ABSTRACT
One of the valuable interventions in controlling chronic kidney disease is the dietary management. The study was designed to test effect of Persea Americana on adenine induced kidney injury. Four groups of mice were used: control group; adenine group: received adenine (0.2% w/w) daily with normal chow for 4 weeks; diet I group: received adenine then avocado [50% w/w in food] for 2 weeks; diet II group: received adenine then avocado [100% w/w] for 2 weeks. Serum levels of creatinine and potassium were measured in addition to oxidative stress biomarkers. Renal tissue injury and collagen deposition were scored. Renal expression of TGF-β1, CCL3L1 and IL-2 were assigned. Results showed significant decrease (p < 0.01) in serum creatinine level in diet I group. Serum potassium was elevated (p < 0.01) in diet I and II groups. Level of MDA was decreased (p < 0.01) while reduced glutathione and catalase were elevated (p < 0.05) by diet I and to a lesser extent by diet II. Both diet I and II markedly reduced fibrosis and tubular injury. Levels of TGF-β1 and CCL3L1 were decreased in both tested groups (p < 0.001) while IL-2 was increased. Enriching diet with Persea Americana fruit can attenuate renal fibrosis progression through anti-fibrotic, anti-oxidant and anti-inflammatory pathways.
Introduction
Chronic kidney disease (CKD) is described a process of gradual, prolonged and continuous loss of kidney function [Citation1]. Typically, CKD is progressive and in its terminal stage the patients require dialysis or kidney transplantation [Citation2]. One of the most valuable interventions in controlling CKD is the dietary management as it may help in slowing the disease progression and limit the accompanying risks. In CKD, the kidneys fail to adjust electrolytes level in blood such as potassium and could increase the risk of hyperkalemia. Hyperkalemia highly disturbs the cardiovascular function and may lead to cardiac arrest increasing mortality rates [Citation3,Citation4]. Restriction of dietary potassium intake is recommended to decrease the risk of hyperkalemia in CKD but undesired sequalae due to deprivation from the health benefits of many high potassium containing foods as fruits and vegetables can occur.
Some studies demonstrated that potassium blood level is controlled mainly by 90% kidney and 10% colonic fecal excretion. The fecal excretion of potassium is directly proportional to the impaired kidney function. In case of end stage renal failure, fecal excretion becomes the only route for potassium excretion [Citation5]. The kinetics of the gastrointestinal tract plays a very important role in the regulation of potassium fecal excretion and hence potassium blood level, i.e. slow peristalsis decreases fecal potassium excretion and allows more absorption [Citation5]. This suggests that motility, instead of potassium dietary load, is the main determinant of potassium blood level in kidney impaired patients [Citation6].
Some authors claim that high potassium-containing diets provide potential health benefits of for CKD patients. The scientific base of such claim is related to the alkalinizing effects of such foods. These effects may protect against metabolic acidosis a common disturbance among CKD patients resulting from kidney mass reduction and renal acid excretion defect associating the disease progression. Additionally, potassium rich plants could enhance the intracellular distribution of potassium as well as increasing potassium fecal excretion due to the effect of their high fibers content [Citation6,Citation7].
In this study, effect of Persea Americana (avocado) as a plant-based diet on adenine induced kidney injury in mice was tested. Persea Americana is characterized by high potassium and fiber content and many beneficial bioactive compounds. Fruit of Persea was tested in two percentages: 50% w/w in diet resembling vegetarian diet and 100% w/w resembling vegan diet. The present work was designed to share our experiment-based opinion in the debate whether high potassium containing plant foods is harmful to CKD patients or beneficial to such patients. Additionally, we tested potential effect of Persea Americana in slowing down kidney fibrosis progression and possible underlying mechanisms.
