ABSTRACT
Chronic wounds aggravate the patient’s indisposition by causing mobility, economic and social problems. Chitosan is a natural polymer that is considered a potent healing agent for skin lesions and has been used previously to manufacture dressings for wounds. The aim of this work was to evaluate the curative effect of chitosan membranes on cutaneous ulcers. The chitosan was characterized by measuring the degree of deacetylation and FTIR. The membranes were obtained by a solvent evaporation technique. Four patients with cutaneous ulcers were recruited to evaluate the effectiveness of the topical membranes. At the beginning of the in vivo assay, patients presented with infected wounds. By the end of treatment, the infection subsided, the granulation process was promoted and complete skin coverage was observed in most patients. The use of chitosan as a treatment for the healing of skin ulcers is considered highly promising for its medicinal properties, as well as for its affordability compared to traditional treatments.
Graphical abstract
Introduction
Venous and pressure ulcers tend to be the most frequent chronic wounds in patients with a comorbidity. People who suffer from venous ulcers can experience disability or low productivity, as well as venous hypertension [Citation1]. Pressure ulcers are secondary complications from ischemia that appear on the skin and can affect the epidermis, dermis, subcutaneous tissue and muscle and can damage the joint and bone [Citation2]. Traumatic ulcers (open and closed) occur from trauma or external aggression in an uncontrolled environment, with healing occurring by primary intention [Citation3].
Several of the factors that affect the healing process of ulcers and wounds are humidity, pressure, infection, necrotic tissue, and age, nutritional status and concomitant diseases of the patient [Citation4]. The mechanism of skin repair is induced by an injury that alters the skin surface. This initial injury is followed by three phases: inflammation, cell proliferation and tissue remodeling [Citation5]. For the local treatment of wounds, dressings are applied to achieve second intention healing [Citation6]. Current recommendations to promote wound healing are to use affordable natural materials to reduce infection and cause less pain for the patient.
Chitosan (poli β-1,4-amino-2-deoxi-D-glucopyranose) is a natural polysaccharide obtained by chitin deacetylation [Citation7]. Recent studies suggest that chitosan has antimicrobial and antifungal properties and is biocompatible, biodegradable, nontoxic and adherable [Citation8]. These properties make chitosan ideal for use in medical and pharmaceutical devices [Citation9], specifically in the biomedical field of drug encapsulation [Citation10,Citation11], in the development of artificial organs, orthopedic materials, and wound dressings, and as scaffolds for tissue regeneration [Citation12]. Kumirska et al. [Citation13] reported that chitosan can lower blood cholesterol levels and lacks allergenicity. The antimicrobial activity of chitosan has been reported under acidic conditions, and is attributed to the positively charged – NH3+ groups that interact with the negative charge of the bacterial membrane. This activity enables chitosan to interfere with the permeability and integrity of the cellular structure and determines the bactericidal or bacteriostatic effect [Citation14].
According to clinical studies conducted by various authors, the chitosan can be used in diverse type of lesions, ulcers or burners. Cárdenas et al. [Citation15] applied chitosan films in three clinical cases and the complete epithelialization was observed after a week. Other authors report that chitosan has also been used as a skin graft with good results [Citation16]. On the other hand, Azad et al. [Citation17] report that chitosan films have the ability to stimulate to the cells to repair the skin by promoting healing, hemostasis and re-epithelialization of the ulcers.
Traditional treatments are generally passive scaffolds with prolonged efficacy time and are characterized by having very high prices. Therefore, the aim of this work was to evaluate the curative effect of chitosan membranes on cutaneous ulcers. All membranes were characterized by physicochemical methods. Chitosan membranes are considered as advanced dressings because they generate a slightly acidic and humid microenvironment, increasing the antimicrobial effect and favoring the healing process. Likewise, it is not necessary to replace them daily, since they must have an adequate time of stay on the ulcer to cause autolytic debridement.
