ABSTRACT
Ampicillin is one of the most commonly prescribed antibiotics against intra-renal infections. Prolonged use of ampicillin has been found to be associated with a high rate of free-radical generation and oxidative stress induced nephrotoxicity. In the present study, we report the comparative effects of melatonin and vitamin-C (Vit-C) in ameliorating the biochemical and structural alterations in renal tissues caused by ampicillin sodium. Biochemical parameters such as acid phosphatase (ACP), alkaline phosphatase (ALP), creatinine and urea levels in urine and serum as well as oxidative stress parameters such as superoxide dismutase (SOD) activity, catalase activity, total antioxidant status (TAS%) and lipid peroxidation in the renal cortical tissues were accessed upon treatment of melatonin and Vit-C following ampicillin sodium administration in Funambulus pennanti. Ampicillin treatment increased ACP, ALP, creatinine and urea levels in serum and urine, indicating renal damage. It also induced oxidative stress by increasing lipid peroxidation and decreasing the activity of antioxidative enzymes. However, the exogenous melatonin or Vit-C treatment inhibited ampicillin mediated renal damages. Melatonin or Vit-C pre-treatment significantly decreased ACP, ALP, creatinine, urea, TBARS level and increased SOD, catalase activity and TAS%. Our results suggest that the antibiotic induced nephrotoxicity can be ameliorated by melatonin or Vit-C via reduction of oxidative stress in renal tissues.
Introduction
Prolonged clinical use of antibiotic drugs continues to be a concern for the development of hepatotoxicity and nephrotoxicity. Nephrotoxicity is one of the most common symptoms, because the kidneys are constantly engaged in the removal of toxic substances and maintaining the metabolic homeostasis. The incidence of nephrotoxicity due to antibiotics has been reported to be up to 36% [Citation1–3]. Drugs associated with renal toxicity mainly include aminoglycosides, beta-lactams, quinolones, rifampin, amphoterin B and cisplatin. These drugs reportedly show toxic effect as a result of oxidative reactions that take place during the secretory process of excretion which attracts neutrophils, granulocytes and macrophages in glomerular vesicular epithelial cells (GVEC), proximal convoluted tubule (PCT) and basolateral membrane of kidney. These activated neutrophils and macrophages produce oxidative stress through a myriad of mediator proteins at the renal level, reflected in blood biochemistry and renal filtrates.
Ampicillin, a beta-lactam broad spectrum antibiotic, has been extensively used to treat bacterial infections. Commonly reported side effects associated with ampicillin toxicity include allergic reactions that range in severity from rashes to potentially lethal anaphylaxis, diarrhea, nausea and vomiting, painful micturition and seizures [Citation4]. It has been reported that intra-renal distribution of ampicillin in normal rats and in non-diseased human kidneys presented a moderate concentration between serum, cortex and medulla [Citation5,Citation6]. Acute doses of ampicillin given for several days induced crystalluria, oligourea and intratubular obstructive renal failure in patients. However, the underlying mechanism by which ampicillin causes renal toxicity remains unexplored. It is largely apparent that ampicillin induces excessive ROS generation, thereby impairing the cell’s protective machinery [Citation7]. Clinically, oxidative stress has been proposed as a leading cause of tissue damage in many pathophysiological disorders. Natural antioxidants such as Vit-C are effective against oxidative stress-related pathology. Melatonin secreted by the pineal gland also exhibits its efficacy as a free-radical scavenger [Citation8–10]. It stimulates the cellular antioxidant defense system by modulating the activities of several important antioxidative enzymes while Vit-C, is a hydrophilic redox catalyst that quenches free radicals because of its characteristic chemical structure. It acts both directly by reacting with aqueous peroxyl radicals and indirectly by enhancing the antioxidant properties of fat-soluble Vitamin-E [Citation11,Citation12]. The bioavailability, tissue uptake, metabolism and biological functions determine its clinical adequacy in modulating oxidative stress [Citation13]. The present study revisited the nephrotoxicity induced by ampicillin administration and evaluated the protective effect of melatonin and ascorbic acid in terms of biochemical and histopathological parameters in renal tissue of a tropical rodent, Funambulus pennantii.
Materials and methods
Animals and maintenance
Thirty healthy adult male squirrels, F. pennanti, weighing 110 ± 10 g were collected from the vicinity of Varanasi, India (Lat. 25º18ʹ N; Long. 83º01´ E) in the month of February. All the squirrels were kept in wire net cages (25´´ × 25´´ × 30´´) and acclimatized for 1 week in a room fully exposed to ambient environmental conditions (light 12.50 h; temperature 20–25ºC; humidity 29–50%). The squirrels were fed with soaked gram seeds (Cicer arietinum), grains, seasonal fruits and nuts, etc., along with water ad libitum. All the experiments were conducted in accordance with the institutional practice and guidelines of committee for the purpose of control and supervision of experimental animals (CPCSEA), 2007 of Government of India.