Materials and methods
Animals: Adult male CD-1 Swiss albino mice (age: 3 weeks) were purchased from VACSERA [Agouza, Giza, Egypt] and allowed food and water ad libitum for 2 accommodation weeks until reaching 25–30 g in weight. The study protocol was in accordance to the guidelines and ethical principles for the care and use of laboratory animals approved by the Scientific Research Ethics Committee, Faculty of Pharmacy, Mansoura University [approval number 2020–23]. These guidelines comply with EU Directive 2010/63/EU. Fruits of Persea Americana were purchased from local market in Mansoura, Egypt. Chemicals: Adenine (99%) as fine white powder (Oxford laboratory)
Experimental design
Mice were housed 10/cage and food was restricted to 50 g food/day/cage during the study. Mice were randomly divided into four groups (10/group): Control group; Adenine group: received adenine (0.2% w/w) daily with food [normal chow] for 4 weeks [Citation8]; Avocado 50%: received adenine as described then received avocado in food [50% w/w] for 2 more weeks; Avocado 100%: received adenine as described then avocado [100% w/w] for 2 more weeks. Schematic presentation of the experimental design is shown in .
Table 1. Experimental design
At the end of the study, urine samples were collected along 24 h before sacrifice through transferring mice to metabolic cages for 24 h. Mice were then anesthetized using thiopental (70 mg/kg, i.p) [Citation9], blood samples were collected and serum was separated by centrifugation at 4°C and both kidneys were isolated.
Measurement of creatinine and serum potassium
Levels (mg/dl) of serum and urine creatinine was measured spectrophotometrically at 492 nm according to the manufacturer’s instructions (Biomed Diagnostics, Egy-Chem, Egypt). Level of potassium (mEq/L) was measured in serum at 623 nm by a spectrophotometric method [Citation10] using diagnostic reagent kit supplied by (Biomed Diagnostics, Egy-Chem, Egypt).
Measurement of oxidative stress
Isolated kidneys were washed, cut into pieces and minced in ice cooled 10% w/v phosphate buffer saline manually to prepare 0.1% w/v kidney homogenate. The resultant suspension was then centrifuged at 4000 rpm for 15 minutes at 4°C; the supernatant was separated and divided into aliquots.
Malondialdehyde as the end product of lipid peroxidation and catalase activity were measured in the prepared kidney homogenate as previously described [Citation11,Citation12]. Reduced glutathione and total antioxidant capacity were measured using commercially available kits according to the manufacturer’s instructions (Bio-diagnostic, Giza Egypt)
Measurement of renal CCL3L1, TGF- β1, and IL-2
Concentrations of CCL3L1, TGF-β1 (Cloud Clone Corp., USA), IL-2 (eBioscience, Vienna, Austria) were measured in kidney homogenate using sandwich ELISA technique as directed by the manufacturers.
Histopathological examination
Pieces of isolated kidneys were fixed in neutral buffered formalin (8% v/v) for 24 hours for standard histopathological techniques and preparation of paraffin blocks.
For tubular injury, sections (4 μm thick) were prepared, stained with hematoxylin-eosin stain and examined at magnification 400× in randomly selected 20 fields using light microscope. According to the modified scoring system described by [Citation13], the histopathological changes (necrosis – vacuolar degeneration – swelling and desquamation from the tubular basement membrane) were scored as follows: normal: 0; involvement of <20% of the tubules: 1; 20–40%: 2; 40–60%: 3; 60–80%: 4; 80–100%: 5
For interstitial fibrosis, 20 randomly selected fields of masson trichrome stained sections were examined with the color image analyzer Image J 1.32. Fibrotic area was quantified in the OSOM and cortico-medullary junction avoiding the blood vessels [Citation14].
Statistical analysis
Results (n = 10) are expressed as mean ± S.E.M and were compared using one way analysis of variance (ANOVA), followed by Tukey–Kramer multiple comparison test as the post-Hoc test. Tubular injury scores were analyzed with Kruskal-Wallis test by rank followed by Dunn’s multiple comparison test. Statistical analysis and figures construction were performed using GraphPad Prism V5.01 (GraphPad Software Inc, CA, USA).
