ABSTRACT
One of diabetes mellitus complications is the disturbance in the structure and functions of male reproductive organs. The present study aimed to evaluate the ameliorative role of Azadiracta indica leaves extract against adverse effects of diabetes on the structure and function of the testicular tissue of rats neonatally induced by streptozotocin. Twenty-four male Albino rats at 6 weeks old were used in the present study and divided into four groups (n=6). Group 1: control group, group 2: neem leaves extract supplemented group. Group 3: diabetic group that received a single I.P dose of Streptozotocin and group 4: diabetic group supplemented with neem leaves extract. The obtained results revealed significant decreased levels of testicular antioxidants, serum testesterone and significant increased levels of serum cholesterol, triglycerides, and low density lipoprotein but a remarkable decrease in high density lipoprotein in diabetic rats. Also, the testicular tissues of diabetic rats displayed several histological and ultrastructural changes especially in the spermatogenic and interstitial cells as well as overexpression of COX-2 protein if compared with control. Post-supplementation of neem leaves extract to STZ- induced diabetic rats, revealedremarkable amelioration in most of disrupted estimated parameters.
Introduction
Diabetes Mellitus (DM) is a metabolic dysfunction characterized by the elevated level of blood glucose due to either insulin deficiency, insulin resistance, or others such as environment, infections, genetic defects, and specific drug [Citation1].
It had been indicated that induction of diabetes by streptozotocin to neonatal rats could result in a transient effect on insulin levels that followed by spontaneous recovery of the β-cells through the first 2 weeks of life, resulting in normoglycemia until at least 6–8 weeks of life [Citation2]. Furthermore, re-growth of β-cell is often incomplete resulting in reduced β-cell mass and consequentially impaired glucose tolerance and decrease plasma insulin in adulthood [Citation2].
Diabetes is associated with impairment of reproductive capacity in both sexes [Citation3]. Seshagiri [Citation4] reported that diabetes can induce male infertility in about 13–18% of couples. Moreover, hyperglycemia can induce oxidative stress through the production of Reactive Oxygen Species (ROS) in various tissues especially testis [Citation5]. Adewole et al. [Citation6] found that diabetes has a deleterious significant effect on the physiological functions of the male reproductive system. Experimentally, injection of high doses of streptozotocin (STZ) leads to deficiency in testicular testosterone level through the destruction of Leydig cells [Citation7]. Ali et al. [Citation8] recorded a remarkable reduction in levels of serum FSH, LH, and growth hormone in the diabetic males. Another study on animal models strongly suggested that diabetes mellitus affects semen parameters and impairs spermatogenesis in male rats [Citation9].
Searching for new drugs for the treatment of DM continues because the present synthetic drugs have various side effects. The anti-diabetic action of some herbal drugs is commercially formulated until now as advanced medicines, in spite of they have been acclaimed for their therapeutic properties in the traditional systems of medicine [Citation10].
The beneficial role of some plant extracts has been extensively used as alternative medicines in protection or as health-promoting agents [Citation11]. One of such plants is Azadircta indica species (Neem) which have commercially and traditionally been used as a supplement for the protection and treatment of various diseases, including diabetes [Citation12]. For instance, Neem oil and leaves extracts can decrease the blood glucose level in normal rats, STZ-induced diabetic rats and alloxan-induced diabetic rabbits [Citation13].
Although the physiological disorders of reproductive derangements in young diabetic females have been widely investigated [Citation14], little studies have been conducted in males. Accordingly, the present work aimed to evaluate the ameliorative role of Azadircta indica leaves extract against diabetes-induced deleterious testicular structure and functions in male albino rats.
Material and methods
Chemical
Streptozotocin (STZ) was purchased from a local agent of Sigma Aldrich (Saint Louis, Missouri, USA).
Neem (Azadiracta indica) leaves extract
The neem tree around the campus of Faculty of Science, Mansoura University, Egypt, was identified and classified by the staff members in the Botany Department, Faculty of Science, Mansoura University. The leaves of the neem tree were collected, washed, and dried in shady condition for 3–4 days. The leaves were then crushed into powder. A sample of 300 g. dried powder was soaked in 70% ethanol, incubated for 48 hours then filtered and the filtrate was exposed to evaporation dish. Then, it was exposed to the air in the presence of mild sun heat to get a semi-solid extract [Citation15]. The obtained percentage yield of neem extract is about 30% from the dried powder (3 g). This extract was kept frozen at 4°C till used in the experiment.
Experimental animals
Twenty adult albino rats (15 virgin females and 5 healthy males) weighing 120–130 g were obtained from breeding stock in the Laboratory Animal Department, Faculty of Pharmacy, Mansoura University, Egypt. The animals were received standard diet and water ad libitum throughout the experiment. After 1 week of acclimatization; adult virgin females were made pregnant by keeping them with healthy adult male overnight. After ensuring of pregnancy by observation of vaginal plugs, pregnant animals were separated and preserved in their cages until parturition.
Ethical considerations
All experiments inclusive of animal handling and sacrifice were conducted as per the guidelines of the Bioethics Committee of Mansoura University.
Induction of diabetes
After parturition, twenty-five newborn at 2 days old was injected intraperitoneally by a signal dose of streptozotocin (80 mg/kg, diluted with buffer, pH 4.5) and 100 mg nicotinamide/Kg body weight to induce type2 diabetes mellitus [Citation16]. After weaning (21 days postnatal), male offspring were isolated from their mothers. The blood sample was collected from the tail vein to measure blood glucose level. Hyperglycemic offspring above 150 mg/dl was isolated and considered as a diabetic individual and represented by 12 male offspring; however, 13 non-diabetic individuals were excluded.