Drugs
Vit-C (ascorbic acid) and melatonin were purchased from Sigma Chemical Co. (St. Louis, USA). Ampicillin sodium (equivalent to anhydrous ampicillin) for injection was procured from Biocilin® (BIOCHEM Pharma Ltd., Mumbai, India). Ascorbic acid was reconstituted in normal saline (0.9%) and ampicillin sodium powder was reconstituted in sterile triple-distilled water. Melatonin stock was prepared freshly after every 2 days by dissolving it in 0.01% ethanolic saline. As per experimental requirement, the working concentration was prepared from the stock by diluting in freshly prepared normal saline (0.9% NaCl). All the other reagents used in this study were of analytical research grade. The doses of ampicillin sodium, melatonin and Vit-C were chosen based on the earlier studies [Citation14–17].
Experimental design
The squirrels were randomly divided into six groups (n = 5 each).
Group I: Control group; saline treated
Group II: Ampicillin sodium treated (100 mg/kg B.Wt., i.v. via subclavian vein for 10 days).
Group III: Melatonin treated (10 mg/kg B.Wt., s.c. for 10 days)
Group IV: Vit-C (ascorbic acid) treated (200 mg/kg B.Wt., i.v. for 10 days).
Group V: Melatonin + Ampicillin (Mel pre-treatment for 7 days followed by co-treatment with Amp for following 10 days)
Group VI: Ascorbic acid (Vit-C) + Ampicillin (Vit-C-pre-treatment for 7 days followed by co-treatment with Amp for following 10 days)
Sample collection
Twenty-four hours after the last injection the squirrels were sacrificed by decapitation under deep ether anesthesia. The urinary bladder was ligated and surgically extruded and emptied by supra-pubic puncture in a sterile tube. The kidneys were removed through a midline abdominal incision and were stripped from their capsule, weighed and slit by longitudinal incision. Cortical, medullary (outer medulla) and papillary (inner medulla-papillary tip) components were separated under a dissecting microscope. The accuracy of the dissection was verified by light microscopy. The kidney tissues were fixed in 10% neutral formalin for histology. Trunk blood was collected by cardiac puncture and centrifuged at 2500 rpm for 20 min at 4ºC. The serum separated was kept at −20◦C till the biochemical estimations such as ACP, ALP, BUN, creatinine and total blood protein were performed.
Biochemical analysis
Assessment of renal function
Estimation of acid phosphatase and alkaline phosphatase activity
The serum and urine acid and alkaline phosphatase activity was estimated using commercially available kits, based on the methods of King et al. and Kind et al. [Citation18,Citation19] according to manufacturer’s protocol (Crest Biosystems, Goa, India). Serum and urine acid and alkaline phosphatase activity was expressed as KA units.
Estimation of creatinine levels
The serum and urine creatinine level was estimated by the alkaline picrate method [Citation20,Citation21] using commercially available kits (Span Diagnostic’s Ltd. Surat, Gujrat, India).
Estimation of blood urea nitrogen and urinary urea content
The urinary and blood serum urea nitrogen was estimated by enzymatic urease (Berthelot) method [Citation22] using urea Berthelot test kit (Span Diagnostics Ltd.) as per manufacturer’s protocol.
Total antioxidative status
The total antioxidative status of kidney cortical tissue was evaluated by ABTS assay [Citation23] with slight modifications. Briefly, a stock solution of ABTS radical cation was prepared by mixing 5 ml of 7 mM ABTS with 1 ml of 14.7 mM potassium persulfate and was kept in dark at room temperature for 16 h. A quantity of 2.95 ml of ABTS radical cation solution was mixed with 50 µl of 10% tissue homogenate and the decrease in absorbance was monitored for 60 min at 6 min intervals at 734 nm. The activity was compared with the original (ABTS˙+) solution using ascorbic acid as standard. Appropriate solvent blanks were run in each assay. The total antioxidant capacity (TAS) was expressed as percent inhibition of the bleaching in relation to aqueous control following the equation: Inhibition of A734 (%) = (1-Af/Ao) × 100 where Ao is the absorbance of uninhibited radical cation and Af is the absorbance measured 6 min after addition of the test sample.