Results
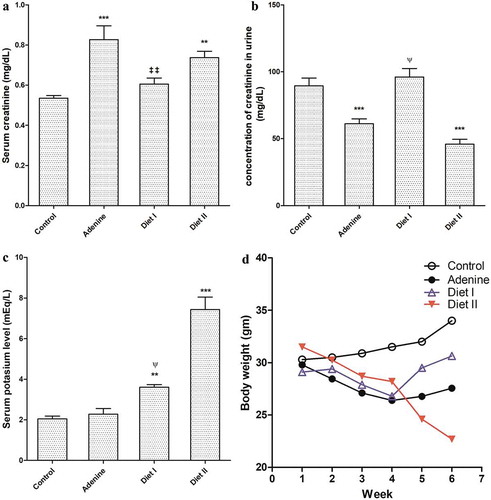
Administration of adenine in diet for 4 weeks resulted in significant (p < 0.001) elevation in creatinine level compared to its level in control group. Addition of avocado to diet in a percentage 50% w/w for two weeks (diet I group) significantly (p < 0.01) lowered creatinine compared to adenine alone. When mice received avocado 100% w/w (diet II group) did not lowered creatinine level in serum compared to adenine group as it remained significantly (p < 0.01) higher than control group (). Concentration of creatinine in urine was significantly (p < 0.001) low in adenine and diet II groups compared to control group. In diet I group, urine concentration of creatinine was significantly (p < 0.001) high compared to adenine group and diet II group as well ().
Serum potassium was significantly increased in both diet I and diet II groups (p < 0.01, 0.001 respectively) compared to control and adenine groups ().
Mortality percentage in adenine group was 10%. When mice received avocado 50% w/w or 100% w/w after adenine mortality was is increased to 20 and 40% respectively. Weekly body weight is shown in () with marked decrease in body weight in adenine group compared to control. In diet I group, mice weight started to increase in week 5 and 6 after starting receiving avocado in contrast from mice that received diet II in which body weight sharply decreased.
Figure 1. Effect of Persea Americana fruit (50% and 100% w/w in diet) on (a) serum creatinine level, (b) urine creatinine concentration, (c) serum potassium level, and (d) mice weight change/week in adenine induced kidney injury in mice. **, *** p < 0.01, 0.001 respectively compared to control group; ‡‡ p < 0.01 compared to adenine group, ψ p < 0.05 compared to diet II group (n = 10)
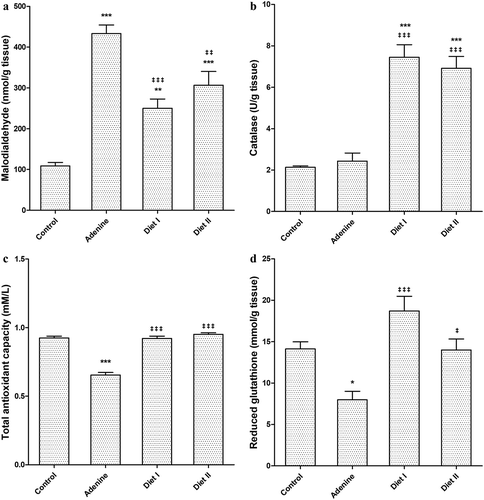
Adenine in diet significantly (p < 0.001) increased MDA renal tissue level compared to control group (). The elevated MDA level was significantly (p < 0.001, 0.01) lowered in diet I and II groups respectively but remained significantly higher than control group level in both groups (p < 0.05).
Renal catalase activity () was significantly increased (p < 0.001) in Diet I and diet II groups compared to both control and adenine groups. Total antioxidant capacity () was significantly (p < 0.001) decreased by adenine compared to control group. Receiving avocado 50% and 100% increased total antioxidant capacity significantly (p < 0.001) compared to adenine group and become comparable to control group capacity. Similar results were obtained for GSH level () which was decreased significantly (p < 0.05) by adenine compared to control group. Diet I significantly increased GSH level (p < 0.001) while (p < 0.05) in diet II group.
Figure 2. Effect of Persea Americana fruit (50% and 100% w/w in diet) on renal (a) malondialdehyde level, (b) catalase activity, (c) total antioxidant capacity, and (d) reduced glutathione concentration in adenine induced kidney injury in mice. *, **, *** p < 0.05, 0.01, 0.001 respectively compared to control group; ‡, ‡‡, ‡‡‡ p < 0.05, 0.01, 0.001 respectively compared to adenine group (n = 10)
Representative photographs for kidney sections stained with hematoxylin-eosin stain are shown in (). The stained sections show marked tissue injury and infiltration of inflammatory cells. Histopathological changes score is shown in . Sections isolated from adenine group showed markedly high tubular injury score that was significantly lowered by diet I (p < 0.001) and diet II (p < 0.05).
