ABSTRACT
In this study, a flavan-3-ol glycoside, (+)-catechin-3ꞌ-O-rhamnopyranoside isolated from the stem bark extract of N. macrophylla was evaluated for its antivenom, analgesic, anti-inflammatory, and antimicrobial activities. The compound (at 1, 5 and 10 mg/kg) was able to protect mice against lethality induced by Naja nigricollis venom with 100%, 40%, and 20% survival rate, respectively; it was also found to be 33.7% effective in combating the hydrolytic actions of N. nigricollis PLA2 enzyme; the compound (at 2.5 and 10 mg/kg) exhibited significant (p < 0.05) analgesic effect in the formalin test which was higher than that observed by the standard drug, piroxicam (10 mg/kg) and there was 45.0% and 41.2% inhibition of inflammation by the compound at the 3rd & 4th hours. In addition, the compound was found to be effective against S. aureus, VRE, S. feacalis, E. coli, P. aeruginosa, P. vulgaris, C. albicans, and C. krusei with mean zone of inhibition ranged between 28 and 36 mm and an MIC and MBC/MFC values ranging from 6.25 to 25.0 µg/mL and 12.5–50.0 µg/mL, respectively. Thus, bioactivities of (+)-catechin-3ꞌ-O-rhamnopyranoside were reported for the first time.
Introduction
Snakebite is a global medical problem especially in the rural areas of the tropics with about 5.4 million bites each year resulting in 1.8 to 2.7 million cases of envenomings, accompanied by 81,1400 to 137,880 deaths and three times as many amputations and other permanent disabilities each year [Citation1,Citation71]. The highest burden of snakebites is in Asia and Sub-Saharan Africa; studies have indicated that, over 314,000 bites, 7300 deaths, and nearly 6000 amputations occur from snakebites annually in Sub-Saharan Africa and Nigeria, by poisonous snakes from two different families of elapids and viperids [Citation2–Citation4]; but the precise incidence is grossly under-estimated due to lack of data and low access to community health facilities [Citation4-Citation6]. Bites by venomous snakes may lead to complications such as, tissue necrosis, severe pain, swelling, bruising, abdominal pain, diarrhea, blindness resulting from spitting cobra, fetal loss, edema, wound infection, tetanus, severe paralysis that may prevent breathing, bleeding disorders that can lead to fatal hemorrhage, irreversible kidney failure and severe local tissue destruction that can cause permanent disability and limb amputation [Citation1,Citation7–Citation10]. However, mortality rate has been reported to be higher in children due to larger amount of venom injected per body weight [Citation11–Citation14]. Due to the wide spectrum of clinical symptoms, therapy using multiple drugs such as anticholinesterase, antibiotics, and analgesics is required [Citation15]. The currently used Antisnake venoms are accompanied by severe side effects and are largely unavailable due to access and cost [Citation4]. These drawbacks necessitate the search for newer agents with improved therapeutic effects.
Most communities in the sub-Saharan Africa, with poor access to health services resort to alternative therapy, which involve the use of extracts from medicinal plants as antivenoms [Citation16]. Several medicinal plants have been screened for their antivenom property [Citation16–Citation17] (Walter et al., 2000; Gomes et al., 2010), but researches mostly end up at preliminary stage of biological testing of the crude extracts leaving behind the crucial stages of isolation and characterization of bioactive constituents responsible for the observed effect.
Neocarya macrophylla (Sabine) Prance (Chrysobalanaceae) is widely distributed along coastal savannahs from Senegal to Liberia, woody savannahs of Southern Mali, Niger and Northern Nigeria [Citation18,Citation19]. It has been used extensively in the Northern part of Nigeria in ethno-medicine to treat numerous diseases which include; asthma, skin infections, treatment of wounds, dysentery, inflammations, pain, snake bite, diarrhea, pulmonary troubles, ear and eye infections, among others [Citation20,Citation21]. Chemical investigations on the stem bark of the plant were limited to steroids such as stigmasterol and flavanols; dimer of flavan-3-ol glycoside i.e. Bis-(5,7-diacetyl-catechin-4’-α-rhamnopyranoside) has hitherto been isolated [Citation22]. GC-MS analysis of the chloroform leaf extract of N. macrophylla revealed the presence of flavonoids, steroids, palmitoleic acid, tannins, glycosides, alpha and beta tacopherol [Citation25]. Pharmacological actions of the stem bark of N. macrophylla has been confined to analgesic [Citation23], antimicrobial [Citation24] and antivenom [Citation25] activities; Isaka et al. [Citation25] has reported the antimicrobial activity of the ethanol leaf extract of the plant; the extracts and fractions of N. macrophylla leaf exhibited remarkable antibacterial and antibiofilm activities against S. aureus and P. aeruginosa [Citation26]. Antimicrobial activity of stigmasterol from the stem bark of the plant has also been reported [Citation27]. The effect of the saponin-rich fractions of the stem and root bark of the plant on murine models of pain and inflammation has been reported [Citation28]. This is a follow-up work to isolate bioactive compound(s) from the stem bark of the plant. We now report the isolation and characterization of (+)-Catechin-3ꞌ-O-rhamnopyranoside from the stem bark of Neocarya macrophylla and its bioactivities.
Materials and methods
General procedures
NMR data were recorded on a Bruker AVANCE spectrometer (600 MHz) with residual solvent as an internal standard. Melting point was determined on an Electrothermal melting point apparatus. TLC was carried out using silica gel 60 GF254 pre-coated aluminum sheets manufactured by Sigma Aldrich, Germany. Column chromatography was conducted using LOBA Cheme silica gel (60–200) mesh while gel filtration chromatography was performed using sephadex LH-20 (Sima, Spruce street, St. Louis, MO, USA). Spots on TLC plates were visualized by spraying with 10% H2SO4 followed by heating at 105°C for 10 min.