Estimation of total kidney cortical protein
The tissue of cortical region was separated from the medullary portion of each kidney under a dissecting microscope and homogenized. The total membrane protein content from kidney cortex was estimated by the Bradford method [Citation24] and expressed as total kidney cortical protein.
Antioxidant enzyme activity
Catalase activity
The indirect catalase activity was measured using hydrogen peroxide (H2O2) as a substrate and was expressed as mM/mL/mg of protein [Citation25]. The supernatant of 10% homogenates in phosphate-buffered saline (PBS) was processed for the assay in a reaction mixture comprising 0.8 mM H2O2 and potassium dichromate in glacial acetic acid heated in a water bath for 10 min and then OD was taken at 570 nm and the decrease in H2O2 content was calculated. The standard curve was calibrated with varying concentrations of 0.2 mM H2O2 in PBS.
Superoxide dismutase assay
Ten percent homogenates of all kidney cortical tissues were prepared in 150 mM PBS (pH 7.4) and centrifuged at 12,000 g for 30 min at 4ºC. The protein level in the tissue homogenate was measured using BSA as standard. The supernatant was processed for total Cu, Zn-Superoxide dismutase (SOD) and Mn-SOD activity. The reaction was stopped by adding freshly prepared Griess Reagent (1% sulfanilamide, 5% orthophosphoric acid, 0.1% N-1-naphthylethylenediamine dihydrochloride) and absorbance was measured at 543nm. SOD activity is expressed as specific activity (units/mg protein) [Citation26].
Lipid peroxidation assay by TBARS (thiobarbituric acid reactive substances) level estimation
A 10% (w/v) homogenate of the kidney cortical tissue was made in 20 mM Tris hydrochloride (HCl) buffer pH 7.4 and centrifuged at 12,000 g for 30 min at 4ºC. The supernatant was then subjected to thiobarbituric acid (TBA) assay by mixing it with 8.1% SDS, 20% acetic acid, 0.8% TBA and boiling for 1 h at 95ºC. The reaction mixture was immediately cooled in running water and vigorously shaken with n-butanol and pyridine reagent (15:1) and centrifuged for 10 min at 1500 g [Citation27]. The absorbance of upper phase was measured at 534 nm. LPO was expressed as TBARS in nmol/g tissue weight by taking 1,1,3,3-tetraethoxy propane (TEP) as standard. The standard curve was calibrated using 10 nM concentration of TEP.
Histological analysis
The kidney tissues were excised and immediately fixed in 10% neutral formalin for 48 h. The kidneys were gradually dehydrated and embedded in paraffin. Sections of 5 µm thickness were stretched on 1% gelatin-coated slides. The sections were then stained with hematoxylin and eosin. The histopathological changes were finally observed and photographed under a research microscope (Nikon, E200, Yurakucho, Tokyo, Japan). The morphometric analysis of capsular space and cellular integrity (PCT, DCT and glomerulus) of kidney cortical region were done in 20 serial sections randomly selected from each squirrel with the help of Filar Ocular micrometer (Webcon Pvt. Ltd., India).
Statistical analysis
All the data were expressed as mean ± SEM of at least five animals per group. Statistical analysis of the data was performed using SPSS 17.0 (SPSS Corp., Armonk, NY, USA) with one-way ANOVA followed by Tukey’s multiple range tests for multiple comparisons. The differences were considered statistically significant when p ≤ 0.05.
Results
Effect of ampicillin sodium on kidney function tests
Acid phosphatase level in urine and serum
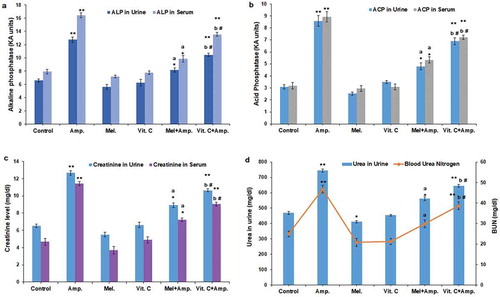
Ampicillin sodium significantly increased the acid phosphatase level in urine and serum (p < 0.01) when compared with other groups. The pre-treatment of melatonin as well as Vit-C reduced the ACP levels in both urine and serum. However, melatonin pre-treatment significantly (p < 0.05) restored the increased ACP levels in urine and serum compared to the ascorbic acid pre-treated groups ().