This work is part of a greater study examining the design and characterization of chitosan biomaterials for their application in in vitro and in vivo tests with patients who require skin treatment, specifically those who have diabetic foot ulcers.
Materials and methods
Production of chitosan
The chitosan was produced by thermoalkaline hydrolysis from shrimp chitin according to the procedure proposed by Sánchez–Duarte et al. [Citation18]. First, the raw chitin was purified in two steps, demineralized with 0.1 N HCl at room temperature and deproteinized with 4.5% NaOH at 60°C. After, to obtain chitosan, the purified chitin was treated with 45% NaOH at 110°C to remove the acetyl residues (deacetylation). Then, the purified chitosan was dried at room temperature, crushed and sieved for physicochemical characterization.
Degree of deacetylation
The degree of deacetylation in the chitosan was determined by spectrophotometry, as proposed by Liu et al. [Citation19]. Standard solutions of glucosamine 7.49 mM (0.08084 g in 500 mL of HCl 0.1 N) and N-acetilglucosamine 0.49 mM (0.1106 g in 1000 mL of HCl 0.1 N) were prepared. From these suspensions, working solutions were prepared to create a 12-point calibration line. Each point consisted of a mixture with a different concentration of the original patterns of N-acetylglucosamine and glucosamine prepared in a 50 mL volumetric flask with 0.1 M HCl. The absorbance of each mixture was measured on a Genesys 10 UV-VIS spectrophotometer (Madison, lowa, USA) at 201 nm. Likewise, the absorbance of each sample solution was measured with 2 mg in 10 mL of HCl 0.1 M. The degree of acetylation of the samples was later calculated with the following equation: DA = ((161.1 * A * V) – (c * m))/((k * m) – (42.1 * A * V)), where "k" and "c" are "a" and "b" of the calibration line equation, A is the absorbance of the sample, V is the total volume in liters where the sample was dissolved and m is the amount of sample in mg. Finally, to estimate the percentage of the degree of deacetylation the following relation was applied: %DA = ((1 – DA) * 100).
Viscosity
The viscosity was evaluated by Ilyina et al. [Citation20] proposed method with several modifications. Fifteen milliliters of 2% chitosan solution were added to an Ostwald viscometer, which was placed in a water bath at 21 ± 1°C to measure the time required for the chitosan to flow from point A to point B. The value is determined by the following equation: N2 = P2T2N1/P1T1, where N2 is the viscosity of chitosan, P2 is the density of chitosan, T2 is the time required for the chitosan to flow, N1 is the viscosity of acetic acid, P1 is the density of acetic acid and T1 is the time required for the acetic acid to flow.
Molecular weight
To determine the molecular weight of the chitosan, a curve was prepared in a concentration range of 0.0006 to 0.0014 mg/ml. Then, the molecular weight was calculated by the equation of Mark-Houwink, [n] = KMa [Citation21]. Where [n] is the intrinsic viscosity that corresponds to the ‘b’ value of the calibration line equation (which is obtained by means of the average value of viscosities at different concentrations), M is the relative molecular weight, and K and ‘a’ are dependent constants of the polymer, solvent and temperature. The values of K and ‘a’ correspond to 0.074 mL/g and 0.76 at 30°C, respectively.
Fourier transform infrared spectroscopy
To identify the functional groups of the chitosan membranes, the spectra were recorded in an FTIR-ATR (Thermo Scientific, Madison, WI, USA). The spectral resolution was 4 cm−1 with 64 scans in a frequency range between 600 and 4000 cm−1 [Citation22].
Preparation of the chitosan gel and membranes
The gel was prepared with 2% w/v chitosan dissolved in 1% (v/v) acetic acid, and the mixture was homogenized for 12 h with a magnetic stirrer to finish dissolving the chitosan. The chitosan membranes were obtained by the coating or solvent evaporation method. Specifically, the chitosan membranes were prepared using 10 mL of the 2% chitosan gel poured into polypropylene plates and dried at 40°C for 24 h in an oven (Felisa, Zapopan, Jalisco, México) [Citation23]. The membranes were carefully detached from the mold, and characterization followed. Functional groups were identified in the FTIR to confirm the structural integrity of the chitosan in the membrane.