Plant material
The plant sample of N. macrophylla was collected in October 2015 at Jega Local Government Area of Kebbi State. It was identified by Namadi Sanusi at the Herbarium section, Department of Biological Sciences, Ahmadu Bello University by comparing with herbarium reference voucher specimen (No. 3197). The stem bark was shade-dried, pulverized to powder, labeled and stored at room temperature for use.
Extraction and isolation
The dried powder (3000 g) was extracted with 90% methanol using maceration method for 6 days. After evaporation of the solvent with the aid of rotary evaporator at 40°C, the extract was suspended in distilled water, filtered and successively partitioned with n-hexane (1 L), dichloromethane (1 L), ethylacetate (2.5 L) and n-butanol (2.5 L) to obtain hexane, dichloromethane, ethylacetate, n-butanol and the residual aqueous fractions. The ethylacetate fraction (13.0 g) was gradiently eluted in a silica gel-packed column (5 × 75 cm) using different solvent combinations starting with n-hexane: ethylacetate (1:1), ethylacetate (100%) to ethylacetate: methanol (8:2). Thirty (30) mL each of a total of 426 fractions was collected and combined based on their TLC profile to give (12) major fractions coded EA-EL. Repeated gel filtration of fraction EH with sephadex LH-20 using methanol as eluting solvent gave three major fractions EH1–EH3. Fraction EH1 further purified using gel filtration repeatedly yielded Compound 1 (82.5 mg) and it was subjected to Ferric chloride, Fehling’s tests and spectroscopic analysis to elucidate its structure.
Pharmacological studies
Experimental animals
Swiss albino mice and adult Wister rats of either sex weighing (18–22 g) and (170–200 g), respectively, were obtained from the Animal House Facility of the Department of Pharmacology and Therapeutics, Ahmadu Bello University, Zaria, Nigeria. They were fed with laboratory diet and water ad libitum and maintained under standard conditions (12 h light and 12 h dark cycle) in propylene cages at room temperature. All experimental procedures were approved by the Animal Right and Ethics Community of the University.
Venom sample
The venom of an adult Naja nigricollis was collected by the milking method of Markfalane [Citation29]. It was lyophilized and stored at 4°C until required.
Standard drugs
Piroxicam (Pfizer, Pakistan). Lyophilized polyvalent snake venom antiserum by VINS Nioproduct Limited, Andra Pradesh, India. Batch. No: 01AS14054, MD: July 2015, ED: June 2018.
Microbial organisms
Clinical isolates were obtained from the Department of Medical Microbiology, Ahmadu Bello University Teaching Hospital, Zaria, Nigeria and comprised of Staphylococcus aureus, Vancomycin-Resistant Enterococci, Streptococcus pyogenes, Streptococcus faecalis, Escherichia coli, Corynebacterium ulcerans, Streptococcus pneumoniae, Pseudomonas aeruginosa, Proteus vulgaris, Candida albicans Candida tropicalis Candida krusei. All the isolates were checked for purity and maintained in slants of nutrient agar (for bacteria) and in slants of sabouraud dextrose agar for fungi
Antisnake venom studies
Lethality assay of the venom
The LD99 of the venom was determined using the method described by [Citation30]; the N. nigricollis venom was reconstituted with normal saline and concentrations ranging from 0.1 to 0.6 mg/mL were obtained. Thirty-five (35) mice were divided into seven (7) groups of five (5) mice each and were injected i.p. with different doses of the reconstituted venom. The control group received only normal saline (0.2 mL each, i.p.). The LD99 was calculated by probit analysis [Citation31] of death occurring within 24 h of venom injection.
Venom detoxification by the compound
The method described by [Citation32] was employed in this study. Twenty (20) mice were divided into four groups (n = 5). Group 1 (control) received the MLD (LD99, 5.75 mg/kg) of N. nigricollis venom only. Groups 2, 3 and 4 (treatment groups) received an equivalent of the MLD of the venom containing 1, 5 and 10mg/kg of compound 1, previously incubated at 37°C for 10 min and the animals were observed for mortality for 24 h. The route of administration was intraperitoneal.
Phospholipase A2 enzyme assay
Acidimetric method, as reported by Tan and Tan [Citation33], was used to evaluate the PLA2 enzyme inhibition potential of compound 1.
Analgesic studies – formalin test in rats
Fifteen rats were divided into five groups of three rats and were administered with either normal saline (1 mL/kg, i.p.), compound 1 (2.5, 5.0 and 10.0 mg/kg, i.p.) or piroxicam (10 mg/kg, i.p.). Thirty minutes after this treatment, 50 µL of a freshly prepared 2.5% solution of formalin was injected subcutaneously under the plantar surface of the left hind paw of each rat. The rats were placed individually in an observation chamber and monitored for 1 h. The severity of pain response was recorded for each rat based on the following numerical scale: (0) rat walked or stood firmly on the injected paw; (1) the injected paw was favored or partially elevated; (2) the injected paw was clearly lifted off the floor; (3) the rat licked, chewed or shook the injected paw. Anti-nociceptive effect was determined in two phases. The early phase (phase 1) was recorded during the first 5 min, while the late phase (phase 2) was recorded during the last 45 min with a 10 min lag period in-between both phases [Citation34].
Anti-inflammatory studies – formalin-induced inflammation in rats
Formaldehyde 2.5% v/v was used as inflammogen [Citation35]. Rats were divided into five groups of three rats each. Thirty minutes before injection of 2.5% v/v formalin (50 µL volumes in the subplantar region of the left hind paw of the rat), the groups were treated i.p. as follows:
Group I: received (1 mL normal saline per kg, as negative control).
Groups II, III and IV: received (compound 1, at 2.5, 5.0 and 10.0 mg per kg, respectively).
Group V: received (10 mg piroxicam per kg, as positive control). The paw diameter (cm) was measured using vernier caliper at an interval of 1 h for 4 h.
