially explain the higher level of sucrose in leaves of B73 compared to other genotypes. The high level of sucrose in B73 most likely contributes to its improved biomass accumulation and overall growth because of its well-known biological significance as the central sugar in carbon assimilation in maize source leaves and as the major transported form of carbohydrates from source tissues to non-photosynthetic tissues [Citation63].
Interestingly, hybrids had relatively similar levels of most photosynthetic pigments and carbohydrate fractions to their parental inbreds. These results are in partial agreement with the reported similar sucrose concentration in source leaf tissues in seedlings of B73 × Mo17 and its parental inbreds [Citation23]. These results thus suggest either intensive utilization of carbohydrates for building up more biomass in hybrids or mobilization of carbohydrate components to sink tissues where they get stored to support the upcoming vigorous growth in hybrids, particularly during the rapid growth phase.
We also tested any possible correlation between growth of the tested genotypes and their foliar N (). Our results revealed three important findings: (1) the tested inbreds had significant natural variability in total N and N fractions in their leaves, (2) B73 accumulated the highest amounts of amino-N, total N concentration (mg N/g) and total N content (g N in leaves) among inbreds, and (3) Sids7 × Sids63 attained consistently higher N fractions than B73 × Mo17. Such natural N heterogeneity among genotypes is attributed to the differential efficiencies of many physiological processes such as N uptake efficiency and remobilization of nitrogenous compounds from green leaves to sink tissues (non-emerged leaves & growing meristems). In fact, our analysis of total N content revealed that the tested genotypes do have differential capacities to absorb soil N () and that the biomass accumulation was positively and strongly correlated to leaf N content (0.73*). The rest of our N analysis suggests that a combination between N content and efficient N utilization stand behind the improved growth of superior maize genotypes.
The tested hybrids exhibit different mode of interaction of their parental genomes
The tested hybrids showed common as well as specific phenotypic patterns compared to their parental inbreds (). For example, both hybrids showed overdominance phenotypes in most critical growth-related traits such as leaf features (length, breadth, area) and biomass accumulation-related traits. Similar common overdominance relations were also observed in physiological traits such as Cars, and total pigments. In contrast, the rest of the tested physiological traits reflected hybrid-specific phenotypic patterns that were differentially distributed among underdominance, LP-dominance, non-additive, additive, and BP-dominance, in both hybrids. Interestingly, unique overdominance appeared in amino-N, total N concentration and total N content in Sids7 × Sid63. The magnitudes of above heterotic records and the different phenotypic patterns of both B73 × Mo17 and Sids7 × Sid63 depends on the output of the interactions (complementation, intralocus, inter-loci epistatic) between alleles contributed by each of their parents’ genomes. These interactions, altogether, can qualitatively explain the observed heterotic phenotypes in the tested morphological and physiological traits; however, the relative contribution of each of these interactions to the observed heterotic variations is unclear. The above quantitative differences in the heterotic records of both B73 × Mo17 and Sids7 × Sid63 may be driven, in part, by quantitative differences in the expression of genes involved in controlling these traits. Similar patterns of interactions were observed in heterotic hybrids when gene expression was used to phenotypically express heterosis [Citation28,Citation64,Citation65]. Further, comparative gene expression in B73, Mo17, and B73 × Mo17 identified a large number of differentially expressed genes that showed all possible modes of interaction in the hybrid [Citation21,Citation66]. These differentially expressed genes most likely drive a significant difference in protein metabolism which in turn drives better growth and development of maize hybrids [Citation18]. Interestingly, genes that exhibited overdominance include those that potentially affect a wide variety of regulatory steps that can significantly influence growth and its related downstream metabolic and physiological processes [Citation28]. Therefore, these genes most likely contribute to the overdominance mode of heterosis observed in the tested hybrids in the current study.