Figure 1. Comparative effect of melatonin and Vit-C treatment on (a) acid phosphatase activity (b) alkaline phosphatase activity (c) the levels of creatinine in urine along with blood serum and (d) urinary urea and blood urea nitrogen (BUN) in the model of ampicillin sodium-induced nephrotoxicity. The renal function was assessed in terms of histograms; data are represented as mean ± SEM (N = 5). Vertical bars on each histogram represent standard error of mean. Significance of difference **p < 0.01 and *p < 0.05 control vs. other groups; a: p < 0.01 Amp vs. Mel + Amp; b: p < 0.05 Amp. vs.Vit-C + Amp. and # p < 0.05 Mel + Amp vs.Vit-C+ Amp
Effect of ampicillin sodium on alkaline phosphatase level in urine and serum
The alkaline phosphatase level was significantly high in the ampicillin-treated groups (p < 0.01). The pre-treatment of melatonin decreased significantly the ALP levels (p < 0.05) in both urine and serum when compared to Vit-C pre-treated groups ().
Effect on urine and serum creatinine levels
Both urine and serum creatinine levels were significantly (p < 0.01) higher following ampicillin sodium administration when compared to other groups. Melatonin pre-treatment significantly (p < 0.05) decreased urine and serum creatinine levels when compared to Vit-C pre-treated group ().
Effect on urea in urine and blood urea nitrogen
Urea levels in urine and the blood urea nitrogen showed a significant increase in the ampicillin-treated groups (p < 0.01) when compared with other groups. Melatonin pre-treated groups significantly restored (p < 0.05) the urea and BUN levels to near normal range than Vit-C pre-treated groups ()).
Effect on total kidney cortical protein and total antioxidant status
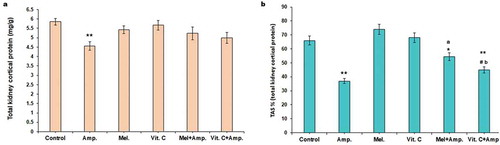
Total kidney cortical tissue protein decreased significantly (p < 0.01) following ampicillin treatment while an increase was noted in melatonin pre-treated animals (). The ampicillin sodium treatment significantly lowered (p < 0.01) the TAS while both the melatonin and Vit-C increased the TAS level. Restoration of the TAS was comparatively more significant in melatonin-treated groups compared to Vit-C pre-treated group ().
Figure 2. Comparative effect of melatonin and Vit-C treatment on (a) total kidney cortical protein and (b) total antioxidative status (TAS%) in F. pennantii model of ampicillin sodium-induced nephrotoxicity. Histograms represent Mean + SEM (N = 5). Vertical bars on each histogram represent standard error of mean. Significance of difference **p < 0.01 and *p < 0.05 Control vs. other groups; a: p < 0.01 Amp vs. Mel+Amp; b: p < 0.05 Amp. vs.Vit-C+ Amp. and # p < 0.05 Mel + Amp vs.Vit-C+ Amp
Changes in antioxidant enzyme activity and lipid peroxidation
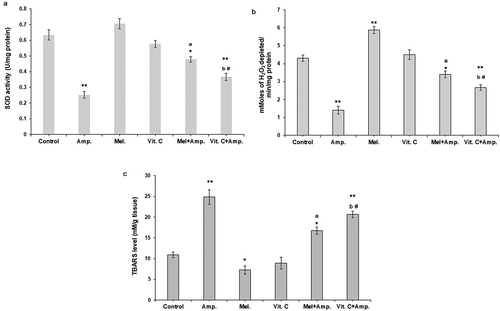
A significant (p < 0.01) decrease in the SOD and catalase activity in kidney cortical tissues was observed in ampicillin-treated groups. Consistent with the decreased antioxidant enzyme activity the MDA (malondialdehyde) levels were significantly higher in ampicillin sodium-treated groups (p < 0.01) when compared with other groups (, , ). Treatment with melatonin and Vit-C significantly increased the antioxidant enzyme activity (p < 0.01) in ampicillin treated groups and significantly reduced (p<0.01) the MDA levels. However, melatonin pre-treatment significantly reduced (p<0.05) oxidative damage in renal cortical tissues than Vit-C.