Antimicrobial screening
Compound 1 was tested for antimicrobial activity against clinical isolates of S. aureus, VRE, S. pyogenes, S. feacalis, E. coli, C. ulcerans, S. pneumonia, P. aeruginosa, P. vulgaris, C. albicans, C. tropicalis and C. krusei, as previously described by Vollekova et al. [Citation36]. Mueller Hinton agar was used as the growth medium for the microbes. The medium was prepared according to the manufacturer’s instructions and sterilized at 121°C for 15 min. Nutrient broth and Sabdroud dextrose broth were used for antibacterial and antifungal evaluations, respectively. It was poured into sterile petri dishes, allowed to cool and solidify. The sterilized medium was seeded with 0.1 mL of standard inoculum of the test microbe; the inoculum was spread evenly over the surface of the medium by the use of a sterile swab. Standard sterile cork borer of 6 mm in diameter was used to bore a well at the center of each inoculated medium. The wells were filled with 0.1 mL of the solution of the compound and allowed to diffuse for 1 h. Incubation of the inoculated medium was made overnight at 37°C and 25°C for bacteria and fungi, respectively, after which the medium was observed for the zone of inhibition of growth; the tests were done in duplicates and the zone of inhibition was measured with a transparent ruler. The mean of the results was recorded in millimeters (mm). The MICs of the compound were determined using the broth dilution method. Two-fold serial dilutions of the compound in the sterile broth were made to obtain the concentrations of 100, 50, 25, 12.5, 6.25 and 3.13 µg/mL; 0.1 mL suspension of the standard inoculum of the test microbe was then inoculated into the different concentrations of the compound. The tubes were incubated at 37°C for 24 h and 25°C for 48 h for bacteria and fungi, respectively, after which the plates were observed for turbidity (growth). The MIC was defined as the lowest concentration of the compound inhibiting the visible growth of each micro-organism. MBC/MFC was carried out to determine whether the test microbes were killed or only their growth was inhibited. Mueller Hinton agar broth was prepared, sterilized at 121°C for 15 min, and transferred into sterile petri dishes to cool and solidify. The contents of the MIC in the serial dilution were sub-cultured into the prepared medium and incubated at 37°C for 24 h; the plates were observed for colony growth; the MBC/MFC was the plate with lowest concentration of the compound in serial dilution without colony growth [Citation36].
Statistical analysis
The data were analyzed using Chi-Square and One-Way ANOVA followed by post hoc test using the SPSS software (version 22). The results were expressed as Mean ±SEM. Values of p < 0.05 were considered significant.
Results
Characterization of compound 1
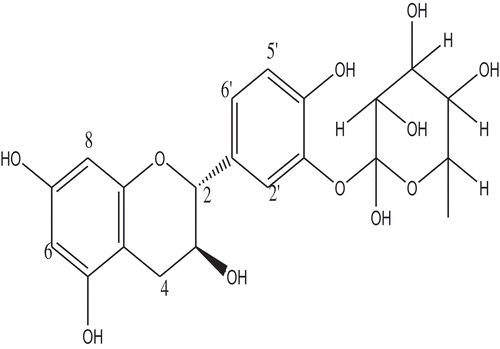
(+)-Catechin-3ꞌ-O-rhamnopyranoside. Dark brown solid substance, m.p. 206–208°C; The 1H-NMR spectrum (CD3OD) of compound 1 revealed the following chemical shift values/integration: δH 5.91 (1H, d, J = 1.9 Hz, H-6), 5.97 (s, H-8), 7.14 (1H, d, J = 8.3 Hz, H-5ꞌ),6.95 (1H, d, J = 2.0 Hz, H-2ꞌ),6.86 (1H, dd, J = 2.0, 8.3 Hz, H-6ꞌ), 4.65 (1H, d, J = 7.5 Hz, H-2),4.03 (m, H-3), 2.89 (IH, dd, J = 5.4, 16.1 Hz, H-4b), 2.57 (1H, dd, J = 8.2, 16.1 Hz, H-4a), 1.23 (J = 6.0 Hz, H-6ꞌꞌ), 4.15 (1H, dd, 1.3, 2.1 Hz, H-2ꞌꞌ), 5.42 (1H, d, J = 1.3, H-1ꞌꞌ), 3.98 (1H, J = 3.3, 9.5 Hz, H-6ꞌꞌ). 13C-NMR (600 MHz, CD3OD); δC 156.1 (C-9), 155.4 (C-5), 156.4 (C-7), 99.8 (C-10), 134.4 (C-1ꞌ), 147.2 (C-4ꞌ) and 144.1 (C-3ꞌ), 81.2 (C-2), 67.4 (C-3), 27.1 (C-4), 94.0 (C-6), 95.0 (C-8), 118.5 (C-6ꞌ), 117.1 (C-5ꞌ), 114.8 (C-2ꞌ), 100.0 (C-1ꞌꞌ), 70.6 (C-2ꞌꞌ), 70.8 (C-3ꞌꞌ), 72.6 (C-4ꞌꞌ), 69.4 (C-5ꞌꞌ), 16.6 (C-6ꞌꞌ).
Antisnake venom studies
The minimum lethal dose (LD99) of the venom estimated by probit was 5.75 mg/kg. Compound 1 has demonstrated significant (p < 0.05) antivenom activity against N. nigricollis venom in mice. Maximum protection (100%) against venom-induced lethality was observed at the lowest dose (1 mg/kg) compared to the control group; 40% and 20% survival rate was recorded at 5 and 10 mg/kg of compound 1, respectively ().
Table 1. 1D and 2D NMR spectral data summary for compound 1 (CD3OD).
Table 2. 1H and 13C-NMR data of compound 1 compared with reported literature.
Table 3. In vitro detoxifying effect of compound 1 against N. nigricollis venom.
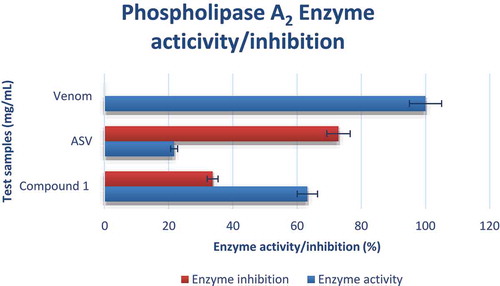
The compound was able to inhibit the action of N. nigricollis PLA2 enzyme activity with 33.7% inhibition which was lower compared to the standard ASV (72.9%) ().