Figure 3. Representative photographs showing recruitment of inflammatory cells (arrows) and tubular injury in kidney sections (hematoxylin-eosin stain) isolated from (a) control group, (b) adenine group, (c) diet I group (Persea Americana fruit 50% w/w in diet), and (d) diet II group (Persea Americana fruit 100% w/w diet)
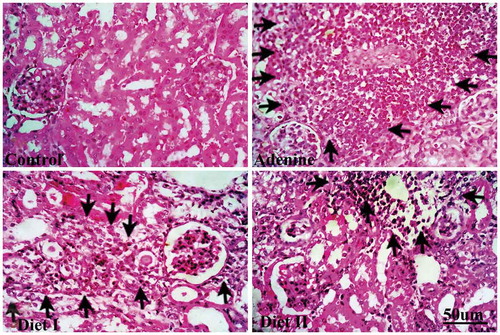
Table 2. Effect of Persea Americana on tubular injury and fibrosis scores
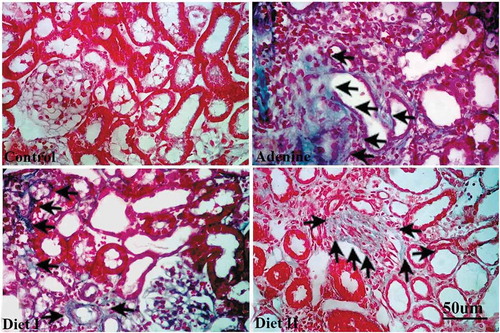
Fibrosis and collagen deposition was scored in sections stained with masson trichrome ( and ). Receiving adenine resulted in markedly high fibrosis score (p < 0.001) compared to control group. Both diet I and II decreased fibrosis about twofold compared to adenine group score but still significantly (p < 0.05, 0.001 respectively) higher than control group score.
Figure 4. Representative photographs showing collagen deposition (arrows) in masson trichrome stained kidney sections isolated from (a) control group, (b) adenine group, (c) diet I group (Persea Americana fruit 50% w/w in diet), and (d) diet II group (Persea Americana fruit 100% w/w diet)
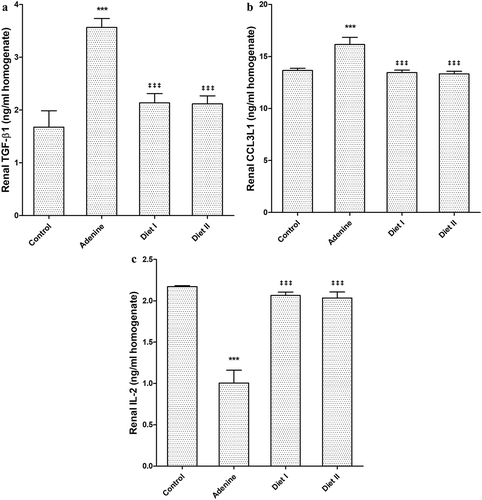
Transforming growth factor-β1 level in renal tissue was significantly (p < 0.001) decreased by adenine administration compared to control group. The fibrotic marker level was significantly (p < 0.001) decreased to control group level in diet I and diet II groups compared to adenine group (). Similar to TGF-β1 results, CCL3L1 level was changed ().
Adenine alone significantly decreased IL-2 in renal tissue compared to control group (p < 0.001). After adding avocado to diet, IL-2 level was significantly increased (p < 0.001) in diet I and II groups compared to its level in mice received adenine alone ().
Figure 5. Effect of Persea Americana fruit (50% and 100% w/w in diet) on renal level of (a) transforming growth factor (TGF)-β1, (b) C-C motif ligand 3 like protein-1 (CCL3L1), and (c) Interleukin (IL)-2 in adenine induced kidney injury in mice. *** p < 0.001 compared to control group; ‡‡‡ p < 0.001 respectively compared to adenine group (n = 10)
Discussion
The continuous deterioration in quality of life and the decreased life expectancy with their related burden on the economy, represent the main dilemma of chronic kidney disease in low, middle and even high-income countries [Citation15].
Traditionally, dietary recommendations for patients with chronic kidney disease prohibit the consumption of plant foods containing high potassium content. However, recently this belief begins to change due to the possible benefits derived from a basically vegetarian diet. This kind of diet can improve gut ecological system due to the reduction in transforming non-harming symbiont into pathogenic ones (pathobionts) and protein-fermenting species. As a result, production of harmful uremic toxins is decreased in addition to the benefits obtained from enhancing short chain fatty acid production [Citation16]. Notably, the exact benefits and the possible risk of a vegetarian/vegan diet-based lifestyle for CKD patients still need more investigations.