In conclusion, the tested genotypes exhibited significant variability in the tested growth and physiological traits. The superior growth of B73, Sids7, and both hybrids was associated with improved leaf features (larger leaf area, leaf breadth, LAI), biomass accumulation, total chlorophyll and total pigments. Other measurements of carbohydrates and nitrogen metabolism-related components suggest that efficient utilization of the existing and newly acquired resources, rather than the content of these resources, contribute to the superior growth of B73, Sids7, and both hybrids. However, it would be interesting to test whether such scenario will be maintained during the rapid growth and flowering stages under limited and sufficient N inputs. Finally, the inbreds B73 and Sids7 have a set of morpho-physiological traits that can form the foundation of production of new hybrids with desirable growth traits.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Verheye W. Growth and production of maize: traditional low-input cultivation. In: Verheye W, editor. Land use, land cover and soil sciences. UNESCO-EOLSS Publishers; 2010. p. 1–24.
- Oaks A. Efficiency of nitrogen assimilation in C3 and C4 cereals. Plant Physiol. 1994;106:407–414.
- Betran FJ, Ribaut JM, Beck D, et al. Genetic diversity, specific combining ability, and heterosis in tropical maize under stress and nonstress environments. Crop Sci. 2003;43:797–806.
- Liu K, Goodman M, Muse S, et al. Genetic structure and diversity among maize inbred lines as inferred from DNA microsatellites. Genet. 2003;165:2117–2128.
- Srikanth M, Amanullah M, Muthukrishnan P. Influence of plant density and fertilizer on yield attributes and grain quality of hybrid maize. J Madras Agric. 2009;96:139–143.
- Ghannoum O, Evans JR, Caemmerer SV. Nitrogen and water use efficiency of C4 plants. In: Raghavendra AS, Sage RF, editors. C4 photosynthesis and related CO2 concentration mechanisms. Dordrecht, The Netherlands: Springer Science and Business Media; 2010. p. 129–146.
- Mengel K, Kirkby E. Principles of plant nutrition. 5th ed. Dordrecht: Kluwer Academic Publishers; 2001.
- Näsholm T, Kielland K, Ganeteg U. Uptake of organic nitrogen by plants. New Phytol. 2009;182:31–48.
- Masclaux-Daubresse C, Daniel-Vedel F, Dechorgnat J, et al. Nitrogen uptake assimilation and remobilization in plants: challenges for sustainable and productive agriculture. Ann Bot. 2010;105:1141–1157.
- Yoneyama T, Ito O, Engelaar WM. Uptake metabolism and distribution of nitrogen in crop plants traced by enriched and natural 15 N: progress over the last 30 years. Phytochem Rev. 2003;2:121–132.
- Huppe H, Turpin D. Integration of carbon and nitrogen metabolism in plant and algal cells. Ann Rev Plant Physiol Plant Mol Biol. 1994;45:577–607.
- Wang R, Okamoto M, Xing X, et al. Microarray analysis of the nitrate response in Arabidopsis roots and shoots reveals over 1000 rapidly responding genes and new linkages to glucose trehalose-6-phosphate iron and sulfate metabolism. Plant Phys. 2003;132:556–567.
- Lopes M, Araus J. Comparative genomic and physiological analysis of nutrient response to NH4+, NH4+: NO3− and NO3− in barley seedlings. Physiol Plant. 2008;134:134–150.
- Oseni TO, Masarirambi MT. Effect of climate change on maize (Zea mays) production and food security in Swaziland. Am-Eurasian J Agric Environ Sci. 2011;11:385–391.
- Shull GH. The composition of a field of maize. J Heredity. 1908;1:296–301.
- Fu D, Xiao M, Hayward A, et al. Utilization of crop heterosis: a review. Euphytica. 2014;197:161–173.
- East EM. Heterosis. Genetics. 1936;4:375–397.
- Goff SA. A unifying theory for general multigenic heterosis: energy efficiency protein metabolism, and implications for molecular breeding. New Phytol. 2010;189:923–937.
- Blum A. Heterosis, stress, and the environment: a possible road map towards the general improvement of crop yield. J Exp Bot. 2013;16:4829–4837.
- Hoecker N, Keller B, Piepho HP, et al. Manifestation of heterosis during early maize (Zea mays L.) root development. Theor Appl Genet. 2006;1:421–429.