Analgesic studies
The result of the analgesic activity of compound 1 is shown in . Compound 1 exhibited significant (p < 0.05) analgesic effect in both phases; the compound at the highest (10 mg/kg) and medium (5 mg/kg) doses was able to inhibit the pain response after injection of formalin in the first phase with mean pain scores of 2.33 ± 1.2 and 2.67 ± 2.1, respectively, which was better than the effect (4.67 ± 2.7) observed for the standard drug, piroxicam at 10 mg/kg ().
Table 4. Effect of compound 1 on formalin-induced pain in rats.
Anti-inflammatory studies
Compound 1 and the standard drug, piroxicam reduced the increase in paw size induced by formalin at all the tested doses (2.5, 5.0 and 10 mg/kg) when compared to the negative control (). Highest inhibition of inflammation was observed at the highest dose (10 mg/kg); however, the lowest dose (2.5 mg/kg) had 45.0% and 41.2% inhibition of inflammation at the 3rd and 4th hours, respectively. The standard drug, piroxicam at 10 mg/kg had 65% inhibition of inflammation ().
Table 5. Effect of compound 1 on formaldehyde induced inflammation in rats.
Antimicrobial studies
The result of antimicrobial activity of compound 1 is shown in . Twelve microorganisms (including nine bacteria and three fungi) were screened. The compound was active against all the test organisms except S. pyogenes, C. ulcerans, S. pneumoniae and C. feacalis with zone of inhibition ranged between 28 and 36 mm; the most sensitive organism was P. aeruginosa (36 mm) while the least sensitive was the fungi, C. albicans (28 mm); the zones of inhibitions were slightly lower than those observed for the standard drugs (ciprofloxacin and fluconazole). The compound exhibited higher inhibition zone against C. krusei (35 mm) compared to the standard antifungal agent (fluconazole) with mean zone of inhibition of 32 mm. The MIC values ranged from 6.25 to 25.0 µg/mL while MBC/MFC ranged from 12.5 to 50 µg/mL.
Table 6. Antimicrobial activity of compound 1 (100 µg/mL).
Discussion
The study was inspired by the widespread ethnomedicinal use of the plant N. macrophylla in the management of snakebite, microbial infections, pain, and anti-inflammatory diseases. Preliminary results provided promising results and validated the ethnomedicinal claims [Citation25] . Inspired by these results, the ethylacetate soluble fraction of the methanol stem bark extract which appeared to be promising was subjected to chromatographic studies in order to isolate and evaluate bioactive compound(s) responsible for the observed antivenom, analgesic, anti-inflammatory, and antimicrobial effects. Thus, the study led to the isolation of a flavonoid. Compound 1 was isolated as a dark brown solid substance. It reacted positively to ferric chloride reagent suggesting the presence of phenolic nucleus and the presence of sugar moiety was confirmed via Fehling’s test [Citation37]. The basic skeletal structure of compound 1 was confirmed from the 1H-NMR data; the presence of two meta-coupled protons (ring A) at δH 5.91 (1H, d, J = 1.9 Hz) and 5.97 (brs) corresponding to H-6 and H-8, respectively, was observed from the calculation of J values. An ABC ring system was clearly discerned from the proton signals at δH 7.14 (1H, d, J = 8.3 Hz, H-5ꞌ), δH 6.95 (1H, d, J = 2.0 Hz, H-2ꞌ) and δH 6.86 (1H, dd, J = 2.0, 8.3 Hz, H-6ꞌ) and the protons at δH 4.65 (1H, d, J = 7.5 Hz, H-2) and δH 4.03 (m, H-3) were due to the presence of an oxygenated aliphatic moiety and the 2, 3-trans stereochemistry was depicted by the high coupling constant value (JH-2-H-3 = 7.5 Hz) of the heterocyclic ring C which confirmed compound 1 to be a catechin rather than an epicatechin [Citation38–Citation41]. Two methylene proton signals at δH 2.89 (IH, dd, J = 5.4, 16.1 Hz) and δH 2.55 (1H, dd, J = 8.2, 16.1 Hz) representing H-4b and H-4a, respectively, further confirmed the presence of an AX-type ring C typical of 3-flavan-type flavonoids [Citation35,Citation36]. The sugar moiety was defined by the resonances at δH 3.51–5.42 with integral intensities of five protons and a doublet at δH 1.23 (J = 6.0 Hz) typical of the – CH3 group which suggests the sugar to be a rhamnoside [Citation42–Citation43]. The anomeric configuration was assigned based on the coupling constant (J = 1.3 Hz) of the anomeric proton at δH 5.42. Proton signal at δH 4.15 (1H, dd, 1.3, 2.1 Hz) was assigned to H-2” and H-3” appeared as a dd at δH 3.98 (1H, J = 3.3, 9.5 Hz). H-4” resonated as a triplet (δH 3.51) with coupling constant J = 9.5. Protons were observed with J values (J = 3.4 Hz) all across at δH 3.84 and was assigned to H-5” of the sugar.