Figure 3. Comparative effects of melatonin and Vit-C treatment on antioxidant enzyme activity (a) superoxide dismutase activity (SOD) (b) catalase activity and (c) lipid peroxidation in kidney cortical tissues in F. pennantii model of ampicillin sodium-induced nephrotoxicity. Histograms represent Mean + SEM (N = 5). Vertical bars on each histogram represent standard error of mean. Significance of difference **p < 0.01 and *p < 0.05 Control vs. other groups; a: p < 0.01 Amp vs. Mel + Amp; b: p < 0.05 Amp. vs.Vit-C + Amp. and # p < 0.05 Mel + Amp vs.Vit-C + Amp
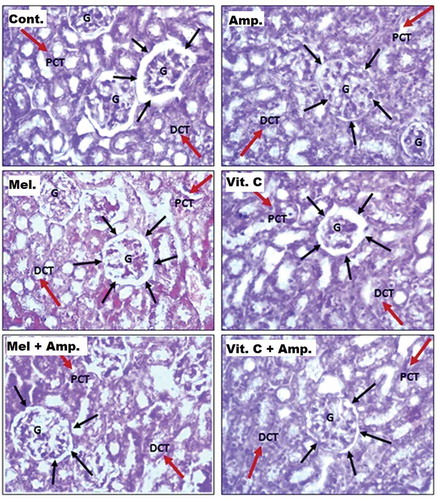
Histology and morphometric analysis
The kidneys from the control, melatonin- and Vit-C-treated groups showed no abnormality, while the kidneys from the ampicillin-treated group showed marked histological changes such as decrease in capsular space, tubular atrophy and interstitial inflammation (). Melatonin and Vit-C pretreatment decreased the ampicillin-induced effects on kidney tissues ().
Table 1. Measurement of capsular space (CS) of Bowman’s capsule and luminal space of PCT (20 sections/group)
Discussion
Ampicillin, a beta-lactam broad spectrum antibiotic, is the most widely prescribed antibacterial drug, for the treatment of urinary tract infections, respiratory tract infections, meningitis, salmonella infections and endocarditis. Although it is one of the safest and well-tolerated second-generation drugs, its continuous exposure is reported to be associated with renal dysfunction such as intra-renal obstruction, interstitial nephritis, alteration of intra-glomerular hemodynamics and inflammatory changes in renal tubular cells [Citation28]. Ampicillin has a basic role in the production of oxidants by neutrophils as a mechanism of its antibiotic action. Neutrophils are an important source of free oxygen radicals and therefore are considered to be the major effectors in the tissue damage that occurs in many inflammatory disorders. Transient exposure to ampicillin can be attributed to the buildup of this drug in the renal tubular epithelial cells and vascular endothelial cells, followed by the production of reactive oxygen species; thus exhibiting induction of nephrotoxicity. The present study demonstrates that both melatonin and Vit-C exert a renal protective effect in combination with ampicillin in a tropical seasonally breeding rodent F. pennanti. The nephrotoxicity in the animals was induced by administering high doses (100mg/kg body weight) of ampicillin sodium intravenously through the subclavian vein for 10 consecutive days. The nephrotoxicity was concluded from a marked increase in the levels of ACP, ALP, urea and creatinine in urine and serum while reduced antioxidant activity in kidney tissues. Nephrotoxicity caused due to ampicillin sodium was further supported by the histopathological evaluation of treated kidneys which exhibited morphological changes in the glomerulus and tubular structure.
Acid phosphatase is a ubiquitous lysosomal enzyme that hydrolyzes organic phosphatases at an acidic pH [Citation5]. On the other hand, alkaline phosphatases catalyze the hydrolysis of phosphate esters and it plays a significant role in transportation across cell membranes [Citation29]. Both the enzymes are present in many tissues including bone, intestine, liver spleen kidney and WBCs [Citation30]. Damage to these tissues causes the release of ACP and ALP into the bloodstream. Thus, the elevated levels of these enzymes in urine and serum can be associated with clinical renal dysfunction.
We found ampicillin sodium treatment increased the levels of these enzymes in urine and serum. Melatonin and Vit-C pre-treatment decreased the elevated levels of these enzymes, but melatonin pre-treatment was found to be more potent in reducing the nephrotoxic effects of ampicillin sodium when compared to ascorbic acid. Blood urea nitrogen is another measure of wastes (urea) in the blood. Under normal conditions it is removed from the blood by kidneys; however, in case of kidney dysfunction the BUN level rises. Ampicillin sodium administration increased the blood urea nitrogen (BUN) level indicating kidney dysfunction (nephrotoxicity). A similar observation was made in the urine and serum creatinine levels, which were high in the animals treated with ampicillin. The increased levels of urea and creatinine were normalized by the melatonin and ascorbic acid pre-treatment.