The present study was conducted to investigate the potential therapeutic benefits of administrating Persea Americana fruit (avocado) in diet with different percentages on inflammatory response and oxidative stress in adenine induced kidney injury in mice. Consumption of adenine in high doses leads to its oxidization into 2, 8-dihydroxyadenine. The later has very low solubility and precipitates in the renal tubules as stones causing inflammation and tubulo-interstitial fibrosis [Citation8]. Potassium serum level and markers of kidney function, inflammation fibrosis in addition to oxidative stress were measured.
Our results revealed an increase in creatinine serum concentration and histopathological changes in kidney tissue after administration of adenine in food as reported by previous studies [Citation17]. Addition of avocado decreased creatinine serum concentration showing a superior effect of diet I to diet II as diet I almost normalized creatinine concentration compared to control group. In line with our results, Anshar et al. (2018) demonstrated that avocado juice decreases level of blood urea nitrogen and creatinine in white rats exposed to toxic doses of meloxicam [Citation18].
It was observed that mice fed diet I regained weight in contrast to mice fed diet II in which weight reduction continues after adenine administration. Weight reduction may be explained by the decrease in mice appetite and daily food consumption when avocado is the sole element of diet. High fat content in avocado (about 23%) and its low carbohydrate content (about 5%) may lower insulin activity and in turn may explain elevated serum potassium in diet II received mice. Insulin effect on inhibiting cellular uptake of serum potassium was investigated by several studies where lowered insulin activity in insulin resistance patients causes an elevation in serum potassium level [Citation19–21].
On the other hand, addition of avocado to normal chow in diet I (50% W/W) showed a beneficial effect on kidney despite of the significant increase in potassium serum concentration. In chronic kidney disease, kidneys are able to keep potassium homeostasis even at low rates of glomerular filtration (up to 15–20 mL/min/1.73 m2). Meanwhile in case of renal failure, the proportion of the fecal excretion of potassium is increased as a compensatory mechanism. Such compensatory mechanism assures relatively normal potassium level in the serum under stable conditions even in case of more deteriorated kidneys. However, this adaptation may be insufficient at end stage CKD so that any increase in external potassium load will significantly increase the serum potassium concentration [Citation22]. The abrupt increase in serum potassium is usually fatal while the gradual increase which occurs on a relatively long time is usually accompanied by an accepted adaptation of the renal excretory mechanisms as increased fractional potassium excretion. Edible plants containing high potassium content like avocado appear to be healthier when compared with meals containing high potassium content like sea food, chicken, and turkey. These plants have the advantage of enhancing intracellular potassium uptake and its excretion in stool as well through increasing fecal bulk (dietary fiber) [Citation23,Citation24].
Adenine showed a significant elevation in malondialdehyde (MDA) level in kidney homogenate when compared to control group. In addition, a decrease in total antioxidant capacity and kidney content of reduced glutathione were observed. Reduced GSH is an endogenous antioxidant which is oxidized by reactive oxygen species thus it protects from tissue damage through consuming free radicals. Administration of avocado decreased MDA level significantly, increased total antioxidant capacity and restored renal GSH content. This may be attributed to the abundance of C7 sugars especially mannohepulose in Persea Americana fruit. Mannohepulose has a polyol structure possessing a strong antioxidant activity. Moreover, avocado is rich in many other natural antioxidants as vitamin C, vitamin E, carotenoids, and phenolic compounds [Citation25,Citation26]. Notably, tubular injury score and renal content of the lipid peroxidation marker MDA were observed to be higher in diet II group compared to diet I group. As a result, consumption of GSH may increase in diet II group decreasing renal tissue content of GSH in this group compared to diet I group.