Experimental design
Twenty-four male rats were used in the present work and divided into four groups as follows:
Group I (served as a control group): included six male rats without treatment.
Group II (served as neem group): included six male rats that received a daily oral dose of neem leaves extract (250 mg/kg b. wt) suspended in distilled water by gastric tube from the 6th week age for 2 weeks [Citation17].
Group III (Diabetic): included six neonatal diabetic male rats and kept as a diabetic without treatment until the 8th week.
Group IV (Diabetic and Neem): diabetic rats supplemented with a daily oral dose of 250 mg/kg.bwt of neem leaves extract from the 6th week age for 2 weeks.
Sample collection and tissue preparation
At the end of the experimental period (8 weeks), the fasted rats were weighed and sacrificed under diethyl ether anesthesia. Blood samples were collected; serum was separated by centrifugation at 860 Xg and kept frozen at −20°C for further biochemical analysis. Rats were then dissected, and the two testes were removed. Right testis from each rat was processed for histological, immunohistochemical and ultrastructural examinations. The left testis was homogenized by tephlon homogenizer in a 10-fold volume of ice-cold distilled water, centrifuged at 860 Xg for 20 min and the resultant supernatants were frozen at −20°C for biochemical analysis.
Investigated parameters
Blood analysis
Assessments of glucose level
The level of blood glucose in serum was estimated based on using glucose kits from spinreact S, A, U Ctra. Santa Coloma, SPAIN according to the method of Trinder [Citation18].
Estimation of serum lipid profiles
Blood cholesterol was determined by the method of Meiattini [Citation19], and plasma triglycerides by the method of Young and Friedman [Citation20]. High-density lipoprotein (HDL) cholesterol and low-density lipoprotein (LDL) cholesterol were measured with phosphotungstate and magnesium, according to Naito and Kaplan [Citation21].
Estimation of hormones in serum
The level of follicle-stimulating hormone (FSH) in serum was determined using rat FSH ELISA KIT purchased from MyBiosoruce Company (Cat. NO. MBS281287). Serum luteinizing hormone (LH) level was estimated using rat LH ELISA KIT purchased by My Biocource Company (Cat.NO.MBS2509833). ELISA KIT from MyBiosoruce Company was used to determine the level of testosterone hormone in serum based on (Cat.NO.MBS026898). The level of insulin hormone in serum was estimated by the rat insulin (TMB) ELISA KIT which manufactured by Shibayagi CO., Ltd, based on (Cat.NO.RSHAKRIN010TR).
Estimation of tissue malondialdehyde and antioxidant in testis
The level of malondialdehyde (MDA) was estimated by using rat MDA ELISA Kit (Cat.NO.MBS738685) purchased by MyBiosoruce Company. Superoxide Dismutase (SOD) activity was determined by using Rat SOD Elisa kit from My Biosource company (Cat.No. MBS722675). Catalase (CAT) activity in testis was estimated by using rat CAT ELISA Kit (Cat No. MBS2600683) purchased from MyBiosoruce Company.
Histological and immunohistochemical examination of the testis
The right testes were removed from the animals and fixed in 10% neutral buffered formalin, then dehydrated in an ascending series of ethyl alcohol. The processed testes were embedded in paraffin wax. The prepared tissue blocks were sectioned on glass slides (5–6 μm thick) using microtome. The paraffin sections were further processed and stained with hematoxylin and eosin [Citation22].
Other embedded paraffin sections were processed for immunohistochemical demonstration of Cyclooxygenase-2 (Cox-2) in the testis. The slides were deparaffinized, hydrated in alcohol. The sections were immersed in citrate buffer (10 mM, pH 6.0) for 3.5 min, then treated with H2O2 in dark for about 30 min. (in water solution 6%; Merck). The slides were put in PBS (10 mM, pH 7.2), then incubated overnight in a moist chamber, at 4°C, containing polyclonal anti-rabbit (COX-2) antibody (Cell Signaling #4842, dilution 1:50). The sections were incubated in Poly HRP conjugate for 20 minutes (Zymed, USA, CAT No. 87–8963) at 37°C. After that, the slides were immersed in diaminobenzidine (Sigma, USA, D-5637) for 5 min at 37°C. Finally, the tissue sections were counterstained with Harris Hematoxylin, then dehydrated using ascending grades of ethanol and covered with mounting medium.
The histological and immunohistochemical prepared sections were examined, and microphotographed using an Axioscop 2 plus microscope (Zeiss, Germany) with a Leica camera (DFC 320 digital, Germany).
Transmission electron microscope
For all examined groups, small pieces of testes were fixed in 5% buffered glutaraldehyde (pH 7.3) at 4°C. After 48 h, postfixation the pieces of testes were washed in 5% buffered sucrose (pH 7.3). The pieces of testes were cut into fine blokes less than 1 mm2 and then fixed in 1% osmium tetroxide for 2 hr followed by washing twice in phosphate buffer (pH 7.3) for 10–15 min [Citation23]. After that, the sections were dehydrated in ascending series of alcohol, propylene oxide and then embedded in Araldite. The semi-thin sections (0.6–0.7 µm thick) were cut with aid of glass knives on ultra-microtome. The obtained sections were stained with toluidine blue and examined by a binocular light microscope. According to the investigated semi-thin sections, the ultrathin sections were cut at 600–700A° and collected on cleaned copper grids. The prepared grids were stained in uranyl acetate and lead citrate. Finally, the grids were investigated and photographed in a JEOL 1200 EXIL TEM in EM center of Mansoura University, Egypt.