The 13C-NMR (600 MHz, CD3OD) and DEPT experiments of compound 1 indicated the presence of seven aromatic carbon peaks. The 13C-NMR spectrum showed a resonance at δC 81.2 which appeared in a lower field further suggesting the compound to be a catechin [Citation38–Citation41]. The chemical shift values at δC 100.0, 70.6, 70.8, 72.6 and 69.4 were characteristic of sugar absorptions and the – CH3 absorption at δC 16.6 suggests the sugar to be a rhamnose which is in consistency with the 1H-NMR data [Citation44,Citation45]. All protonated carbons were assigned by the HSQC spectra (); the 2D 1H-1H COSY correlation spectrum of compound 1 revealed the protons that are adjacent to one another. Major correlations observed are; δH 7.14 (H-5ʹ) and 6.86 (H-6ʹ) which confirmed the assignment on ring B; Correlations at δH 4.65 (H-2) and 4.03 (H-3), δH4.03 (H-3) and 2.89, 2.55 (H-4b, a), δH 2.89 (H-4b) and 2.55 (H-4a) confirmed the presence of ring C, typical of flavan-3-ols. The anomeric proton of the sugar moiety δH 5.42 (H-1”) correlated with the proton at δH 4.15 (H-2”). Other correlations observed within the sugar moiety include; δH 3.98 (H-3”) and 3.51 (H-4”), δH 3.84 (H-5”) and 3.51 (H-5”). The HMBC between the protons and carbons was used to establish the connectivity between the sub-structures in the molecule. Long-range correlation was observed at δH7.14 and δC 147.2 (C-4ʹ), 144.1 (C-3ʹ) and 134.4 (C-1ʹ); δH 6.95 and δC 81.2 (C-2), 118.5 (C-6ʹ), 144.1 (C-3ʹ) and 147.2 (C-4ʹ); δH 6.86 and δC 81.2 (C-2), 114.8 (C2ʹ), 144.1 (C-3ʹ) which confirmed the presence of ring B system. The presence of an ABC-system was ascertained via the correlations observed at δH 5.97 and δC 156.4 (C-9) and 99.8 (C-10); δH 4.65 and δC 156.4 (C-9), 134.4 (C-1”), 118.5 (C-6ʹ), 114.8 (C-2ʹ), 67.4 (C-2) and 27.1 (C-4); δH 2.89, 2.55 (H-4b, a) and δC 155.4 (C-7), 99.8 (C-10), 81.2 (C-2) and 67.4 (C-4). The anomeric proton signal at δH 5.42 (H-1”) exhibited a long-range correlation with δC 144.1 (C-3ʹ) and 70.8 (C-3”) confirming the linkage of the aglycone to the rhamnosyl chain at C-3. Other HMBC correlations for the sugar moiety are δH 3.98 and δC 72.6 (C-4”); δH 3.51 and δC 70.6 (C-2”) and 16.6 (C-6”) (). Based on the 1D and 2D-NMR spectral data () and comparison with existing data in the literature [Citation46, ], the structure of compound 1 () was confirmed as (+)-Catechin-3ꞌ-O-rhamnopyranoside.
Snakebite envenomation is a major public health problem especially in the rural areas of the savannah region of West Africa notably Nigeria, Ghana among others; the African cobras (Naja spp) is one of the major venomous snakes frequently causing envenomation in the northern part of Nigeria [Citation7]. Cobra bites is accompanied by sharp pain (which spread within few minutes), blisters, necrosis, tenderness, inflammation, and severe bacterial infection to mention but few [Citation47–Citation48]. Several bioactive compounds have been evaluated for their antisnake venom [Citation16,Citation49] and other effects. However, to the best of our knowledge, there is no report yet on the antivenom, analgesic, anti-inflammatory and antimicrobial properties of (+)-ZCatechin-3ꞌ-O-rhamnopyranoside. In this study, the effect of compound 1 was evaluated on snakebite, pain, inflammation and microbial infection was investigated.
The survival rate of mice injected with the pre-incubated mixture of an equivalent of the LD50 of N. nigricollis venom and the isolated compound 1 was used to deduce the antivenom effect of the plant [Citation30]. The findings indicated that compound 1 gave maximum protection against N. nigricollis venom-induced actions in a dose-dependent manner with the lowest dose having the highest effect; the possible explanation to this phenomena could be due to saturation at the higher doses. Catechin and gallocatechin were reported to have antivenom effect [Citation50].
Phospholipase A2 enzyme assay conducted using acidimetry [Citation33]; compound 1 was able to inhibit the action of N. nigricollis PLA2 enzyme activity with 33.7% inhibition which was lower compared to the standard ASV (72.9%).
Studies have shown that PLA2 inhibitors constitutes structural features similar to those of phenolic compounds with aromatic ring substituted by one or more hydroxyl groups [Citation51]; which include, phenolic hydroxyl group on carbon 5, the pyronic carbonyl, the double bond, and double-bonded oxygen in the oxane ring [Citation52–Citation53]; for selective inhibition of PLA2-II, hydroxyl groups in positions 3ʹ and 4ʹ are required (these features are important for the overall capacity of a compound to inhibit PLA2 activity) [Citation54–Citation55]. Lower effect of compound 1 might be due to the absence of the pyronic carbonyl, the double bond, and double-bonded oxygen in the oxane ring of most flavonoids; however, the sugar moiety might contribute to the observed effect of compound 1 [Citation16].
Formalin acts via two mechanisms in which the first phase involves neurogenic pain that is initiated when harmful stimuli agitate nociceptors while the second phase involves anti-inflammatory reactions [Citation56]. Studies have shown that drugs that inhibit both phases possessed central acting effect and peripheral acting drugs only inhibit the second phase. The ability of the compound to diminish the nociceptive response induced by formalin in both phases suggests that compound 1 may possess central acting analgesic effect with shorter onset and duration of action [Citation56–Citation58].
Formalin is a pro-anti-inflammatory agent that acts by inhibiting acute inflammatory mediators [Citation59]. Formalin-induced inflammation is believed to be biphasic, the early phase (1–2 h) is mediated by histamine, serotonin and increased synthesis of prostaglandins while the late phase involves the release of prostaglandins and it is mediated by bradykinin, leukotrienes and related substances [Citation61]. The ability of compound 1 to significantly (p < 0.05) decreased inflammation induced by formalin suggests that the mechanism of action is related to the inhibition of histamine, serotonin, and related substances as well as the inhibition of prostaglandins synthesis [Citation61].
