?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
Research evidence attempting to evaluate the safety and efficacy of herbs use by pregnant women against malaria in Africa are seriously lacking. This study seeks to determine efficacy of 3 antimalarial plants commonly used by pregnant women in Nigeria on placental malaria and pregnancy outcome in murine model. The experiment was carried out in 110 experimental pregnant mice randomly distributed into 22 groups and treated with extracts of M. lucida, E. chlorantha, and C. citatrus at a graded dose of 100, 200, and 300 mg/kg. The positive control was treated with fansidar at 10 mg/kg while the negative control was not treated. Extract of M. lucida and E. chlorantha recorded over 70% chemosuppression at the highest dose of 300 mg/kg body weight. While extract of E. chlorantha induced abortion in pregnant mice at all concentrations, extract of M. lucida and C. citatrus caused miscarriage at the highest dosage. Malaria related pathological damages were more pronounced in placenta of mice treated with C. citatrus, it was reduced to the barest minimum in mice treated with extracts of M. lucida. The use of herbs was significantly associated with poor pregnancy outcomes (pups weight, pups mortality, and litter size) in this study.
Introduction
Immunity of pregnant women against many parasitic diseases including malaria become suppressed as a result of physiological changes that accompanies pregnancy. Malaria infection during pregnancy have been acknowledged as a major problem in sub-Saharan Africa. This is because the most predominant malaria parasite species (Plasmodium falciparium) in sub-Saharan Africa are capable of sequestration in the placenta of infected pregnant woman. The implication of this sequestration is that while maternal peripheral blood may be free of Plasmodium parasites, the parasites can be seen in the placenta, causing damages to the placenta and consequently on the developing fetus.
The placental sequestration of P. falciparum (placental malaria) leads to the colonization of parasitized erythrocytes in the intervillous space. Placental malaria has long been regarded as a major complication of malaria during pregnancy. Placental malaria is a big problem in sub-Saharan Africa and is associated with poor pregnancy outcomes, such as low birth weight (LBW), stillbirth and preterm delivery. Studies have suggested that Plasmodium parasites can evade the placenta barrier, leading to mother-to-child transmission of malaria, (a condition known as congenital malaria) [Citation1,Citation2]. Studies in Africa have shown that at least 7–10% of newborns have malaria parasite-infected placentas, and a significant part of the transmission of parasites from the mother to the child occurs in utero [Citation3,Citation4]. Despite the devastating effect of placental malaria, the main choice of prevention is through the use of intermittent preventive treatment with Sulphadoxine pyremethamine (IpT-Sp). However, the truth is that malaria parasites are currently developing resistance to IpT-Sp. Recently, exploration of natural products of plant sources have been the center of focus to detect a new, safer and more effective bioactive compounds with medicinal properties [Citation5]. The use of medicinal plants in the treatment and prevention of malaria have been on a rise in various parts of the world. One of the aims of this study is to explore the potential of some known antimalarial plants in preventing placental malaria.
Apart from the issue of resistance, IpT-Sp is only available and useful for those who attend hospitals for their antenatal care (ANC). Studies have documented that, a lot of pregnant women in Nigeria preferred to use Traditional Birth Homes (TBHs) instead of hospital for ANC [Citation6,Citation7]. Some of the random surveys carried out by other researchers in Nigeria, indicated high level of patronages ranging from 35% [Citation7] to as high as 75% [Citation8] depending on the region. Despite this high level of patronage, studies documenting efficacy and safety of herbs used by pregnant women in Nigeria and other African countries are lacking. Few studies that assessed the management of malaria during pregnancy among pregnant women attending TBHs revealed that the traditional birth attendants (TBAs) often depends on herb for management of malaria during pregnancy [Citation9,Citation10].