- Stupar R, Gardiner J, Oldre A, et al. Gene expression analyses in maize inbreds and hybrids with varying levels of heterosis. BMC Plant Biol. 2008;8:33.
- Flint-Garcia SA, Buckler ES, Tiffin P, et al. Heterosis is prevalent for multiple traits in diverse maize germplasm. PLoS ONE. 2009;10:e7433.
- Ko DK, Rohozinski D, Song Q, et al. Temporal shift of circadian-mediated gene expression and carbon fixation contributes to biomass heterosis in maize hybrids. PLOS Genet. 2016;12:e1006197.
- Paschold A, Marcon C, Hoecker N, et al. Molecular dissection of heterosis manifestation during early maize root development. Theor Appl Genet. 2010;120:383–388.
- Schnable P, Springer N. Progress toward understanding heterosis in crop plants. Annu Rev Plant Biol. 2013;64:71–88.
- Reif JC, Hallauer AR, Melchinger AE. Heterosis and heterotic patterns in maize. Maydica. 2005;50:215–223.
- Li Z, Luo L, Mei H, et al. Overdominant epistatic loci are the primary genetic basis of inbreeding depression and heterosis in rice I. Biomass and grain yield. Genetics. 2001;158:1737–1753.
- Swanson-Wagner R, Jia Y, DeCook R, et al. All possible modes of gene action are observed in a global comparison of gene expression in a maize F1 hybrid and its inbred parents. Proc Natl Acad Sci USA. 2006;103:6805–6810.
- Barber WT, Zhang W, Win H, et al. Repeat associated small rNAs vary among parents and following hybridization in maize. Proc Natl Acad Sci USA. 2012;26:10444–10449.
- Chen ZJ. Genomic and epigenetic insights into the molecular bases of heterosis. Nat Rev Genet. 2013;7:471–482.
- Ng DW, Miller M, Helen HY, et al. A role for CHH methylation in the parent-of-origin effect on altered circadian rhythms and biomass heterosis in Arabidopsis intraspecific hybrids. Plant Cell. 2014;26:2430–2440.
- Coors J, Pandey S. The genetics and exploitation of heterosis in crops. Madison, WI: American Society of Agronomy; 1999.
- Tollenaar M, Lee E. Dissection of physiological processes underlying grain yield in maize by examining genetic improvement and heterosis. Maydica. 2006;51:399–408.
- Sharopova N, McMullen MD, Schultz L, et al. Development and mapping of SSR markers for maize. Plant Mol Biol. 2002;48:463–481.
- Lee M, Sharapova N, Beavis WD, et al. Expanding the genetic map of maize with the intermated B73 x Mo17 (IBM) population. Plant Mol Biol. 2002;48:453–461.
- Schnable PS, Ware D, Fulton RS, et al. The B73 maize genome: complexity diversity and dynamics. Science. 2009;2009(326):1112–1115.
- Mikel M, Dudley J. Evolution of North American dent corn from public to proprietary germplasm. Crop Sci. 2006;46:1193–1205.
- Adawy SS, Assem SK, Hussein EH, et al. Development of AFLP markers and genotyping of elite maize inbred lines. Arabian J Biotech. 2004;7:53–64.
- Sadek SE, Ahmed MA, El-Ghaney HA. Correlation and path coefficient analysis in five parents inbred lines and their six white maize (Zea mays L.) single crosses developed and grown in Egypt. J Appl Sci Res. 2006;2:159–167.
- Mousa WME, Sadek SE, El-Nahrawy MM. Silage yield and quality of some maize and teosinte genotypes and their hybrid. Am-Eurasian J Agric Environ Sci. 2017;17:373–378.
- AOAC. Association of official analytical chemists. 17th ed. Maryland: A.O.A.C. international; 2000.
- Sadasivam S. Biochemical methods. 1st ed. New Delhi: New Age International; 1996.
- Hunt R. Plant growth analysis: studies in biology. London: Edward Arnold London; 1978.