Determination of testicular cells apoptosis by flow cytometry
Testicular cell apoptosis was determined by using flow cytometry technique with kits purchased from BD bioscience company (Cat. NO: 558,662). In further, testicular cells were harvested by centrifugation, washed in ice-cold PBS, and fixed in 80% ethanol that had been prechilled to 20°C. They were then repelleted and re-suspended at a concentration of 0.1–0.3 106/ml in PBS containing 18 mg/ml propidium iodide (PI; Sigma) and 8 mg/ml RNase A (Sigma; PI solution). After incubation in the dark for at least 1 h, apoptotic cell analysis was performed on 10,000–20,000 cells on a FACSort flow cytometer using the Cell quest analysis program (Becton Dickinson, Sunnyvale, CA, USA).
Statistical analysis
The results obtained in the present work were calculated by One Way ANOVA (analysis of variance) test. Then the data were expressed as a mean ± standard error (mean ± SE), where p ≤ 0.05 is considered statistically significant.
Results
Body weight changes
In neem feeding group, the mean body weight showed no significant change compared with control however, the mean body weight of diabetic rats was significantly (P˂0.001) lowered compared with control. In diabetic rats post-supplemented with neem leaves extract, the mean body weight was significantly (P˂0.001) increased if compared with diabetic rats but showed low significant decrease compared with control ()).
Serum analysis
Glucose level
No significant change was noticed between the neem supplemented group and control for the serum glucose level; however, in STZ-induced rats, the mean level of serum glucose appeared significantly higher (P < 0.001) than control. On the other side, the mean level of serum glucose of group4 (diabetic and neem) showed a significant decrease in comparison to STZ-induced group ()).
Insulin hormone concentration
In Azadiracta indica supplemented group, the levels of insulin hormone showed no significant change with the control; however, in STZ-induced diabetic rats, the level of insulin hormone was significantly lowered (P < 0.001) than that of control. Moreover, in the diabetic rats post-supplemented with neem extract for 2 weeks, the level of serum insulin appeared significantly lower than control ()).
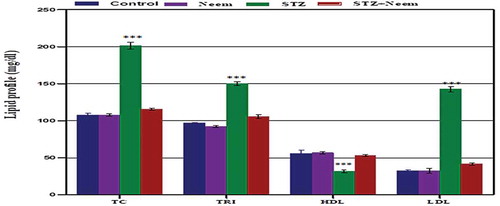
Serum total cholesterol (TC), triglycerides (TG), high-density lipoprotein (HDL) and low-density lipoprotein (LDL)
As shown in , the levels of serum TC, TG, and LDL in diabetic rats appeared significantly higher than control (P < 0.001) while the level of HDL was significantly decreased. On another hand, a remarkable significant decrease for serum lipid profiles was noticed in the diabetic rats that supplemented with neem while the HDL level was increased compared to diabetic rats.
Serum testosterone, FSH and LH
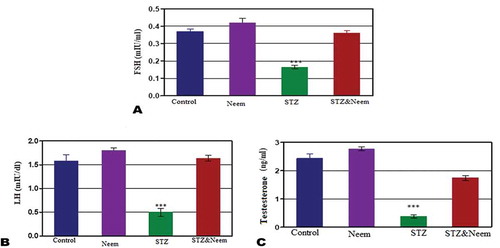
In STZ-induced diabetic rats, the levels of serum FSH, testosterone and LH showed significant decrease (P < 0.001) compared to the control; however, post-supplementation of neem extract to diabetic rats the levels of these hormones were significantly increased and return to the normal levels as in control ().
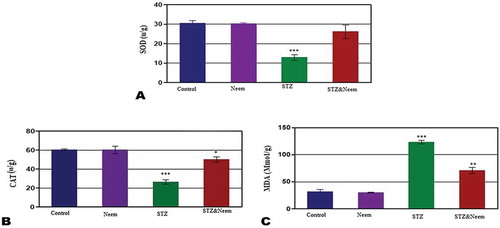
The level of testicular antioxidants (SOD and CAT) and lipid peroxidation; MDA
The obtained data presented in revealed that the levels of antioxidants; SOD and CAT were significantly lowered, but the MDA level was significantly higher in diabetic rats compared with control (P < 0.001). In ameliorated group (diabetic and neem) both SOD and CAT levels were significantly increased while MDA level was significantly decreased if compared with the diabetic rats alone but the levels of CAT and MDA still showing low significant change with control.
Figure 1. Represents the body weight changes (a), serum glucose levels (b) and serum insulin levels among the different studied groups. Note: a highly significant decrease in the mean body weight and serum insulin and significant increase in the serum glucose level (P < 0.001) of STZ-induced diabetic rats in comparing with control
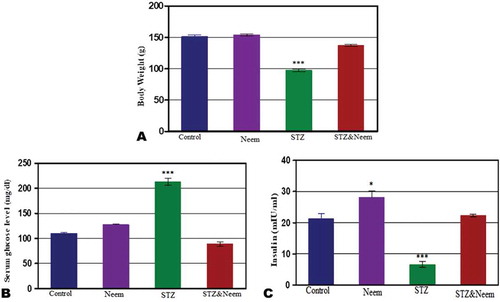
Figure 2. Represents the changes of serum levels of total cholesterol (TC), Triglycerides (TRI), high-density lipoprotein (HDL) and low-density lipoprotein (LDL) among the different studied groups. Note: a highly significant increased levels of TC, TRI & LDL and high significant decreased level of HDL (P < 0.001) in STZ-induced diabetic rats compared with control
Histological observations
In control and neem supplemented groups, the testicular sections showed the normal histological pattern whereas, the seminiferous tubules appeared rounded or oval and surrounded by a thin basal lamina (BL). The tubules were lined by stratified germinal epithelium, spermatogonia, primary spermatocytes, secondary spermatocytes, spermatids and spermatozoa. In-between the tubules, the interstitial tissue present blood vessels with clusters of Leydig cells with their characteristic oval shape and spherical nuclei ().