The thickness was measured with a manual micrometer (Mitutoyo América Corporation, Kawasaki-shi, Kanagawa, Japan), making five measurements on each membrane, specifically one in the center and four at the perimeter of the membrane. The transparency of the membranes was determined per the method proposed by Rodríguez-Núñez et al. [Citation24], whereby rectangular membranes (2 cm x 4 cm) were cut and placed in the cells of the UV-VIS spectrophotometer (Genesys 10 UV, Madison, lowa, USA) for measurement at 600 nm. This procedure was repeated three times for each of the membranes. The value was estimated with the following equation: transparency = A600/T, where A600 is the absorbance at 600 nm, and T is the thickness of the membranes in mm.
Medical application of the chitosan membranes
Initially, the lesion was lavaged with 0.9% NaCl (PiSA, Guadalajara, Jalisco, México) physiological saline solution and dried thoroughly with a sterile gauze. Next, a small amount of chitosan gel was spread over the ulcer with a sterile needleless syringe and allowed to stand for 1 min. A chitosan membrane was later applied to the ulcer. Lastly, a bandage was placed to protect the ulcer from the external environment. The procedure was repeated every 48 h throughout the study. Patients were followed for a period of 3 months after the study. Patients with a variety of ulcers were studied from November 2014 to March 2015 at the Sports Medicine Clinic of the Sonora Institute of Technology in Ciudad Obregón, Sonora, Northwest Mexico. All patients and staff involved with the study confirmed their participation through informed consent [Citation25].
Patient 1. A 62-year-old male patient with a diabetic chronic infected wound in the upper pectoral region. The injury presented with torpid evolution with no tendency to improve or worsen and did not respond to the application of alginate-type desiccants.
Patient 2. A 73-year-old female patient with a diabetic ulcer on the third toe of the right foot treated with microdacyn®60 (hypochlorous acid and sodium hypochlorite).
Patient 3. A 92-year-old female patient with cavitated ulcers in the right and left glutes. The ulcers had been present for 1 year with infectious-appearing secretions.
Patient 4. A 63-year-old male patient with diabetic cavited ulcer in first toe of right foot. The ulcers had been present for 1 month with abundants secretions.
Results
Obtaining and characterizing chitosan
Purification of chitin to remove minerals and proteins is the initial step for the production of chitosan. Next, chitin is deacetylated by thermoalkaline hydrolysis. The yield of chitin purification and chitosan production is presented in .
Table 1. Percentage yield of chitin purification and chitosan production.
The analytical characteristics of the spectrophotometric method used to measure the degree of deacetylation of chitosan are shown in . This methodology was based on the two UV chromophore groups of chitosan, D-glucosamine and N-acetylglucosamine.
Table 2. Linearity, precision and applicability from method to measure degree of deacetylation.
The average viscosity of the 2% chitosan solution was 938.29 cps and was in the range of 851.58 to 1056.05 cps. The molecular weight of chitosan was 153.15 kDa (n = 3), calculated per the Mark-Houwink equation, which relates the intrinsic viscosity to the molecular weight.
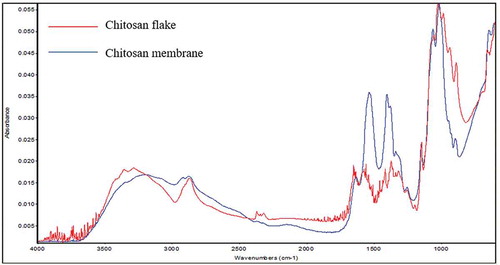
Chitosan flake and membranes were characterized by FTIR (). The spectra show the characteristic bands at 3255.31 cm−1 corresponding to the – OH, as well as at 2923.43 and 2869.40 cm−1, which are related to the vibrations of the CH2 groups. The bands at 1603.62 and 1526.42 cm−1 correspond to the side chains of the amino group. Additionally, the bands located at 1375.92 and 1329.61 cm−1 are assigned to the vibrations of the -OH, -NH2 and -CO groups. The band at 1603.33 cm−1 is attributed to the stretching of groups C-C and C-O. Lastly, the functional group found at 889.67 cm−1 is attributed to the C-O-C of the pyranose ring.