Based on the postulation that mean zone of inhibition diameter >18 mm [Citation62], compound 1 can be said to possess a good broad spectrum of activity. Also, Tang et al. [Citation63–Citation64] reported that compounds with MIC values less than 100 µg/mL are regarded as very good antimicrobial agents. Catechins and sugars have been reported to have good in vitro antibacterial activity [Citation65–Citation68]. Mori et al. [Citation69] suggested that the ring B of the flavonoids may intercalate or form hydrogen bond with the stacking of nucleic acid bases and further lead to inhibition of DNA and RNA synthesis in bacteria while most antifungal agents act by interfering with the production of fungal protein, DNA replication and via different cellular metabolisms [Citation70], hence the compound might act via the same mechanism. S. aureus, E. coli, and Enterococcus spp were the most common pathogens identified in wound infections associated with snakebites [Citation48]; compound 1 has demonstrated good activity against the pathogens.
In conclusion, the results of this study indicated that catechin-3-O-rhamnopyranoside isolated from the ethylacetate soluble fraction of the stem bark of N. macrophylla has demonstrated significant antivenom, analgesic, anti-inflammatory, and antimicrobial activities, thus providing a new theory in the search for better therapeutic agents in the treatment of snakebite, pain, inflammation and microbial infections. To the best of our knowledge, this is the first detailed biological activity of this compound. Thus, further detailed phytochemical studies is still ongoing in our laboratory with a view to isolate more bioactive compound(s) from the plant.
Acknowledgments
We would like to acknowledge the efforts of Mr. Dilip Jagjivan, School of Chemistry and Physics, University of Kwa-Zulu Natal-Durban, South Africa for running the 1D & 2D-NMR spectroscopy; Dr Mika’ila of the Institute of Leather Research, Samaru, Zaria, for rendering assistance and bench space to conduct the antimicrobial studies and Pharmacist Aminu A. Biambo for running the statistical analysis.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- World Health Organization, WHO. 2019. Snake envenomings. WHO Fact sheets; [cited 2019 Aug 23]. Available from: https://www.who.int/news-room/fact-sheets/detail/snakebite-envenoming.
- Omogbai EK, Nworgu ZA, Imhafidon MA, et al. Snake bites in Nigeria: a study of the prevalence and treatment in Benin City. Trop J Pharm Res. 2002; 1:39–44.
- Chippaux JP. Estimate of the burden of snakebites in sub-Saharan Africa: a meta-analytic approach. Toxicon. 2011; 57:586–599.
- Habib AG. Public health aspects of snakebites care in West Africa: perspectives from Nigeria. J Venomous Anim Toxins Incl Trop Dis. 2013; 19: 27.
- Kshirsagar VY, Ahmed M, Colaco SM. Clinical profile of snake bite in children in rural India. Iran J Pediatr. 2013; 23: 632–636.
- Ghosh R, Mana K, Gantait K, et al. A retrospective study of clinico-epidemiological profile of snakebite related deaths at a tertiary care hospital in Midnapore, West Bengal, India. Toxicol Rep. 2018; 5: 1–5.
- Warrell DA, Ormerod LD. Snake venom ophthalmia and blindness caused by the spitting cobra (Naja nigricollis) in Nigeria. Am J Trop Med Hyg. 1976; 25(3): 525–529.
- Habib AG. Tetanus complicating snake bite in northern Nigeria: clinical presentation and public health implications. Acta Trop. 2003; 85(1): 87–91.
- Habib AG, Abubakar SB, Abubakar IS, et al. Envenoming after carpet viper (Echis ocellatus) bite during pregnancy: timely use of effective antivenom improves maternal and foetal outcomes. Trop Med Int Health. 2008; 13(9):1172–1175.
- Abubakar SB, Habib AG, Mathew J. Amputation and disability following snakebite in Nigeria. Trop Doc. 2010; 40(2): 114–116.
- Rusell FE. Snake venompoisoning. Philadelphia: J.B. Lippincott Company; 1980. Pp. 235–285.
- Looareesuwan S, Viravan C, Warrell DA. Factors contributing to fatal snake bite in the rural tropics: analysis of 46 cases in Thailand. Trans R Soc Trop Med Hyg. 1988; 82:930–934.
- Hansdak SG, Lallar KS, Pokharel P, et al. A clinico-epidemiological study of snake bite in Nepal. Trop Doct. 1998; 28:223–226.
- Mathew JL, Gera T 2015. Ophitoxaemia (venomous snake bites). [cited 2015 Aug 19]. Available from: http://www.priory.com/med/ophitoxaemia.htm.
- Aghahowa SE, Ogbevoen RN. Incidence of snake bite and utilization of antivenom in the University of Benin Teaching Hospital Benin City, Nigeria. Niger J Exp Clin Biosci. 2017; 5:5–10.
- Gomes, A., Das, R., Sarkhel, S., Misra, R. and Mukherjee, S. Herbs and Herbal Constituents active against snake bite. India J. Exp. Biol. 2010; 48: 865–878.
- Walter, B. M., Maria, C. N., Bettima M. R. P. and Nuno, A. P. Plant natural products active against snake bite – the molecular approach. Phytochemistry. 2000; 55: 627–642.
- Burkill HM. The useful plants of West Tropical Africa.1; 1985. Vol 3, Royal Botanic Gardens, Kew, UK. Pp. 1–3.
- Arbonnier M. Trees, Shrubs and Lianas of WestAfrican dry zones. Cidrad: Margraf Publishers; 2004. p. 250–251.
- Warra AA, Umar RA, Sani I, et al. Preliminary phytochemical screening and physicochemical analysis of ginger bread plum (Parinari macrophylla) seed oil. J Pharmacogn Phytochem. 2013; 1(2):20–25.
- Yusuf, A. J., Abdullahi, M. I., Haruna, A. K., Idris, A.Y. and Musa, A. M. Preliminary Phytochemical Screening, Toxicological and Antivenin property of the stem bark of Neocarya macrophylla on Naja nigricollis venom. African Journal of Pharmaceutical Research and Development. 2015a; 7(1): 6–10.