In the diabetic group, the sections of the testicular seminiferous tubules showed severe deleterious histological changes including, hemorrhage, infiltrated Leydig cells and wide intertubular spaces. Moreover, the germinal epithelium, spermatocytes, and spermatids were lost in some area of the sections with multiple vacuoles among them. In addition, the lumen of seminiferous tubules showed low density of sperms. Also, Sertoli cells appeared atrophied and disorganized with pyknotic nuclei ().
On the other side, the structure of seminiferous tubules showed remarkable amelioration post-supplementation of neem leaves extract to diabetic rats. Further, the germinal epithelium and spermatogenic cells were appeared organized in spite of little hemorrhage and vacuoles still present in some area of the sections. Also, the spermatogenic cells appeared adjacent to each other. Sertoli cells become highly organized with their spindle-shaped characteristic appearance ()).
Immunohistochemical observations of cyclo-oxygenase (COX-2) activity
In both control and Azidracta indica leaves extracts groups, the seminiferous tubules displayed negative to weak immune expression for COX-2 protein (). However, the sections of seminiferous tubules of diabetic rats showed intense reaction for COX-2 protein especially in the basal lamina and spermatogonia as well as in Leydig cells (). In the diabetic group that supplemented with neem leaves extract, the tubule section was displayed a moderate expression for a COX-2 protein that more confined to the interstitial cells and basal lamina of seminiferous tubules ()).
Ultrastructural observations
In the control group, the ultrastructural investigation of seminiferous tubules showed normal fine structure for the spermatogenic cells whereas their cytoplasm appeared homogenated with intact cellular membranes. Moreover, the cytoplasm contains rounded to oval mitochondria, dispersed SER and Golgi bodies (), A1). The Interstitial cells (Leydig cells) were normal with granulated cytoplasm and normally distributed cell organelles ( A2). Moreover, the sperms were appeared normal with distinct spindle-shaped head and tail. The head contains apical cap-shaped acrosome and centrally located nucleus. The tail is supported by the elongated axial filament ().
In diabetic rats, several ultrastructural changes were noticed in the spermatogenic cells in form cytoplasmic lysis, dispersed vacuoles and fragmented basal lamina. Also, some pyknotic nuclei were noticed inside the seminiferous tubules. The vacuolated mitochondria were noticed in the cytoplasm especially in the primary and secondary spermatocytes (), B1). The Leydig cell revealed cytoplasmic lysis, vacuolated area and disorganized cell organelles. Also, the nucleus appeared pyknotic (Figure 7B2). Moreover, the head of sperms appeared irregular with disintegrated acrosome and nucleus ().
Post-supplementation of neem leaves extract to diabetic male rats, the structure of both primary and secondary spermatocytes was enhanced whereas; the cytoplasm appeared homogenated. Also, the cell organelles were well organized and distributed in the cytoplasm in spite of little vacuoles still present in some cells (,c1)). In addition, the interstitial cell showed obvious recovery in their fine structure whereas the cytoplasm, nucleus and cell organelles appeared normal (Figure 7C2). Also, the sperm structure showed a normal head with an oval nucleus and elongated acrosome ()).
Figure 5. Photomicrograph of histological sections through the testes of control (a), neem (b), diabetes (c,d) and diabetes with neen (e) groups. In (a) and (b), the seminiferous tubules are showing the normal histological architecture. In (c) and (d), the seminiferous tubules are showing remarkable degeneration with obvious wide inter-tubular spaces, infiltrated Leydig cells, hemorrhage, separated basement membrane (star) and vacuoles. In (e), the seminiferous tubules are showing remarkable amelioration in their architecture (H&EX: 400)
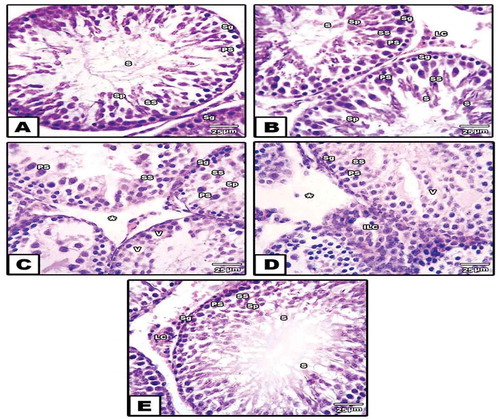
Figure 6. Photomicrograph of paraffin-embedded sections through the testes of control (a), neem (b), diabetic (c,d) and diabetic with neem (e) stained with anti-COX-2 antibody. Note: Weak to moderate immunoreactivity for COX-2 protein in groups (a) and (b), a strong positive reaction in (c) and (d) and moderate immune expression for COX-2 in group (e)
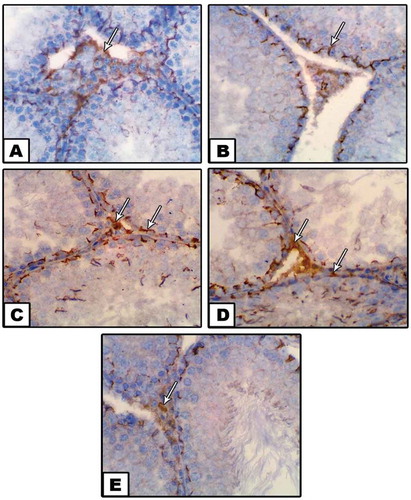
Figure 7. Transmission electron micrograph (TEM) through the seminiferous tubule of control (a-a2), diabetes (b-b2), diabetes with neem (c-c2). Note: In A &A1, the spermatogonia is showing normal nucleus (N), widely distributed mitochondria (M) with tight junction cell membrane. In B &B1, the spermatogenic cells are showing scattered vacuoles (V), highly condensed nucleus. In C&C1, the spermatogenic cells are showing remarkable amelioration in spite of moderate destruction of mitochondria and few vacuoles still present. In A1, the primary & secondary spermatocytes and spermatid are showing normal nucleus (N), mitochondria (M) and Golgi apparatus condensation over the nucleus of spermatid. In A2, the Leydig cell appears normal with homogenate cytoplasm and cell organelles. In B2, the Leydig cell is showing irregular nucleus membrane with peripherally accumulated chromatin, vacuolated cytoplasm, depleted mitochondria and wide cisterme rough endoplasmic reticulum. In C2, the Leydig cell is showing normal nucleus, oval mitochondria and rough endoplasmic reticulum (X: 3000)
