ABSTRACT
Multilineage differentiating stress enduring (Muse) cells is an adult rare pluripotent subpopulation within mesenchymal stem cells (MSCs). Therefore, the need for multiple passages without losing cell pluripotency is required to satisfy the demand for translation medicine. Hence, this study aims to determine the efficiency of Muse cells as a pluripotent cells during in vitro passaging. confocal microscope analysis and cell count of the expression of stage-specific embryonic-3 (SSEA-3) surface marker and the expression of pluripotent genes by reverse transcriptase-qPCR (RT-qPCR) were used for cultured Muse cells at passage 3, 5, 7, 9 and 11. The results showed gradual down-regulation of SSEA-3 surface marker and pluripotent genes, while at passage 3, cells showed higher efficiency compared to other passages. Taken together, these findings indicate that passage 3 is the peak of Muse cell pluripotency.
Introduction
Stem cells can be divided as embryonic and non-embryonic stem cells. Embryonic stem cells are the gold standard for pluripotent stem cells which can differentiate into the three germ layers (ectoderm, endoderm and mesoderm). In the developing embryo, pluripotent stem cells are the origin of somatic and germline cells [Citation1]. Adult stem cells as embryonic stem cells are all undifferentiated cells. However, the differentiation capacity of adult stem cells is limited to its origin. Hematopoietic and mesenchymal stem cells are the main identified types of adult stem cells, hematopoietic stem cells can be obtained from bone marrow, umbilical cord blood, and peripheral blood and are capable of generating all cell lineage found in mature blood [Citation2]. While mesenchymal stem cells, in the suitable environment have the ability to differentiate into chondrocytes, adipocytes and osteocytes [Citation3], and can be obtained from bone marrow as a primary source, fat tissue and umbilical cord [Citation4]. In 2006, a scientific breakthrough was performed by Yamanaka and colleagues after generating pluripotent stem cells from somatic cells by genetic manipulation with pluripotent markers, these cells are called induced pluripotent stem cells (iPSCs) [Citation5].
Muse cells are double-positive to CD105, CD90 and SSEA-3 surface markers and considered as a new adult pluripotent subpopulation [Citation6]. In addition to Muse cells, several subpopulations within MSCs showed to have pluripotency function such as SSEA-4 [Citation7] and very small embryonic-like stem (VSEL) cells [Citation8].
Materials and methods
Retrieval of human MSCs
All required approvals for this study were obtained from the ethical committee of Mansoura University. Liposuction aspirates were obtained from three healthy volunteers during elective cosmetic surgeries after providing informed consent. Muse cells were obtained from adipose derived-MSCs and cultured in 2D state as previously reported [Citation9].
RT-qPCR
Total RNA was extracted from all passages by Direct-Zol RNATM Mini Prep kit (ZYMORESEARCH, USA). Three micrograms of total RNA was converted by RT2 First Standard Kit (Qiagen, Germany) to cDNA. Gene expression was evaluated for pluripotent genes; Nanog Homeobox (Nanog), SRY-box2 (Sox2), and POU Class 5 Homeobox 1 (POU5F1 or OCT-4) as referred in . MSCs gene expression for targeted genes served as control and for mathematical calculations. Glyceraldehyde-3-Phosphate Dehydrogenase (GAPDH) was included as a reference gene and for normalization the results. Amplifications were performed in a total 25 µL reaction volume in each well that contains 12.5 µL 2X SYBR Green Rox Master Mix (Qiagen), 100 ng of cDNA template, 10 pmol primers and nuclease-free water. The plate array was inserted in CFX96 real-time system (Bio-Rad, USA) and programmed according to manufacturer instructions by using the cycling parameters of the PCR amplification as follows: initial denaturation at 95ºC for 3 min, followed by 40 cycles of amplification (denaturation at 94°C for 10 s, annealing and extension at 60°C for 30 s). For each sample, the procedure was carried out in triplicate. A mathematical model introduced by M. Pfaffl was used for the relative quantification of target genes [Citation10].