Characterization of chitosan membranes
The diameter of the membranes was 8.5 cm, and the thickness was in the range of 0.132 to 0.166 mm. The weight ranged from 0.2442 to 0.2665 g. The transparency value of the membranes was in the range of 0.256 to 0.371.
Treatment application
The medical reports about the applications the chitosan membranes are presented here, and from –.
Figure 2. Chronological evolution of the ulcer on upper pectoral region. Initial appearance (a), ulcer at 30 days (b).
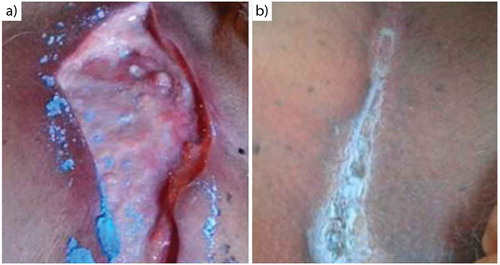
Figure 3. Chronological evolution of the ulcer on the third toe of the right foot. Initial appearance (a), ulcer at 30 days (b).
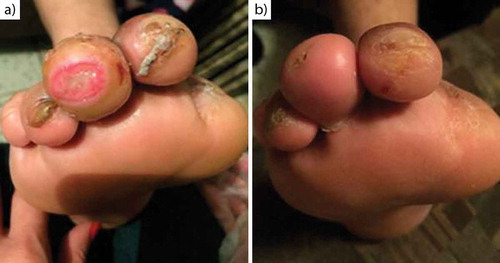
Figure 4. Chronological evolution of the ulcer on the glutes. Initial appearance for right (a) and for left (c), aspect of the ulcer at 70 days for the right (b) and for left (d).
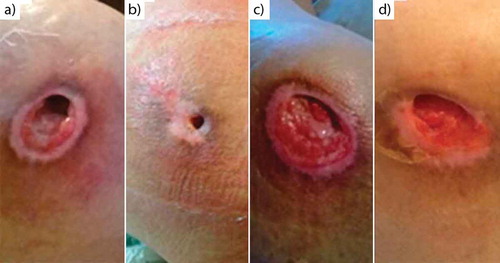
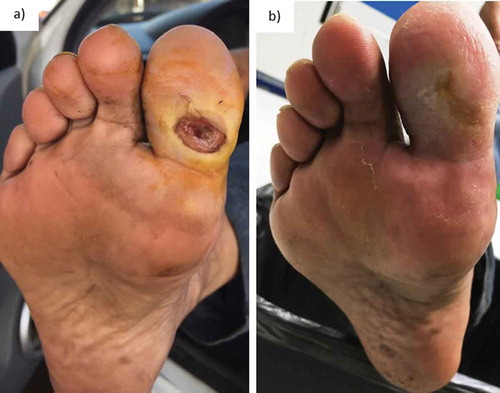
Figure 5. Chronological evolution of the ulcer in first toe of right foot. Initial appearance (a), ulcer at 37 days (b).
Patient 1. There are no infectious secretions in the ulcer, granulation tissue has appeared, the tissue has retracted, and complete skin coverage is noted at 30 days ().
Patient 2. Infectious secretions from the ulcer have resolved, granulation tissue is present, and complete skin coverage is seen after 30 days of treatment ().
Patient 3. Ulcer shows decreased purulent secretion from cavitations, increased granulation tissue and almost full closure by epithelium at 70 days ().
Patient 4. At 37 days, it was observed granulation tissue is present and complete skin coverage with the treatment ().