Ampicillin sodium treatment disturbed the balance between oxidant and antioxidant leading to the accumulation of reactive oxygen species which thereby impairs cellular functioning and viability [Citation31]. Cellular antioxidant enzymes such as SOD and Catalase protect the cell from oxidative stress [Citation32]. In the present study, we observed a significant reduction in the activity of catalase and superoxide dismutase in the kidney cortical tissues of ampicillin sodium-treated animals. The decreased SOD activity in kidney cortical tissue suggests impaired dismutation of superoxide radicals resulting in their accumulation. High level of superoxide radicals, in turn, inhibits the catalase activity. Melatonin and its metabolites enforce the antioxidant system by scavenging free radicals [Citation33,Citation34]. It not only stimulates the synthesis of antioxidant enzymes [Citation35] but also protects antioxidant enzymes from oxidative damage [Citation36,Citation37]. Vit-C, on the other hand, is known to be an outstanding chain-breaking antioxidant as well as a potent free-radical scavenger [Citation38]. Both these antioxidants reversed the effects of ampicillin sodium by upregulating the activities of catalase and SOD. However, melatonin pre-treatment showed a more pronounced effect than Vit–C, suggesting its high efficacy to control oxidative damage.
Our study demonstrated that ampicillin sodium-induced renal toxicity arises as a result of membrane lipid peroxidation due to the stimulation of reactive oxygen entities like H2O2 and O2. MDA is one of the most important end products of lipid peroxidation and is known to be a marker of free-radical damage. The MDA level in ampicillin sodium-treated groups was significantly higher than control, melatonin- and Vit-C-treated groups. Meanwhile, in melatonin + ampicillin and Vit-C + ampicillin group MDA levels significantly decreased. The antioxidant defense system has many components; a deficiency in any of these components can cause a reduction in the overall antioxidant status. Reduction in the total antioxidant status (TAS) has been implicated in several diseases. The other observation of the present study is that there was a significant decrease in TAS% in the ampicillin-treated group; while, the melatonin and Vit-C pre-treatment normalized TAS in kidney tissues. Thus, melatonin and Vit-C scavenge free radicals directly, inhibit biomolecule oxidation, prevent lipid damage and alter antioxidant pathways [Citation39].
In the present study, marked histopathological changes were observed in the ampicillin sodium-treated groups including dilation in tubules, inflammation, reduction of Bowman’s space and tubular atrophy. These changes were significantly reduced in melatonin and Vit-C pre-treated groups. To our knowledge, very few reports are available that demonstrates the effect of antioxidants; melatonin and Vit-C on ampicillin induced nephrotoxicity. Nathanson et.al. demonstrated the potential adverse effects of β-lactam antibiotics (ampicillin and amoxicillin) in the rat kidney development [Citation15].
The antioxidant effects of melatonin are well investigated. Melatonin administration leads to upregulation of several antioxidant enzymes and downregulation of pro-oxidant enzymes in particular 5- and 12-lipoxygenases and NO synthases [Citation40]. Melatonin has been found to be protective against gentamycin- and cisplatin-induced nephrotoxicity and glycerol-induced renal failure because of its antioxidant effects [Citation41,Citation42]. A few studies have compared the antioxidant effects of melatonin with other antioxidants [Citation43–45]. Melatonin was found to be more efficient than Vit-C and E in reducing oxidative stress induced by chlorpyrifos-ethyl in rats [Citation44]. In another study, melatonin was found to reduce markers of oxidative stress than Vit-C or N-acetylcysteine against acetaminophen toxicity in mice.
Vit-C, on the other hand, is an important water-soluble antioxidant present in biological fluids. It readily scavenges reactive oxygen and nitrogen species. Vit-C acts as a co-antioxidant by regenerating α-tocopherol from α-tocopheroxyl radical produced via scavenging of lipid-soluble radicals [Citation46–48]. Although substantial scientific evidence exists regarding the antioxidant and health effects of Vit-C, being a redox-active compound, it not only acts as an antioxidant but also as a pro-oxidant in presence of redox-active transition metal ions [Citation49]. Vit-C can result in the formation of highly reactive hydroxyl radicals via reaction of reduced metal ions with hydrogen peroxide, thereby, indicating that it is apparently less efficient in ameliorating oxidative damages induced by ampicillin sodium treatment than melatonin.
Conclusion
Based on our observations it can be concluded that both melatonin and Vit-C are potent nephro-protective and nephro-curative agents, but the benefits of melatonin are profound and far-reaching and that the melatonin and its metabolites are more efficient free-radical scavengers with an impressive ability to control oxidative damage. These data may open avenues for the clinical implication of melatonin to minimize the oxidative damages induced during prolonged usage of antibiotics.
Acknowledgments
Authors thank University Grants Commission, New Delhi for providing financial assistance. Instrument gift from the Alexander von Humboldt Foundation, Bonn, Germany to Prof. C. Haldar is gratefully acknowledged.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Kaloyanides GJ. Antibiotic-related nephrotoxicity. Nephrol Dial Transplant. 1994;9:130–134.