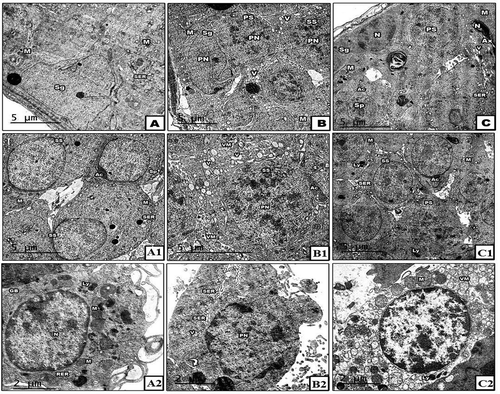
Figure 8. TEM through the sperm of control (A&B), STZ-induced (C&D) and STZ with neem (E) groups. Note: In A&B, the sperm is showing normal structure with spindle-shaped head, axoneme surrounded by mitochondria. In C&D, the sperm is showing remarkable fragmentation in microtubules of axoneme and destructive In E&F, the sperm is showing obvious amelioration in their structure tend to be similar for control X: 5000
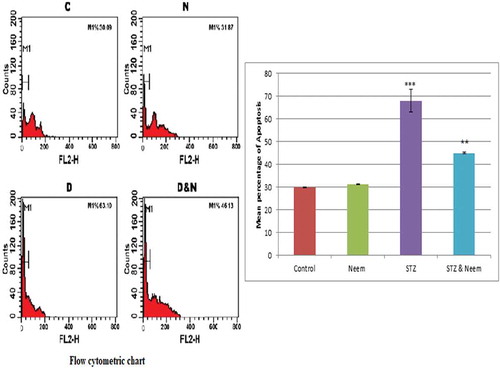
Flow cytometric analysis for apoptosis
The flow cytometric analysis for the apoptosis of testicular tissues was confirmed that the mean percentage of apoptosis in diabetic rats was significantly higher than that of control. On the other side, the diabetic rats that ameliorated with neem leaves extract, the mean percentage value of apoptosis was markedly lowered than that of the diabetic rats but still apparently higher than that of the control group ().
Figure 9. A Flow cytometric chart (left figure) and histogram (right figure) represent the mean percentage value of apoptosis of testicular cells among the different studied groups (C: Control, N: Neem, D: Diabetes, D&N: Diabetes and Neem). Note: a highly significant increase in the mean % of apoptosis in testicular tissues of diabetic group compared with control
Discussion
The results in the present work showed that the mean body weight and serum insulin level were significantly decreased while the serum glucose level was significantly increased in diabetic rats. It has been shown that diabetic individuals have a significant increase in blood glucose level that due to decreased insulin secretion or insulin action that simultaneously is a major factor for the decreased rate of glucose metabolism [Citation24]. Other studies recorded that body weight decreased in pre-pubertal diabetic rats [Citation25]. Ramachandran and Senhalatha [Citation26] explained that insulin deficiency which caused a drastic elevation in glucose levels as a result of excessive production of endogenous changes in body weight. This decrease may be a result of excessive breakdown of protein and lipid by insulin deficiency [Citation27].
In the present work, neem supplementation in the diabetic rats showed a significant elevation in body weight which is parallel with the finding of Das et al. [Citation17] who observed that neem leaves extract has hypoglycemic and body weight gain effect. The modulation of body weight in rats treated with neem leaves extracts may be attributed to the enhancement of glucose metabolism. The reduction in blood glucose and elevation of insulin observed in diabetic rats treated with neem leaf extracts is an indication of its hypoglycemic properties [Citation28]. The hypoglycemic effect of this extract may be attributed to the powerful protective role of antioxidant activity of this plant through the proliferation and regeneration of β-cells. In addition, the neem leaves extract contains phyto-constituents especially terpenoid, glycoside and flavonoids which play a potentiating role in insulin secretion [Citation29].
A highly significant increase in the levels of serum TC and LDL was recorded in diabetic rats, while the level of HDL was significantly lowered. The obtained results were in accordance with previous studies [Citation30]. Increased lipid fractions in the diabetic rats may be attributed to insulin deficiency which affects the liver apolipoprotein production. Moreover, insulin can regulate the enzymatic activity of lipoprotein lipase (LpL) and cholesterol ester transport protein. So, the insulin deficiency reduces the activity of hepatic lipase and several steps in the production of biologically active LpL [Citation31]. Furthermore, the conversion of triglycerides into free fatty acids and glycerol was performed by the action of lipase, an insulin deficiency, and the level of plasma-free fatty acids elevated. The acetyl CoA results from the catabolize of free fatty acid in liver and the elevated level of acetyl CoA turn into triglyceride, cholesterol and ketone bodies which lead to ketosis [Citation30]. Bopanna et al. [Citation32] reported that excess plasma fatty acid level in diabetic individuals promotes the ability of the liver to transform some fatty acids into cholesterol triacylglycerol and phospholipids that may be secreted as lipoproteins in the blood. The significant decrease in HDL of diabetic rats goes parallel with the findings of Khan et al. [Citation33] who found that the decreased level of HDL is associated with the reduction in plasma HDL-cholesterol in diabetic individuals to defect in reverse cholesterol transport. On the other hand, for a diabetic group that supplemented with neem leaves extract, there was a significant decrease in serum level of cholesterol, TG and LDL. Dholi et al. [Citation34] recorded that; supplementation of neem leaves extract to diabetic rats could ameliorate the lipid profiles changes. The hypolipidemic effect of neem extract may be induced by their contents of polyphenolic and flavonoids compounds that enhance the liver apolipoprotein production [Citation35].