- Yusuf, A. J., Abdullahi, M. I., Haruna, A. K., Idris, A. Y. and Musa, A. M. Isolation and Characterization of Stigmasterol and Bis-(5,7-diacetyl-catechin-4’-α-rhamnopyranoside) from the Stem bark of Neocarya macrophylla (Sabine) Prance (Chrysobalanaceae). Nigerian Journal of Basic and Applied Science. 2015b; 21(1): 15–22.
- Yusuf A. J., Abdullahi M. I., Haruna A. K., Musa A. M., Ibrahim Z. Y. Y. and Uba A. Acute Toxicity Studies and Evaluation of Analgesic Property of the Methanol Stem Bark Extract of Neocarya macrophylla. Journal of Applied Pharmaceutical Sciences. 2015c; 5(Suppl1): 061–064.
- Yusuf, A. J., Abdullahi, M. I., Haruna, A. K., Musa, A. M., Abdullahi, M. S. Ibrahim, Z. Y. Y., Halilu, M. E. and Odiba, O. J. Phytochemical and antimicrobial evaluation of the methanol stem bark extract of Neocarya macrophylla. Journal of Chemical and Pharmaceutical Research. 2015d; 7(1): 477–481.
- Isaka I., Gumel, A. M., Muhammad, H. Y. and Kemi, A. F. Phytochemical analysis and antimicrobial activity of Neocarya macrophylla leaves extract. International Journal of Health and Life-Sciences. 2017; Vol. 3 Issue 1, pp. 18– 34. DOI-https://dx.doi.org/10.20319/lijhls.2017.31.1834.
- Olowo-Okere A, Yusuf AJ, Shuaibu AB, et al. Antibacterial and anti-biofilm activities of Neocarya macrophylla against clinical bacterial isolates. Niger J Pharm Res. 2018; 14(1):111–119.
- Yusuf AJ, Abdullahi MI, Aleku GA, et al. Antimicrobial activity of stigmasterol from the stem bark of Neocarya macrophylla. J Med Plants Econom Dev. 2018; 2(1):a38: 1–5.
- Yusuf Amina Jega, Musa Ismail Abdullahi, Amina Idris Rufa’i, Asma’u Baba Bala, Hassan Abubakar, Muntaka Abdulrahman, Celestina Oluranti Alebiosu, Aminu Ahmed Biambo and Millicent Ladi Umaru. Effect of Saponin-rich fractions of Neocarya macrophylla on murine models of pain and inflammation. Asian Journal of Biological Sciences. 2019; 12: 349–355. Doi 10.3923/ajbs.2019.349.355. ISSN 1996-3351.
- Markfarlane, R. G. Russel’s Vipers Venoms, 1963-1964. British Journal of Haemat. 1967; 13: 437–451.
- Theakston, R. D. G. and Reid, H. A. The development of simple standard assay procedures for characterization of snake venoms. Bull. W.H.O. 1983; 61: 946–956.
- Finney, D. J. Probit analysis. 3rd Edition. Cambridge University Press. Cambridge. London. 1977; Pp. 8– 49.
- Abubakar, M. S., Sule, M. I., Pateh, U. U., Abdulrahman, E. M., Haruna, A. K. and Jahun, B. M. In vitro snake venom detoxifying action of the leaf extract of Guiera senegalensis. Journal of Ethnopharmacology. 2000; 69: 253–257.
- Tan NH, Tan CS. Acidimetric assay of phospholipase A2 using egg yolk suspension as substrate. Anal Biochem. 1988; 170(2):282–288.
- Dubuisson, D. and Dennis, S. R. The formalin test: A quantitative study of the analgesic effects of morphine, meperidine and brain stem stimulation in rats and cats. Pain, 1977; 4: 161–174.
- Pateh, I. M. Sule, I. Iliya, A. K. Harun, A. H. Yaro, A. A. Ambi and A. M. Musa. Analgesic and anti-inflammatory activities of the methanolic extract of the rhizomes of Stylochiton lancifolius Pyre and Kotchy (Araceae) in rodents. Journal of Medicinal Plants Research 2011; 5(21), pp. 5203–5207.
- Vollekova A, Kostalova D, Sochorova R. Isoquinoline alkaloid from Mahonia aquifolium stem bark is active against Malsseizia species. Folia Microbiol. 2001; 46:107–111.
- Silva GL, Lee I, Douglas KA. Special problems with extraction of plants. In: Cannell JPR, editor. Natural products isolation. New Jersey USA: Human Publishers; 1998. p. 251–293.
- Petereit F. 1992. Polyphenolische Inhaltsstoffeund Untersuchungen zurentsündung shemmenden Aktivität der traditionellen Arzneipflanze Cistus incanus L. (Cistaceae) [PhD thesis]. Germany: University of Münster.
- Kombal R 1993. Untersuchung der Flavan-3-ole und Flavanoide in Potentilla anserina L [PhD thesis]. Germany: University of Münster.
- De Mello JP, Petereit F, Nahrstedt A. Dictionary of food compounds. Phytochemistry. 1996; 41:807–813.
- Antonelli UTM, Yamaguti E, Uemura LM, et al. Chemical and microbiological study of extract from seeds of guarana (Paullinia cupana varsorbilis). Latin Am J Pharm. 2007; 26(1):5–9.
- Fatima AM, Mohamed SAM. A novel phenylethanoid dimer and flavonoids from Jacaranda mimosaefolia. Z Naturforsch. 2007; 62:1213–1220.
- Hye MA, Taher MA, Ali MY, et al. Isolation of (+) – catechin from Acacia catechu (Cutch Tree) by a convenient method. J Sci Res. 2009; 1(2):300–305.
- Manfred B, Hilmar F, Herbert K. (+)-Catechin-3-Rhamnoside from Erythroxylum novogranatense. Phytochem. 1986; 25(5):1205–1207.