Table 1. List of Manual Primers Sequence
Immunofluorescence
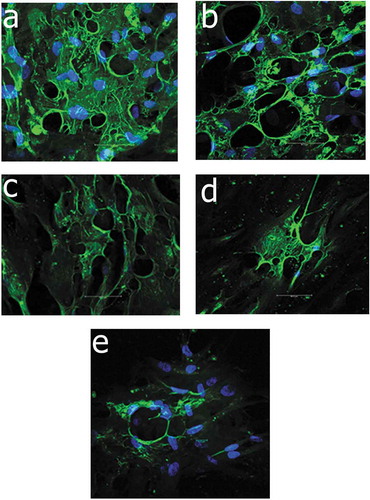
Cultured cells on eight-chamber slides (Nunc, Thermo Scientific, USA) were fixed with 4% paraformaldehyde for 10 min at RT, permeabilized by 100% chilled methanol for 10 min and blocked with 5% normal goat serum for 1 h at RT. Cells were incubated overnight with the primary antibodies anti-SSEA-3 antibody, clone MC-631 (Merk Millipore, USA) at 4ºC. The nuclei were counterstained with DAPI (Invitrogen, UK). Negative controls were obtained by omitting treatment with the primary antibody. Confocal images were captured using Leica TCS SP8microscope (Leica Microsystems, Mannheim, Germany).
Statistical analysis
Statistical analyses were carried out using the program SPSS 16. Data are present as mean and standard error (SE) and the error bar in the bar graph represented SE. Data were examined to determine whether they were normally distributed with the One-Sample Kolmogorov–Smirnov test and were found to be normally distributed.
Results
Gene expression
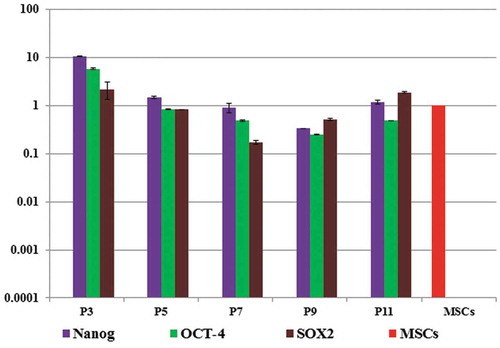
Relative gene expression of pluripotent markers; Nanog, OCT-4 and Sox2 was performed at passage three, five, seven, nine and eleven. At passage 3 the expression of these genes was up-regulated by 28 fold changes for Nanog, 60 folds for Sox2, 12 folds for OCT-4 and started to down-regulate gradually by in vitro passaging till passage eleven, relative to that of the gene expressed in starting whole MSCs ().
Immunofluorescence
The detection of SSEA-3 surface marker was performed at passages three, five, seven, nine and eleven. At passage 3, about approximate 62% of Muse cells was positive to anti-SSEA-3 and the percentage started to gradually down-regulate till 0.75% at passage eleven ().
Discussion
Loss of pluripotent and down-regulation of pluripotent markers was previously reported due to several factors and pathways such as; chromatin modifications [Citation11] and DNA methylation [Citation12]. While maintenance of pluripotency in ESCs and iPSCs is still an obstacle during in vitro culture and the definition of pluripotency gene network is still not fully understood [Citation13]. Several cellular pathways are involved in the maintenance of pluripotency such as; Wnt/β-catenin [Citation14], leukemia inhibitory factor [Citation15], and anus kinase-signal transducer and activator of transcription-3 (JAK-STAT3) pathway [Citation16]. In addition to several methods and factors are used to maintain and regulate pluripotency such as hypoxia condition [Citation17], physiochemical environment of the culture medium [Citation18], and dispensing fetal bovine/calf serum with human serum and knock out serum [Citation19].
The behavior and the identification of MSCs and its subpopulations is still not fully discovered, even the ability to isolate a pure homogenous population of MSCs has not been yet applicable. While our study does not fully investigate the behavior and the characteristics of Muse cells, but this pilot study is required to determine only which passage is the most convenient to start the differentiation and the correlation between the expression of pluripotent genes with SSEA-3 surface marker is not answered by our study. In conclusion, adult pluripotent subpopulations may be the solution to avoid the ethical and religious debates for ESCs and iPSCs, while the maintenance of pluripotency is required for all types of pluripotent stem cells not only adult pluripotent stem cells.