- Zhang C, Zhang J, Zhang H, et al. Mechanisms for the relationships between water-use efficiency and carbon isotope composition and specific leaf area of maize (Zea mays L.) under water stress. J Plant Growth Regul. 2015;77:233–43.
- Lee E, Ahmadzadeh A, Tollenaar M. Quantitative genetic analysis of the physiological processes underlying maize grain yield. Crop Sci. 2005;45:981–987.
- Metzner H, Rau H, Senger H. Untersuchungen zur synchronisierbarkeit einzelner pigmentmangel-mutanten von Chlorella studies on synchronization of some pigment-deficient Chlorella mutants. Planta. 1965;65:186–194.
- Kissimon J Analysis of the photosynthetic pigment composition: In international. Workshop and Training Course on Microalgal Biol. and Biotech. Mosonmagyarouar: 1999; p. 13–26.
- Riazi A, Matsuda K, Arslan A. Water-stress induced changes in concentrations of proline and other solutes in growing regions of young barley leaves. J Exp Bot. 1985;36:1716–1725.
- Handel EV. Direct micro determinations of sucrose. Anal Biochem. 1968;22:280–283.
- Yemm E, Willis A. The estimation of carbohydrates by anthrone. J Biochem. 1954;57:508–514.
- Yemm E, Willis A. The respiration of barely plants. IX. The metabolism of roots during the assimilation of nitrogen. New Phytol. 1956;55:229–252.
- Naguib MI. Effect of sevin (N-methyl-1-naphthyl carbamate) on carbohydrates and nitrogen metabolism during the germination of cotton seeds. Ind J Exp Biol. 1964;2:149–152.
- Muting D, Kaiser E. Spectrophotometric method of determination of α-amino-N in biological material by means of the ninhydrin reaction. Hopper Seyler’s Z Physiol Chem. 1963;332:276–289.
- Chinbal A, Rees M, Williams E. The total nitrogen content of egg albumin and other proteins. Biochem J. 1943;3:354–359.
- Fehr WR. Principles of cultivar development: theory and technique. New York: MacMillan publishing Co; 1991.
- Uziak Z, Borowski E, Blamowski Z. The attempt to explain the various reactions of rape and corn plants on the nitrogen use in the ammonium or nitrate forms. Acta Agrobotanica. 1993;1:39–49.
- Whaley W, Heimsch C, Rabideau G. The growth and morphology of two maize inbreds and their hybrid. Am J Bot. 1950;37:77–84.
- Dias MAN, Mondo VHV, Cicero SM. Maize seed vigor and weed competition. Braz Seed J. 2010;32:93–101.
- Mondo V, Cicero S, Dourado-Neto D, et al. Maize seed vigor and plant performance. Braz J. 2012;34:143–155.
- Hauck AL, Novais J, Grift TE, et al. Characterization of mature maize (Zea mays L.) root system architecture and complexity in a diverse set of Ex-PVP inbreds and hybrids. SpringerPlus. 2015;4:424.
- Burkholder PR, McVeighm I. Growth and differentiation of maize in relation to nitrogen supply. Am J Bot. 1940;27:414–424.
- Fujimoto R, Taylor J, Shirasawa S, et al. Heterosis of Arabidopsis hybrids between C24 and Col is associated with increased photosynthesis capacity. Proc Natl Acad Sci USA. 2012;109:7109–7114.
- Lemoine R, La Camera S, Atanassova R, et al. Source-to-sink transport of sugar and regulation by environmental factors. Front Plant Sci. 2013;4:272.
- Hoecker N, Keller B, Muthreich N, et al. Comparison of maize (Zea mays L.) F1-hybrid and parental inbred line primary root transcriptomes suggests organ-specific patterns of non-additive gene expression and conserved expression trends. Genetics. 2008;179:1275–1283.
- Ding H, Qin C, Luo X, et al. Heterosis in early maize ear inflorescence development: a genome-wide transcription analysis for two maize inbred lines and their hybrid. Int J Mol Sci. 2014;15:13892–13915.
- Springer N, Stupar R. Allelic variation and heterosis in maize: how do two halves make more than a whole? Genome Res. 2007;17:264–275.