Interleukin (IL)-2 has an initial proinflammatory effect and then shows anti-inflammatory effects. It specially increases the activity of regulatory T cells in addition to a possible role in activation of Fas-mediated cell death [Citation27]. Obtained results demonstrated a decrease in IL-2 expression with adenine administration and its elevation when avocado was administered after adenine. This observation may indicate an anti-inflammatory role of IL-2 in certain kidney tissues, specifically in the renal tubular epithelium the site of adenine induced renal injury [Citation28].
de Frutos et al. (2019) reported an increase in transforming growth factor (TGF)-β1 activity in the renal tissue of adenine-fed mice [Citation29]. Also, activity of TGF-β1 was found to be decreased in adenine induced CKD after treatment with omega-3 polyunsaturated fatty acids [Citation30]. In line with these studies, our results revealed a substantial rise in renal TGF-β1 expression in adenine fed group. Addition of avocado in diet I and diet II decreased renal tissue expression of TGF-β1 which may be attributed to its high content of poly unsaturated fatty acids [Citation31].
Another cytokine involved in renal inflammatory responses is C-C ligand 3-like protein 1 (CCL3L1). It is a pro-inflammatory chemokine, that induces macrophage recruitment, activation of leukocytes like lymphocytes and monocytes [Citation32]. Some pro-inflammatory chemokines are controlled genetically by nuclear factor-κB and activate protein-1 through the phosphorylation of the transcription factor p38/MAPK. It was found that P38/MAPK activity is diminished by polyunsaturated fatty acids found in fish oil [Citation33]. Accordingly, we can assume that polyunsaturated fatty acids in avocado could affect P38/MAPK activity leading to decreasing CCL3L1 and diminishing its pro-inflammatory role.
Conclusion
Enriching diet of CKD patients with Persea Americana fruit can represent a safe beneficial source of potassium. High fiber content can regulate potassium balance in such patients. Furthermore, antioxidants and polyunsaturated fatty acids in avocado fruit can attenuate the progression of CKD through anti-inflammatory and antifibrotic mechanisms.
Acknowledgments
Authors introduce their gratitude to Prof. Amr El-Karef (professor of pathology, Faculty of Medicine, Mansoura University) for his effort in histopathological examinations.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Eknoyan G. Kidney Diseases. In: SR Q, editor. International encyclopedia of public health. Oxford: Academic Press; 2017. p. 353–357.
- Fleig S, Patecki M, Schmitt R. Chronic kidney disease: what is currently available for treatment? Internist (Berl). 2016;57(12):1164–1171.
- Abuelo JG. Treatment of Severe Hyperkalemia: confronting 4 Fallacies. Kidney Int Rep. 2018;3(1):47–55.
- Thomsen RW, Nicolaisen SK, Hasvold P, et al. Elevated potassium levels in patients with chronic kidney disease: occurrence, risk factors and clinical outcomes-a Danish population-based cohort study. Nephrol Dial Transplant. 2018;33(9):1610–1620.
- Cupisti A, Kovesdy CP, D’Alessandro C, et al. Dietary approach to recurrent or chronic hyperkalemia in patients with decreased kidney function. Nutrients. 2018;10:261.
- St-Jules DE, Goldfarb DS, Sevick MA. Nutrient non-equivalence: does restricting high-potassium plant foods help to prevent hyperkalemia in hemodialysis patients? J Ren Nutr. 2016;26:282–287.
- Chen W, Abramowitz MK. Metabolic acidosis and the progression of chronic kidney disease. BMC Nephrol. 2014;15:55.
- Ali BH, Al-Salam S, Al Za’abi M, et al. New model for adenine-induced chronic renal failure in mice, and the effect of gum acacia treatment thereon: comparison with rats. J Pharmacol Toxicol Methods. 2013;68(3):384–393.
- Gargiulo S, Greco A, Gramanzini M, et al. Mice anesthesia, analgesia, and care, Part I: anesthetic considerations in preclinical research. Ilar J. 2012;53:E55–69.
- Maruna RF, Trinder P. Determination of serum sodium by the magnesium uranyl acetate. Clin Chem Acta. 1958;2:585.
- Ohkawa H, Ohishi N, Yagi K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem. 1979;95:351–358.
- Aebi HE. Catalase. In: Bergmeyer HU, Bergmeyer J, Grabi M, editors. Methods of enzymatic analysis. Verlag Chimie: Weinheim; 1985. p. 273–286.
- Kinomura M, Kitamura S, Tanabe K, et al. Amelioration of cisplatin-induced acute renal injury by renal progenitor-like cells derived from the adult rat kidney. Cell Transplant. 2008;17(1–2):143–158.