Discussion
Characterization from flake and membranes chitosan
The function of a polymer used as a biomaterial is to direct cell growth, either of the adjacent tissues or of the cells planted within it. To be successful, the polymer must provide adequate cell adhesion, promote proliferation and cell differentiation, and even favor cell migration [Citation26]. Chitosan has been shown to be involved in the formation of granulation tissue, angiogenesis, hemostasis and the formation of interleukins which, in turn, promote the proliferation of fibroblasts and keratinocytes [Citation27].
Purification of chitin was performed to remove pigments, proteins and salts, such as calcium carbonate. The conversion of chitin to chitosan is known as deacetylation and aims to remove the acetyl groups from the polymer chain [Citation28]. The yield was less than 100% due to the shrinkage that occurred during the washing process.
Van de Velde et al. [Citation29] mention that a 60% deacetylation is sufficient for chitin to be considered chitosan. Methacanon et al. [Citation30] indicate that the degree of deacetylation depends mainly on the temperature and alkali concentration, explaining that with 60% NaOH at 100°C for 60 min, up to 97.3% degree of deacetylation can be reached. This finding is comparable to the 88.7% degree of deacetylation of chitosan produced in this investigation.
Pilliai et al. [Citation31] reported viscosity ranging from 200 to 2000 cps in a 1% chitosan solutions produced with 45-50% NaOH. These values were lower than those reported in our research. Additionally, Pilliai et al. [Citation31] mention that the viscosity is affected by the degree of deacetylation and observed a range of DD between 60% and 90% in their chitosan.
Molecular weights differed from results found by some researchers. Molecular weights have been reported for chitosan of 483 to 520 kDa [Citation32] and 150 kDa [Citation33], which coincides with the findings in this research. All investigations mention that the conditions of the thermoalkaline hydrolysis at extremely high temperatures and alkali concentrations cause degradation of the polymer.
It was possible to identify bands corresponding to chitosan by means of the FTIR technique. According to Cesano et al. [Citation34], with the FTIR technique, the range of 1050 cm−1 bands corresponds to the vibrations of the glycosidic groups and C-O-C and, taken together, result in the fingerprint of chitosan. However, Hernández et al. [Citation35] mention that the characteristic groups of chitosan correspond to the amino (1621 cm−1), the hydroxyls (3434 cm−1) and the NH (3254 cm−1) groups. The band corresponding to the NH group of the primary amine is best defined when the sample is subjected to the deacetylation process [Citation36].
Chitosan can be solubilized in mineral acids such as hydrochloric and sulfuric acid or in organic acids, such as acetic, lactic and citric acid. Chitosan solutions form membranes with evaporation of the solvent. This phenomenon is attributed to the formation of intra- and intermolecular hydrogen bonds between the chitosan chains [Citation37].
In the chitosan films, it was also possible to identify the bands corresponding to chitosan. Cheng et al. [Citation38] report that the bands that show the structure of the saccharide are at 901 and 1155 cm−1, and the band of the amino group is at 1591 cm−1. The band at 1651 cm−1 corresponds to the amide of chitin, which means that when this peak decreases, the degree of deacetylation is greater. Likewise, Kloster et al. [Citation39] mention that the band at 1333 cm−1 is attributed to the vibrations of CH3 in the amide group and the CH/CH2 vibrations in the pyranose ring. Additionally, the bands found at 1630 and 1515 cm−1 are characteristic of ammonium side chains.
The chitosan membranes were homogeneous and transparent. Transparency is a very important parameter when it is required to give applications in wounds healing to the chitosan membranes. This parameter does not vary considerably when the membranes are made with pure chitosan. However, when combined with other agents, this parameter is modified.
Treatment application
The present study demonstrates the efficacy of chitosan membranes to fight infection and healing of skin ulcers caused by various conditions. Four clinical cases are exposed, where three of the patients have diabetes mellitus, numbered as 1, 2, and 4. The other case corresponds to an elderly patient with multiple compression ulcers. With her, at the end of the treatments, it was observed that the size of the ulcer decreases, with absence of infection and pain.