Acknowledgments
The study was funded by Misr El-Kheir Foundation (a charity nonprofit Egyptian organization), Bank Misr, and National bank of Egypt. The authors would like to thank Romaila Raouf for providing the statistical analysis works.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Thomson JA, Itskovitz-Eldor J, Shapiro SS, et al. Embryonic stem cell lines derived from human blastocysts. science. 1998;282(5391):1145–1147.
- Ogawa M. Differentiation and proliferation of hematopoietic stem cells. Blood. 1993;81(11):2844–2853.
- Pittenger MF, Mackay AM, Beck SC, et al. Multilineage potential of adult human mesenchymal stem cells. science. 1999;284(5411):143–147.
- Hass R, Kasper C, Böhm S, et al. Different populations and sources of human mesenchymal stem cells (MSC): a comparison of adult and neonatal tissue-derived MSC. Cell Commun Signaling. 2011;9(1):12.
- Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. cell. 2006;126(4):663–676.
- Kuroda Y, Kitada M, Wakao S, et al. Unique multipotent cells in adult human mesenchymal cell populations. Proc Nat Acad Sci. 2010;200911647. DOI:10.1073/pnas.0911647107
- Gang EJ, Bosnakovski D, Figueiredo CA, et al. SSEA-4 identifies mesenchymal stem cells from bone marrow. Blood. 2007;109(4):1743–1751.
- Kucia M, Reca R, Campbell F, et al. A population of very small embryonic-like (VSEL) CXCR4+ SSEA-1+ Oct-4+ stem cells identified in adult bone marrow. Leukemia. 2006;20(5):857.
- Fouad AM, Gabr MM, Abdelhady EK, et al. In vitro differentiation of human multilineage differentiating stress-enduring (Muse) cells into insulin producing cells. J Genet Eng Biotechnol. 2018;16: 433–440.
- Pfaffl MW. A new mathematical model for relative quantification in real-time RT–PCR. Nucleic Acids Res. 2001;29(9):e45–e45.
- Song J, Saha S, Gokulrangan G, et al. DNA and chromatin modification networks distinguish stem cell pluripotent ground states. Mol Cell Proteomics. mcp. M. 2012;111:011114. .
- Carlone DL, Lee J-H, Young SR, et al. Reduced genomic cytosine methylation and defective cellular differentiation in embryonic stem cells lacking CpG binding protein. Mol Cell Biol. 2005;25(12):4881–4891.
- Humphrey RK, Beattie GM, Lopez AD, et al. Maintenance of pluripotency in human embryonic stem cells is STAT3 independent. Stem Cells. 2004;22(4):522–530.
- Xu Z, Robitaille AM, Berndt JD, et al. Wnt/β-catenin signaling promotes self-renewal and inhibits the primed state transition in naïve human embryonic stem cells. Proc Nat Acad Sci. 2016;113(42):E6382–E6390.
- Hirai H, Karian P, Kikyo N. Regulation of embryonic stem cell self-renewal and pluripotency by leukaemia inhibitory factor. Biochem J. 2011;438(1):11–23.
- Tang Y, Tian X. JAK-STAT3 and somatic cell reprogramming. JAKSTAT. 2013;2(4):e24935.
- Zachar V, Prasad SM, Weli SC, et al. The effect of human embryonic stem cells (hESCs) long-term normoxic and hypoxic cultures on the maintenance of pluripotency. In Vitro Cell Dev Biol-Anim. 2010;46(3–4):276–283.
- Rajala K, Vaajasaari H, Suuronen R, et al. Effects of the physiochemical culture environment on the stemness and pluripotency of human embryonic stem cells. Stem Cell Stud. 2011;1(1):3.
- Ellerström C, Strehl R, Moya K, et al. Derivation of a xeno‐free human embryonic stem cell line. Stem Cells. 2006;24(10):2170–2176.