- Morin JP, Fillastre JP, Olier B. Antibiotic nephrotoxicity. Chemioterapia. 1984;3(1):33–40.
- Fanos V, Cataldi L. Amphotericin B-induced nephrotoxicity: a review. J Chemother. 2013;12:467–470.
- Singh NP, Ganguli A, Prakash A. Drug-induced kidney diseases. J Assoc Physicians India. 2003;51:970–979.
- Fabre J, Blanchard P, Rudhart MR. Pharmacokinetics of ampicillin, cephalothin and doxycycline in various tissues of rat. Chemotherapy. 1977;23:129–141.
- Whelton A, Sapir DG, Carter GC, et al. Intrarenal distribution of ampicillin in the normal and diseased human kidney. J Infect Dis. 1972;125:466–470.
- Pierrefiche G, Laborit H. Oxygen free radicals, melatonin and aging. Exp Gerontol. 1995;30:213–227.
- Reiter RJ, Tan DX, Pilar TM, et al. Melatonin and its metabolites: new findings regarding their production and their radical scavenging actions. Acta Biochem Pol. 2007;54:1–9.
- Rodriguez C, Mayo JC, Sainz RM, et al. Regulation of antioxidant enzymes: a significant role of melatonin. J Pineal Res. 2004;36(1):1–9.
- Ahmad R, Gupta S, Haldar C. Age dependent expression of melatonin membrane receptor (MT1, MT2) and its role in regulation of nitrosative stress in tropical rodent Funambulus pennanti. Free Radic Res. 2012;46(2):194–203.
- Machlin LJ, Bendich A. Free radical tissue damage: protective role of antioxidant nutrients. Faseb J. 1987;1(6):441–445.
- Chambail S, Dwivedi S, Shukla KK, et al. Vitamin C in disease prevention and cure: an overview. Indian J Clin Biochem. 2013;28(4):314–328.
- Rock CL, Jacob RA, Bowen PE. Update on the biological characteristics of the antioxidant micronutrients: vitamin C, vitamin E and the carotenoids. J Am Diet Assoc. 1996;96(7):693–702.
- Mukherjee A, Haldar C. Melatonin membrane receptor (MT1R) expression and nitro-oxidative stress in testis of golden hamster mesocricetus auratus. Exp Gerontol. 2015;69:211–220.
- Nathanson S, Moreau E, Merlet-Benichou C, et al. In utero and in vitro exposure to beta- lactams impair kidney development in the rat. J Am Soc Nephrol. 2000;11(5):874–884.
- Oksay T, Naziroğlu M, Doğan S, et al. Protective effects of melatonin against oxidative injury in rat testis induced by wireless (2.45 GHz) devices. Andrologia. 2014;46(1):65–72.
- Yousef JM, Chen G, Hill PA, et al. Ascorbic acid protects against the nephrotoxic it y and apoptosis caused by colistin and affects its pharmacokinetics. J Antimicrob Chemother. 2011;67(2):452–459.
- King EJ, Jegatheesan KA. A method for the determination of tartratelabile prostatic acid phosphatase in serum. J Clin Pathol. 1959;12(1):85–89.
- Kind PRN, King EJ. Estimation of plasma phosphatase by determination of hydrolyzed phenol with amino-antipyrine. J Clin Pathol. 1954;7(4):322–326.
- Bonses RW, Taussky HH. On the colorimetric determination of creatinine by the Jaffe reaction. J Biol Chem. 1945;158:581–591.
- Slot C. Plasma creatinine determination. A new and specific Jaffe reaction method. Scand J Clin Lab Invest. 1965;17:381–387.
- Fawcett JK, Scott JE. A rapid and precise method for the determination of urea. J Clin Pathol. 1960;13(2):156–159.
- Re R, Pellegrini N, Proteggente A, et al. Antioxidant activit y applying an improved ABTS radical cation decolorization assay. Free Radical Biol Med. 1999;26:1231–1237.
- Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254.
- Sinha AK. Colorimetric assay of catalase. Anal Biochem. 1972;47:389–394.
- Das K, Samanta L, Chainy NBG. A modified spectrophotometric assay of superoxide dismutase using nitrite formation by superoxide radicals. Indian J Biochem Biophysiol. 1999;37:201–204.
- Ohkawa H, Ohishi N, Yagi K. Reaction of linoleic acid hydroperoxide with thiobarbuteric acid. J Lipid Res. 1978;19:1053–1057.