Ulcers are open lesions located in different parts of the body that are usually accompanied by secretions. It is considered that they are infected when the secretions are purulent or when there are more than two signs of inflammation such as erythema, heat or sensitivity [Citation40], pain, hypersensitivity and unpleasant smell [Citation41]. In the cases evaluated in this investigation, after the application of the treatment, the infection was eliminated, approximately 7 days after treatment, resulting in ulcers without secretions and without fetid odors.
The protonation of chitosan provides antiseptic properties, which restricts bacterial pathogen growth in skin ulcers. The treatment with chitosan favors secondary intention healing by stimulating fibroblast migration, which releases growth factors that favor the cicatricial process [Citation42]
Three of the patients treated with chitosan membranes have diabetes mellitus. Diabetic foot is one of the main complications associated with diabetes that causes morbidity, hospitalization and amputation, due to this the risk of developing an ulcer is 25% [Citation43], as presented in . Physiological failures such as venous, arterial or metabolic factors that occur in the human body, generate chronic ulcers on legs and feet that are usually hard to heal [Citation40].
A polymer acts a as a biomaterial when its function is to lead the cell growth, either by the adjacent tissues or the cells seeded in it. For this, the polymers must provide adequate cell adhesion, promote cell proliferation and differentiation and, in certain cases, to favor cell migration [Citation26]. In all treated clinical cases, was observed granulation tissue, tissue retraction and in most cases complete closure of the skin coverage. Several authors have conducted studies with pure chitosan, and other agents, and the results have been successful. Because they have shown that it improves vascularization, it forms well-organized skin tissue and scarring is generated without abnormalities [Citation44]. Azad et al. [Citation17] reported tests where they removed a part of the skin and applied chitosan membranes, a good adherence to the wound was observed, hemostasis and re-epithelialization of the wound was promoted, and pain was reduced.
Kordestani et al. [Citation45] demonstrated that using chitosan as a dressing allows chitosan to act as a promoter of both fibroblast migration and the angiogenesis necessary for the regeneration of damaged tissues.
Per Weinstein-Oppenheimer et al. [Citation27], natural polymers have often been used for skin treatments because of the similarity of the native cellular environment. A microenvironment is generated, where the chitosan with a pH of 4 to 5 resembles the skin, promoting tissue retraction and controlling moisture in the ulcer.
Conclusion
Based on the results shown in this research, the gel and chitosan membranes are now a promising option in the biomedical area because they efficiently promote the healing process in ulcers. Additionally, the results from our patients show that it is possible to counteract infection and promote the process of tissue regeneration to the maximum. Chitosan offers potential as an alternative material for healing from natural sources and as a more economical option than conventional treatments.
Acknowledgments
AA Escárcega-Galaz gratefully acknowledges the CONACYT by PhD scholarship CVU: 417707
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Castillo P, Sagues R, Urrea C, et al. Colgajo sural en úlceras venosas crónicas de piernas. Rev Chil Cir. 2004;56(5):475–480.
- Sancho A, Albiol R, Mach N. Relación entre el estado nutricional y el riesgo de presentar úlceras por presión en pacientes incluidos en el programa de atención domiciliaria. Atención Primaria. 2014;44(10):586–594.
- Gutiérrez I, Villegas M, López L, et al. Uso de los antisépticos en atención primaria. Atención Primaria. 2014;46(2):10–24.
- Téllez GA, Acero MA, Pineda LA, et al. Larvaterapia aplicada a heridas con poca carga de tejido necrótico y caracterización enzimática de la excreción, secreción y hemolinfa de larcas. Biomédica. 2012;32:312–320.
- Benavides J. Reparación de heridas cutáneas. Rev Asoc Col Dermatol. 2008;16:29–35.
- Lorenzini N, Manterola C, Moranga J. Bioingeniería de tejidos: cultivo de queratinocitos humanos en el tratamiento de la epidermólisis bullosa. Rev Chil Cir. 2014;66(4):359–363.