- Surabhi Y, Bhadoria K. Two dimeric flavonoids from Bauhinia purpurea. Indian J Chem. 2005; 4(4):2604–2607.
- Jung EK, Sang SK, Chang-Gu H, et al. Antioxidant chemical constituents from the stems of Cleyera japonica thunberg. Int J Pharmacol. 2012; 8(5):410–415.
- Garg, A., Sujatha, S., Garg, J., Acharya, N. S. and Chandra P. S. Wound secondary to snakebite. J. Infect. Dev. Ctries, 2009;.30(3): 221–223.
- Chen, C. M., Wu, K. G., Chen, C. J. and Wang C. M. Bacterial infection in association with snakebite: a 10-year experience in a northern Taiwan medical center. J. Microbiol. Immunol. Infect, 2011; 44(6): 456–60. Doi: 10.1016/j.jmii.04.011.
- Mors WB, Nascimento MC, Pereira BM, et al. Plant natural products active against snakebite-the molecular approach. Phytochem. 2000; 55(6):627–642.
- Esmeraldino LE, Souza AM, Sampaio SV. Evaluation of the effect of aqueous extract of Croton urucurana Bailon (Euphorbiaceae) on the hemorrhagic activity induced by the venom of Bothrops jararaca, using new techniques to quantify hemorrhagic activity in rat skin. Phytomed. 2005; 12:7.
- Nicholson RL, Hammerschmidt R. Phenolic compounds and their role in disease resistance. Ann Rev Phytopathol. 1992; 30:21.
- Mors WB. Plants against snake-bites. Memorias Do Instituto Oswaldo Cruz. 1991; 86(Suppl. 2):193.
- Soares AM, Ticlia FK, Marcussi S, et al. Medicinal plants with inhibitory properties against snake venoms. Curr Med Chem. 2005; 12:2625–2641.
- Chippaux JP, Rakotonirina VS, Rakotonirina A, et al. Drug or plant substances which antagonize venoms or potentiate antivenins. Bull Soc Pathol Exot. 1997; 90(4):282–285.
- Lindahl M, Tagesson C. Flavonoids as phospholipase A2 inhibitors: importance of their structure for selective inhibition of group II phospholipase A2. Inflammation. 1997; 21(3):347–356.
- Tominaga M, Numazaki M, Iida T, et al. 2004. Regulation mechanisms of vanilloid receptors. In Symposium on Pathological Pain: From Molecular Clinical Aspects, held at the Novartis Tsukuba Research Institute, Tsukuba, Japan, in collaboration with the Novartis Foundation (Japan) for the promotion of Science, 2003 Sept 30–2003 Oct 2. Chichester: John Wiley & Sons. pp. 4–18.
- Derardt R, Journey S, Delevalcee F, et al. Release of prostaglandins E and F in an algogenic reaction and its inhibition. Eur J Pharmacol. 1980; 51:17–24.
- Bentley GA, Newton SH, Starr J. Studies on the anti-nociceptive action of agonist drugs and their interaction with opioid mechanisms. British J Pharmacol. 1983; 32:295–310.
- Gepdiremen, A., Mshvildadze, V., Suleyman, H. and Elias, R. Acute anti-inflammatory activity of four saponins isolated from ivy: alpha-hederin, hederasaponin-C, hederacolchiside-E and hederacolchiside-F in carrageenan-induced rat paw edema. Phytochemistry, 2005; Volume 12, Issues 6–7 (15): p 440–444.
- Brito ARMS, Antonio MA. Oral anti-inflammatory and anti-ulcerogenic activities of a hydroalcoholic extract and partition fraction of Turnera ulmifolia (Turneaceae). J Ethnopharmacol. 1988; 61:215–228.
- Musa AM, Abdullahi MI, Mahmud MD, et al. Analgesic and anti-inflammatory activities of the methanol leaf extract of Indigofera hirsuta Linn and isolation of stigmasterol. Niger J Pharm Sci. 2012; 11(1):39–48.
- Tania MAA, Axreia FS, Mitzi B, et al. Biological screening of Brazilian medicinal plants. Mem Inst Oswaldo Cruz Rio De Janeiro. 2000; 95 (3):367–373.
- Mahmoud A. Ghannoum and Louis B. Rice. Antifungal agents: mode of action, mechanisms of resistance, and correlation of these mechanisms with bacterial resistance. Clinical Microbiology Reviews, 1996; vol. 12, issues 4: p501– 517.
- Tang T, Bremner P, Kortenkamp A, et al. Biflavonoids with cytotoxic and antibacterial activity from Ochna macrocalyx. Planta Med. 2005; 69: 247–253.
- Borris RP. Natural products research: perspectives from a major pharmacological company. J Ethnopharmacol. 1996; 51(1–3):29–38.
- Moerman DE. An analysis of the food plants and drug plants of native North America. J Ethnopharmacol. 1996; 52(1):1–22.
- Nakayama M, Shimatani K, Ozawa T, et al. A study of the antibacterial mechanism of catechins: isolation and identification of Escherichia coli cell surface proteins that interact with epigallocatechin gallate. Food Sci Biotech. 2013; 33(2):433–439.
- Zhoa L, Zhang H, Hao T, et al. In vitro antibacterial activities and mechanism of sugar fatty acid esters against five food related bacteria. Food Chem. 2015; 15(187):370–377.
- Mori, A., Nishino, C., Enoki, N. and Tawata, S. Antibacterial activity and mode of action of plant flavonoids against Proteus vulgaris and Staphylococcus aureus. Phytochemistry, 1987; vol. 26, no. 8, pp. 2231–2234, View at Google Scholar · View at Scopus.
- Pandima DK, Arif NS, Sakthive R, et al. Eugenol (an essential oil of clove) acts as an antibacterial agent against Salmonella typhi by disrupting the cellular membrane. J Ethnopharmacol. 2010; 130:107–115.
- Chippaux JP. Snakebite envenomation turns again into a neglected tropical disease. J Venom Anim Toxins Incl Trop Dis. 2017; 23:38.