- Shahrbaf FG, Assadi F. Drug-induced renal disorders. J Renal Injury Prev. 2015;4(3):57–60.
- Celik H, Tosun M, Cetinkaya MB, et al. Markedly elevated serum alkaline phosphatase level in an uncomplicated pregnancy. J Matern Fetal Neonatal Med. 2009;22(8):705–707.
- Kaplan MM. Alkaline phosphatase. N Engl J Med. 1972;286(4):200–202.
- Madhu P, Pratap Reddy K, Sreenivasula RP. Melatonin reduces oxidative stress and restores mitochondrial function in the liver of rats exposed to chemotherapeutics. J Exp Zool A Ecol Genet Physiol. 2015;323(5):301–308.
- Reiter RJ, Gultekin F, Flores LJ. Melatonin: potential utility for improving public health. TAF Prev Med Bull. 2006;5:131–158.
- Tan DX, Manchester LC, Terron MP, et al. One molecule, many derivatives: a never-ending interaction of melatonin with reactive oxygen and nitrogen species? J Pineal Res. 2007;42(1):28–42.
- Hardeland R, Backhaus C, Fadavi A. Reactions of the NO redox forms NO+, ●NO and HNO (protonated NO-) with the melatonin metabolite N1-acetyl-5-methoxykynuramine. J Pineal Res. 2007;43(4):382–388.
- Reiter RJ, Tan DX, Osuna C, et al. Actions of melatonin in the reduction of oxidative stress.A review. J Biomed Sci. 2000;7(6):444–458.
- Gitto E, Tan DX, Reiter RJ, et al. Individual and synergistic antioxidative actions of melatonin: studies with vitamin E, vitamin C, glutathione and desferrioxamine (desferoxamine) in rat liver homogenates. J Pharm Pharmacol. 2001;53(10):1393–1401.
- Kilic U, Kilic E, Tuzcu Z, et al. Melatonin suppresses cisplatin- induced nephrotoxicity via activation of Nrf-2/HO-1 pathway. Nutr Metab. 2013;10(1):7.
- Abraham P. Vitamin C may be beneficial in the prevention of paracetamol-induced renal damage. Clin Exp Nephrol. 2005;9(1):24–30.
- Elbe H, Dogan Z, Taslidere CA, et al. Beneficial effects of quercetin on renal injury and oxidative stress caused by ciprofloxacin in rats: a histological and biochemical study. Hum Exp Toxicol. 2016;35(3):276–281.
- Pandi-Perumal SR, Srinivasan V, Maestroni GJ, et al. Melatonin: nature’s most versatile biological signal? Febs J. 2006;273(13):2813–2838.
- Malhotra S, Sawhney G, Pandhi P. The therapeutic potential of melatonin: a review of the science. Med Gen Med. 2004;6(2):46.
- Ferraz FF, Kos AG, Janino P, et al. Effects of melatonin administration to rats with glycerol- induced acute renal failure. Ren Fail. 2002;24:735–746.
- Montilla-Lopez P, Munoz-Agueda MC, Lopez MF, et al. Comparison of melatonin versus vitamin C on oxidative stress and antioxidant enzyme activity in alzheimer’s disease induced by okadaic acid in neuroblastoma cells. Eur J Pharmacol. 2002;451:237–243.
- Gultekin F, Delibas N, Yasar S, et al. In vivo changes in antioxidant systems and protective role of melatonin and a combination of vitamin C and vitamin E on oxidative damage in erythrocytes induced by chlorpyrifos-ethyl in rats. Arch Toxicol. 2001;75:88–96.
- Sener G, Sehirli AO, Ayanoglu-Dulger G. Protective effects of melatonin, vitamin E and N - acetylcysteine against acetaminophen toxicity in mice: a comparative study. J Pineal Res. 2003;35:61–68.
- Carr AC, Frei B. Toward a new recommended dietary allowance for vitamin-C based on antioxidant and health effects in humans. Am J Clin Nut. 1999;69(6):1086–1107.
- Packer L. Vitamin C and redox cycling antioxidants. In: Packer L, Fuchs J, editors. Vitamin C in health and disease. New York: Marcel Dekker Inc; 1997. p. 95–121.
- Bowry VW, Mohr D, Cleary J, et al. Prevention of tocopherol mediated peroxidation in ubiquinol-10-free human low density lipoprotein. J Biol Chem. 1995;270:5756–5763.
- Halliwell B. Vitamin C: antioxidant or pro-oxidant in vivo. Free Radic Res. 1996;25:439–454.