- Kafetzopoulos D, Martinou A. Bioconversión of chitin to chitosan: purification and characterization of chitin deacetylase from Mucor rouxii. J Korean Soc Appl Biol. 1993;90:2564–2568.
- Supaprutsakul S, Chotigeat W, Wanichpakorn S, et al. Transfection efficiency of depolymerized chitosan and epidermal growth factor conjugated to chitosan-DNA polyplexes. J Mater Sci Mater Med. 2010;21:1553–1561.
- Delmar K, Bianco-Peled H. The dramatic effect of small pH change on the properties of chitosan hydrogels crosslinked with genipin. Carbohyd Polym. 2015;27:28–37.
- Alarracín-Hernández W, Valderrama-Hohírquez N. Inclusión de compuestos químicos en matrices poliméricas de quitosano y su efecto en las propiedades de película. Rev Fac Quím Farm. 2014;21(1):49–59.
- Reverchon E, Adami R. Supercritical assited atomization to produce nanostructured chitosan-hydroxyapatite microparticles for biomedical application. Powder Technol. 2013;246:441–447.
- Kumar S, Koh J. Physiochemical and optical study of chitosan-teraphthaldehyde derivative for biomedical applications. Int J Biol Macromol. 2012;51:1167–1172.
- Kumirska J, Weinhold M, Sauvageau J, et al. Determination of the pattern of acetylation of low-molecular-weight chitosan used in biomedical applications. J Pharmaceut Biomed. 2009;50:587–590.
- Cheng S-Y, Wang B-J, Weng Y-M. Antioxidant and antimicrobial edible zein/chitosan composite films fabricated by incorporation of phenolic compounds and dicarboxylic acids. LWT-Food Sci Technol. 2015;63:115–122.
- Cárdenas G, Anaya P, Plessing C, et al. Chitosan composite films. Biomedical applications. J Mater Sci Mater Med. 2008;19:2397–2405.
- Stone CA, Wright H, Clarke T, et al. Healing at skin graft donor sites dressed with chitosan. Br J Plast Surg. 2000;52:601–606.
- Azada AK, Sermsintham N, Chandrkrachang S, et al. Chitosan membrane as a wound-healing dressing: characterization and clinical application. J Biomed Mater Res. 2004;69B(2):216–222.
- Sánchez-Duarte RG, Sánchez-Machado DI, López-Cervantes J, et al. Adsorption of allura red dye by crosslinked chitosan from shrimp waste. Water Sci Technol. 2012;65(4):618–623.
- Liu H, Bao J, Du Y, et al. Effect of ultrasonic treatment on the biochemphysical properties of chitosan. Carbohyd Polym. 2006;64:553–559.
- Ilyina A, Zagorskaya D, Levov A, et al. The use of enzymatic preparation of the production of low molecular-weight chitosan from the king crab hepatopancrease. Appl Biochem Micro. 2009;45(4):374–379.
- Zhang H, Neau S. In vitro degradation enzyme preparation: effect of molecular weight and degree of deacetylation. Biomaterials. 2001;22:1653–1658.
- Li J, Du Y, Yang J, et al. Preparation and characterisation of low molecular weight chitosan and chito-oligomers by a commercial enzyme. Polym Degrad Stab. 2005;87:441–448.
- Sánchez-Machado DI, López-Cervantes J, Rodríguez-Nuñez JR. Antimicrobial effect of different chitosan preparations against selected food borne pathogens. Int J Pharm Biol Sci. 2015;6(1):204–212.
- Rodríguez-Núñez JR, Madera-Santana TJ, Sánchez-Machado DI, et al. ChitosanYhydriphilic plasticizer-based films: preparation, physicochemical and antimicrobial properties. J Polym Environ. 2012;22:41–51.
- Escárcega-Galaz AA, De La Cruz-Mercado JL, López-Cervantes J, et al. Chitosan treatment for skin ulcers associated with diabetes. Saudi J Biol Sci. 2018;25:130–135.