Ali et al. [Citation8] declared that DM could interfere with the hypothalamus-pituitary-testicular axis in several levels. They also added that impairment in sex hormones was induced by reactive oxygen species (ROS) resulted in remarkable inhibition in steroidogenic gene expression [Citation36]. In the current work, the levels of testosterone, FSH and LH were significantly lowered in diabetic rats. Previous studies had suggested that the deficiency in testosterone level in diabetic individuals may be linked to insulin insensitivity at the hypothalamic center where it is associated with the increased concentration of inflammatory proteins and inhibition of gonadotropic releasing hormone (GnRH) [Citation37]. The decreased levels of FSH and LH in diabetic conditions may be related to the direct inhibitory effect of insulin deficiency on the secretory centers of the anterior pituitary gland [Citation36]. Prashanth & Krishnaiah [Citation38] explained that neem leaves extract can ameliorate the level of testicular hormone levels through its scavenging properties for free radical caused by flavonoids, nimbosterol, and limonoids. Such finding goes parallel with fiding of this work.
The obtained results showed that the oxidative stress induced in the testis of diabetic rats is implicated in elevation of MDA and reduction of SOD and CAT activities. Similar findings were recorded by Adewole et al. [Citation6] in the testis of diabetic rats. Hypoinsulinemia is a major factor in elevation of MDA activity as a result of increased activity of fatty actyl coenzyme A oxidase, which induces β-oxidation of fatty acids and lipid peroxidation [Citation6]. Moreover, protein glycation and glucose auto-oxidation can initiate the formation of free radicals, and this can equally increase the process of lipid peroxidation. Increased lipid peroxidation inhibits membrane functions by lowering the membrane fluidity and alteration of membrane-bound enzyme activity and their receptors. The decreased testicular activities of SOD and CAT in diabetic rats have agreed with the finding of Beyazyıldız et al. [Citation39]. Decreased SOD and CAT activities might be attributed to the alterations of cellular integrity of testes result from increased oxidative stress. Pokhre et al. [Citation40] found that the active ingredients especially phenylpropanoids, tannins in neem leaves extract play a vital role in scavenging of free radicals. Such finding goes parallel with our obtained results including pronounced amelioration of antioxidant activities in the testicular tissues post-supplementation of neem leaves extract to diabetic rats.
In the current work, several deleterious histological alterations were recorded in the testicular sections of diabetic rats including fragmented seminiferous tubules, scattered vacuoles, hemorrhage, infiltrated Leydig cells and wide inter-tubular spaces. These changes were also confirmed by observation damaged cell organelles of spermatogenic cells using ultrastrucural technique. On the other side, post supplementations of neem extract to diabetic rats these histological and ultrastrucural alterations were obviously restored. Previous studies on rats have been indicated that oxidative stress induced by diabetes is implicated in disruption of seminiferous tubules leading to inhibition of spermatogenesis [Citation41,Citation42]. The testicular damage in this study may be attributed to low levels of serum FSH and testosterone induced by STZ [Citation43]. Furthermore, the manufacture of stem cell factor (SCF) could decrease due to the low level of FSH. The SCF is a major factor for synthesis of Sertoli cells that has been actually helping in the development and survival of Leydig cells. Also, this factor plays a role in the protection of several types of spermatogenic cells in adult rats [Citation44]. Other alterations like vacuolated cells in seminiferous tubules are considered as a type of cellular defense mechanism against STZ-induced toxicity [Citation45]. The congested seminiferous tubules may be attributed to inhibition of STZ to prostaglandin synthesis maintaining blood flow [Citation46]. Further, a previous study by Prashanth & Krishnaiah [Citation38] proved that the active components of Azidracta indica leaves extract like flavonoids and polyphenols have potential role in rebuilding of testicular damage induced by oxidative stress of diabetes through elevations of antioxidants in the testicular tissues.
Several researches have been confirmed that type2 DM can enhance the inflammatory and apoptotic effects on most body tissues [Citation47,Citation48]. In the current work, the testicular tissues of diabetic rats displayed strong positive reaction for the inflammatory marker COX-2 and significant cellular apoptotic percentage however, post-supplementation of neem extract to the diabetic rats the immuno-reactivity for COX-2 and percentage of apoptosis were apparently decreased. Seibert et al. [Citation49] reported that COX-2 activities are obviously increased in cases of inflamed tissues; however, it is also induced by several factors like steroid hormones, cytokines, and mitogen stimuli. COX-2 expression has been shown in the testes of diabetic rats [Citation50]. Also, it has been noticed that COX-2 is strongly expressed in testicular aspiration of men suffering from impaired spermatogenesis, while it is weakly expressed in the testes of regular spermatogenesis [Citation51]. Further, DM has been found to induce apoptosis in the testis of rats [Citation42]. Oxidative stress and inflammations caused by DM are implicated in activation of glucose auto-oxidation and consequently increased reactive oxygen species (ROS). This sequence can accelerate the apoptotic signals, leading to cellular/tissue damage [Citation52]. It has been reported that the anti-inflammatory effect of neem leaves extract is mainly due to its quercetin component whereas the mechanism of action still unclear [Citation53]. The anti-apoptotic effects of neem extract in this study may be attributed its role in attenuation of the production of inflammatory mediators, including ROS [Citation54].