- Yamate J, Tatsumi M, Nakatsuji S, et al. Immunohistochemical observations on the kinetics of macrophages and myofibroblasts in rat renal interstitial fibrosis induced by cis-diamminedichloroplatinum. J Comp Pathol. 1995;112(1):27–39.
- Tonelli M, Wiebe N, Culleton B, et al. Chronic kidney disease and mortality risk: a systematic review. J Am Soc Nephrol. 2006;17(7):2034–2047.
- Cases A, Cigarrán-Guldrís S, Mas S, et al. Vegetable-based diets for chronic kidney disease? It is time to reconsider. Nutrients. 2019;11:1263.
- Jia T, Olauson H, Lindberg K, et al. A novel model of adenine-induced tubulointerstitial nephropathy in mice. BMC Nephrol. 2013;14:116.
- Anshar AR, Bahar MA, Ikliptikawati DK. The effect of avocado to the profile of blood urea nitrogen (BUN) and creatinine in rats (Rattus norvegicus) induced with meloxicam. Jurnal Riset Veteriner Indonesia (Journal of The Indonesian Veterinary Research). 2018;2(1):1.
- Cohen P, Barzilai N, Lerman A, et al. Insulin effects on glucose and potassium metabolism in vivo: evidence for selective insulin resistance in humans. J Clin Endocrinol Metab. 1991;73(3):564–568.
- Plavinik FL, Rodrigues CI, Zanella MT, et al. Hypokalemia, glucose intolerance, and hyperinsulinemia during diuretic therapy. Hypertension. 1992;19(2_supplement):II26.
- Nguyen TQ, Maalouf NM, Sakhaee K, et al. Comparison of insulin action on glucose versus potassium uptake in humans. Clin J Am Soc Nephrol. 2011;6(7):1533–1539.
- Clase CM, Carrero JJ, Ellison DH, et al. Potassium homeostasis and management of dyskalemia in kidney diseases: conclusions from a kidney disease: improving global outcomes (KDIGO) controversies conference. Kidney Int. 2020;97:42–61.
- Aronson PS, Giebisch G. Effects of pH on potassium: new explanations for old observations. J Am Soc Nephrol. 2011;22(11):1981–1989.
- Noori N, Kalantar-Zadeh K, Kovesdy CP, et al. Dietary potassium intake and mortality in long-term hemodialysis patients. Am J Kidney Dis. 2010;56(2):338–347.
- Bertling I, Tesfay SZ, Bower JP. Antioxidants in ‘Hass’ avocado. South African Avocado Growers’ Association Yearbook. 2007;30:17–19.
- Calderón-Oliver M, Escalona-Buendía HB, Medina-Campos ON, et al. Optimization of the antioxidant and antimicrobial response of the combined effect of nisin and avocado byproducts. LWT-Food Sci Technol. 2016;65:46–52.
- Hoyer KK, Dooms H, Barron L, et al. Interleukin-2 in the development and control of inflammatory disease. Immunol Rev. 2008;226(1):19–28.
- Diwan V, Mistry A, Gobe G, et al. Adenine-induced chronic kidney and cardiovascular damage in rats. J Pharmacol Methods. 2013;68(2):197–207.
- De Frutos S, Luengo A, García-Jérez A, et al. Chronic kidney disease induced by an adenine rich diet upregulates integrin linked kinase (ILK) and its depletion prevents the disease progression. Biochim Biophys Acta. 2019;1865(6):1284–1297.
- Xu J, Feng ZP, Peng HY, et al. Omega-3 polyunsaturated fatty acids alleviate adenine-induced chronic renal failure via regulating ROS production and TGF-β/SMAD pathway. Eur Rev Med Pharmacol Sci. 2018;22:5024–5032.
- Dreher ML, Davenport AJ. Hass avocado composition and potential health effects. Crit Rev Food Sci Nutr. 2013;53:738–750.
- McKinney C, Merriman ME, Chapman PT, et al. Evidence for an influence of chemokine ligand 3-like 1 (CCL3L1) gene copy number on susceptibility to rheumatoid arthritis. Ann Rheum Dis. 2008;67:409–413.
- Abedin M, Lim J, Tang TB, et al. N-3 fatty acids inhibit vascular calcification via the p38-mitogen-activated protein kinase and peroxisome proliferator-activated receptor-γ pathways. Circ Res. 2006;98:727–729.