- Falke G, Atala A. Reconstrucción de tejidos y órganos utilizando ingeniería tisular. Arch Argent Pediatr. 2000;98(2):103–115.
- Weinstein-Oppenheimer C, Aceituno A, Brown D, et al. The effect of an autologous cellular gel-matrix integrated implant system on wound healing. J Transl Med. 2010;8(59):1–11.
- Parada LG, Crespín R, Miranda GD, et al. Caracterización de quitosano por viscosimetría capilar y valoración potenciométrica. Rev Iberoam Polím. 2004;5(1):1–16.
- Van de Velde K, Kiekes P. Structure analysis and degree of substitution of chitin, chitosan and dibutyrylchitin by FT-IR spectroscopy and solid state 13C NMR. Carbohyd Polym. 2004;58:409–416.
- Methacanon P, Prasitslip M, Pothsree T, et al. Heterogeneous N-deacetylation of squid chitin on alkaline solution. Carbohyd Polym. 2003;52:119–123.
- Pillai CKS, Paul W, Sharma CP. Chitin and chitosan polymers: chemistry, solubility and fiber formation. Prog Polym Sci. 2009;34:641–678.
- Yen MT, Yang JH, Mau JL. Physicochemical characterization of chitin and chitosan from crab shell. Carbohyd Polym. 2009;75:15–21.
- Wang QZ, Chen XG, Liu N, et al. Protonation constants of chitosan with different molecular weight and degree of deacetylation. Carbohyd Polym. 2006;65:194–201.
- Cesano F, Fenoglio G, Carlos L, et al. One-step synthesis of magnetic chitosan polymer composite films. Appl Surf Sci. 2015;345:175–181.
- Hernández H, Águila E, Flores O, et al. Obtención y caracterización de quitosano a partir de exoesqueleto de camarón. Superf Vacío. 2009;22(3):57–60.
- Chávez A, Colina M, Valbuena AC, et al. Obtención y caracterización de películas de quitosano elaborado a partir de los desechos de la industria cangrejera. Rev Iberoam Polím. 2012;13(3):77–88.
- Ritthidej GC, Phaechamud T, Koizumi T. Moist heat treatment on physicochemical change of chitosan salt films. Int J Pharmaceut. 2002;232:11–12.
- Cheng M, Deng J, Yang F, et al. Study on physical properties and nerve cell affinity of composite films from chitosan and gelatin solutions. Biomaterials. 2003;24:2871–2880.
- Kloster GA, Marcovich NE, Mosiewicki MA. Composite film based on chitosan and nanomagnetite. Eur Polym J. 2015;66:386–396.
- Howell-Jones RS, Wilson MJ, Hill KE, et al. A review of the microbiology, antibiotic usage and resistance in chronic skin wounds. J Antimicrob Chemother. 2005;55:143–149.
- Mama M, Abdissa A, Sewunet T. Antimicrobial susceptibility pattern of bacterial isolates from wound infection and their sensitivity to alternative topical agents at Jimma University specialized hospital, South-West Ethiopia. Clin Microbiol Antimicrob. 2014;13814:1–10.
- Velazco G, Gonzalez A, Ortiz R. Chitosan films for the diabetic foot treatment. Av Biomedicina. 2012;1(1):38–41.
- Kateel R, Augustine AJ, Prabhu S, et al. Clinical and microbiological profile of diabetic foot ulcer patients in a tertiary care hospital. Diabetes Metab Syndrome. 2018;12:27–30.
- Dai T, Tanaka M, Huang YY, et al. Chitosan preparations for wounds and burns: antimicrobial and wound-healing effects. Expert Rev Anti Infect Rher. 2011;9(7):857–879.
- Kordestani S, Shahrezaee M, Tahmasebi MN, et al. A randomised controlled trial on the effectiveness an advanced wound dressing used in Iran. J Wound Care. 2015;17(7):323–327.