In conclusion: Azadirachta Indica (Neem) leaves extract has a potential role in amelioration of disrupted testicular structure and functions of diabetic male albino rats through exerting hypoglycemic, hypolipidemic and antioxidant effects and modulation of testosterone hormone.
Acknowledgments
The authors would like to express the deepest sense of Gratitude and appreciation to botany department for preparation of Neem extract. We would like to introduce deep thanks to electron microscope unit in Mansoura University for their efforts to process the grade slides.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Maitra A, Abbas AK. Endocrine system Robbins and Cotran management of diabetes mellitus. J Diabetes Metab. 2005;6(5):2–9.
- Kiss AC, Woodside B, Sinzato YK, et al. Neonatally induced mild diabetes: influence on development, behavior and reproductive function of female Wistar rats. Diabetol Metab Syndr. 2013;5(61):1–10.
- Bhasin S, Enzlin P, Coviello A, et al. Sexual dysfunction in men and women with endocrine disorders. Lancet. 2007;369(9561):597–611.
- Seshagiri PB. Molecular insights into the causes of male infertility. J Biosci. 2001;26(4):429–435.
- Ruder EH, Hartman TJ, Blumberg J, et al. Oxidative stress and antioxidants: exposure and impact on female fertility. Hum Reprod Update. 2008;14(4):345–357.
- Adewole OS, Caxton-Martins AE, Salako AA, et al. Effects of oxidative stress induced by streptozotocin on the morphology and trace minerals of the testes of diabetic wistar rats. Pharmacologyonline. 2007;2:478–497.
- Khaki A, Khaki AA, Hajhosseini L, et al. The antioxidant effects of ginger and cinnamon on spermatogenesis dysfunction of diabetes rats. Afr J Traditional Complementary Altern Med. 2014;11(4):1–8.
- Ali ST, Shaikh RN, Ashfaqsiddiqi N, et al. Serum and urinary levels of pituitary-gonadal hormones in insulin-dependent and non-insulin-dependent diabetic males with and without neuropathy. Arch Androl. 1993;30(2):117–123.
- Arikawe AP, Daramola AO, Odofin AO, et al. Alloxan-induced and insulin-resistant diabetes mellitus affect semen parameters and impair spermatogenesis in male rats. Afr J Reprod Health. 2006;10(3):106–113.
- Wadkar KA, Magdum CS, Patil SS, et al. Anti-diabetic potential and Indian medicinal plants. J Herb Med Toxicol. 2008;2(1):45–50.
- Chew EY. Nutrition effects on ocular diseases in the aging eye. Invest Ophthalmol Vis Sci. 2013;54(14):42–47.
- Sharma Y, Dua D, Srivastva SN. Comparative study of different parts of Azadirachta indica (neem) plant on the basis of anti-bacterial activity, phytochemical screening and its effect on rat PC-12 (Pheochromocytoma) cell line. Int J Biotechnol Allied Fields. 2014;2(7):144–154.
- Biswas K, Chattopadhyay I, Banerjee RK, et al. Biological activities and medicinal properties of neem (Azadirachta indica). Curr Sci. 2002;82(11):1336–1345.
- Agathocles T, Jennifer W, Florence B. Diabetes in women: pathophysiology and therapy. Obstet Med. 2011;4(2):86.
- Offor CE. Comparative anti-diabetic effects of the ethanol leaf-extracts of Vernonia amygdalina and Azadirachta indica in albino rats. Int J Curr Microbiol App Sci. 2015;4(1):201–209.
- Ghasemi A, Halifax S, Jedi S. Streptozotocin-nicotinamide-induced rat model of type 2 diabetes. Acta Physiol Hung. 2014;101(4):408–420.
- Das AR, Mostofa M, Hoque ME, et al. Comparative efficacy of neem (azadirachta indica) and metforminhydrochloride (comet®) in streptozotocin induced diabetesmelitus in rats. Bangl J Vet Med. 2010;8(1):75–80.
- Trinder P. Determination of glucose in blood using glucose oxidase with an alternative oxygen acceptor. Ann Clin Biochem. 1969;6(1):24–27.
- Meiattini F. The 4-hydroxybenzoate/4-aminophenazone chromogenic system. Clin Chem. 1978;24(12):2161–2165.
- Young DS, Friedman RB. Effects of disease on clinical laboratory tests. Am Assoc Clin Chem. 2002;48(4):682–685.
- Naito HK, Kaplan A. High-density lipoprotein (HDL) cholesterol. Clin Chem the CV Mosby Co. St Louis Toronto Princeton. 1984;1207–1213.
- Kim SK, Layton C, Bancroft JD. Bancroft’s theory and practice of histological techniques. 8th ed. Elsevier Health Sciences; 2019.
- Hayat MA, Giaquinta R. Rapid fixation and embedding for electron microscopy. Tissue Cell. 1970;2(2):191–195.
- Nagarchi K, Ahmed S, Sabus A, et al. Effect of streptozotocin on glucose levels in albino wister rats. J Pharm Sci Res. 2015;7(2):67–69.
- Mahdi AA, Chandra A, Singh RK, et al. Effect of herbal hypoglycemic agents on oxidative stress and antioxidant status in diabetic rats. Indian J Clin Biochem. 2003;18(2):8–15..
- Ramachandran A, Snehalatha C. Diabetes prevention programs. Med Clinics. 2011;95(2):353–372.
- Kyari F, Tafida A, Sivasubramaniam S, et al. Prevalence and risk factors for diabetes and diabetic retinopathy: results from the Nigeria national blindness and visual impairment survey. BMC Public Health. 2014;14(1):1–12..
- Subramoniam A. Plants with anti-diabetes mellitus properties. 1st ed. CRC Press; 2016.
- Boby RG, Leelamma S. Blackgram fiber (Phaseolus mungo): mechanism of hypoglycemic action. Plant Food Human Nutri. 2003;58(1):7–13.
- Pari L, Latha M. Effect on lipid peroxidation in streptozotocin diabetes. Gen Physiol Biophys. 2005;24:13–26.
- Vinod-Mahato R, Gyawali P, Raut PP, et al. Association between glycaemic control and serum lipid profile in type 2 diabetic patients: glycated haemoglobin as a dual biomarker. Biomed Res. 2011;22(3):375–380.
- Bopanna KN, Kannan J, Sushma G, et al. Antidiabetic and antihyperlipidaemic effects of neem seed kernel powder on alloxan diabetic rabbits. Indian J Pharmacol. 1997;29(3):162–167.
- Khan A, Safdar M, Khan MM, et al. Cinnamon improves glucose and lipids of people with type 2 diabetes. Diabetes Care. 2003;26(12):3215–3218.
- Dholi SK, Raparla R, Mankala SK, et al. In vivo Anti-diabetic evaluation of neem leaf extract in alloxan induced rats. J Appl Pharm Sci. 2011;01(4):100–105.
- Chattopadhyay RR, Bandyopadhyay M. Effect of Azadirachta indica leaf extract on serum lipid profile changes in normal and streptozotocin induced diabetic rats. Afr J Biomed Res. 2005;8(2):101–104.
- Elseed MN, Gaber M, Shatla IM, et al. Protective effect of gherlin on testicular function in adult male diabetic albino rats. AL-Azhar Med J. 2016;45:195–208. DOI:10.12816/0026285
- Onah CE, Meludu SC, Dioka CE, et al. Pattern of male sex hormones in type 2 diabetic patients in Nnewi, South Eastern Nigeria. IOSR-JDMS. 2013;10(4):65–70..
- Prashanth GK, Krishnaiah GM. Chemical composition of the leaves of Azadirachta indica Linn (Neem). Int J Adv Eng Technol Manage Appl Sci. 2014;1:21–31.
- Beyazyıldız E, Çankaya AB, Ergan E, et al. Changes of total antioxidant capacity and total oxidant status of aqueous humor in diabetes patients and correlations with diabetic retinopathy. Int J Ophthalmol. 2013;6(4):531.
- Pokhre B, Rijal S, Raut S, et al. Investigations of antioxidant and antibacterial activity of leaf extracts of Azadirachta indica. Afr J Biotechnol. 2015;14(46):3159–3163.
- Trindade AAT, Simões ACP, Silva RJ, et al. Long term evaluation of morphometric and ultrastructural changes of testes of alloxan-induced diabetic rats. Acta CirBras. 2013;28(4):256–265.
- Kianifard D, Sadrkhanlou RA, Hasanzadeh S. The ultrastructural changes of the sertoli and leydig cells following streptozotocin induced diabetes. Iran J Basic Med Sci. 2012;15(1):623–635.
- Kianifard D, Sadrkhanlou RA, Hasanzadeh SH. The histological, histomorphometrical and histochemical changes of testicular tissue in the metformin treated and untreated streptozotocin-induced adult diabetic rats. Vet Res Forum. 2011;1(2):13–24.
- García MC, Lopez M, Alvarez CV, et al. Role of ghrelin in reproduction. Reproduction. 2007;133(3):531–540.
- Ali AA, El-seify GH, El Haroun HA, et al. Effects of monosodium glutamate on the ovaries of adult female albino rats and the possible protective role of green tea. Menoufia Med J. 2014;27(4):793–800.
- Oforofuo IC, Adebayo EA, Kuye OM. Effects of monosodium glutamate in ovaries of female Sprague-Dawley rats. Int.J.Curr.Microbiol.App.Sci. 2015;4(5):737–745.
- Graves DT, Liu R, Alikhani M, et al. Diabetes-enhanced inflammation and apoptosis – impact on periodontal pathology. J Dent Res. 2006;85(1):15–21.
- Krijnen PA, Simsek S, Niessen HW. Apoptosis in diabetes. Apoptosis. 2009;14(12):1387–1388.
- Seibert K, Zhang Y, Leahy K, et al. Pharmacological and biochemical demonstration of the role of cyclooxygenase 2 in inflammation and pain. Proc Nat Acad Sci. 1994;91(25):12013–12017.
- Kushwaha S, Jena GB. Enalapril reduces germ cell toxicity in streptozotocin-induced diabetic rat: investigation on possible mechanisms. Naunyn Schmiedebergs Arch Pharmacol. 2012;385::111–124.
- Frungieri MB, Gonzalez-Calvar SI, Matzkin ME, et al. Sources and functions of prostaglandins in the testis: evidence for their relevance in male (in)fertility. Anim Reprod. 2007;4:63–69.
- Nna VU, Abu Bakar AB, Ahmad A, et al. Oxidative stress, NF-κB-mediated inflammation and apoptosis in the testes of streptozotocin-induced diabetic rats: combined protective effects of Malaysian propolis and metformin. Antioxidants (Basel). 2019;8(10):1–23.
- Schumacher M, Cerella C, Reuter S, et al. Anti-inflammatory, pro-apoptotic, and anti-proliferative effects of a methanolic neem (Azadirachta indica) leaf extract are mediated via modulation of the nuclear factor-κB pathway. Genes Nutr. 2011;6(2):149–160.
- Alzohairy MA. Therapeutics role of Azadirachta indica (Neem) and their active constituents in diseases prevention and treatment. Evid Based Complement Alternat Med. 2016;2016:1–11.