Therefore, there is a need to understand plants used by pregnant women so as to ensure that pregnant women receive effective treatment, to identify potential toxic effect (especially on the developing fetus), and also to keep valuable record about medicinal plants for pregnant women’s health for the future [Citation11]. The effects of the herbs on pregnancy and neonates have been poorly studied and could not be ascertained [Citation12]. Despite this, some herbalists and herb sellers claimed that herbs could be safely used by pregnant women for the treatment of malaria [Citation13]. However, some experimental investigations suggested that some herbs could be teratogenic both in human and animal models [Citation14,Citation15]. This paucity of scientific information continues to limit the knowledge about the effects of herbs on prevention of pregnancy-associated malaria including placental malaria.
Although malaria in pregnancy has recently enjoyed a lot of attention from researchers and donors, there are still some ethical and logistic issues that restrict studies of human malaria infection during pregnancy. Thus, the specific pathologic bases of different Pregnancy Associated Malaria (PAM) outcomes remain poorly understood [Citation16]. As a result, mice are often used for studying these complex systems and serve as developmental and functional models to understand human pregnancy, as both species have a hemochorial placenta exhibiting similar transport mechanisms [Citation17,Citation18].
We recently carried out a random survey to document plants used to prevent or treat malaria in Pregnancy in Abeokuta, Nigeria. About 17 plants were mentioned to be useful in which Morinda lucida, Cymbopogon citratus and Enathia chlorantha top the list of the most frequently mentioned plants [Citation19]. Thus, this study seeks to determine efficacy of these three plants on placental malaria and pregnancy outcome in murine model.
Materials and methods
Plant collection, identification and extraction of plant extracts
Fresh leaves of M. lucida, C. citratus, and bark of E. chlorantha were collected from locations around Odeda Local Government, Abeokuta. Permission to collect the plants was granted by the state Ministry of Forestry and later by the owners of land where the plants were located. Visual Identification and authentication were carefully done by a plant taxonomist Dr. Ekundayo of the Forestry Research Institute of Nigeria (FRIN).
The plant parts were washed, dried at room temperature (28 ± 2 °C), and then pulverized using plant grinder. These were soaked separately in distilled water for 24 hours and later filtered. The filtrates were afterward evaporated to dryness over water bath at 35°C for further use [Citation20].
Source of animals
A total of 110 female BALB/c mice (each weighing between 23.0 g and 23.2 g) with mean body weight of 23.2 ± 0.2 g and same physiological age commercially obtained from Nigerian Institute of Medical Research (NIMR), Yaba, were used for this study. The mice were free of pathogens, and have not been used for any initial experiment.
Animal housing and husbandry
The mice were kept in plastic cages which contained dried wood shavings as beddings at room temperature (28 ± 2 °C) and were fed with standard ration (Vital Feeds Limited, Ibadan) and clean water in the animal house. The mice were kept at 12 hours light and dark cycle.
Ethical considerations
All experiments inclusive of animal handling and sacrifice were conducted as per the guidelines of the Bioethics Committee of Department of Pure and Applied Zoology, Federal University of Agriculture, Abeokuta.
Experimental design
A total of 110 mice (each weighing between 23.0 g and 23.2 g) were randomly divided into 22 groups of 5 pregnant mice each. Eleven out of the pregnant groups were infected at low inoculum at gestation day (GD) 6. Treatment started at GD 9 and lasted for 6 days; nine (9) groups were given plant extracts. A group was treated with Sulphadoxine-pyrimethamine (Positive control) while a group was not treated (Negative control). Remaining non-infected groups were subjected to the same treatment (). On GD 18, three out of five mice in each group euthanized by cervical dislocation. The placenta(s) were harvested for histopathological studies. The remaining animals were allowed to litter for studies on pregnancy outcome.
Table 1. Experimental groups
Experimental procedures
Treatment of infected and non-infected pregnant mice
Leaves extracts of M. lucida, C. citratus, and bark extract of E. chlorantha were administered to the pregnant mice orally with the aid of an oral cannula at 3 different dosages; 100 mg/kg, 200 mg/kg and 300 mg/kg body weight, respectively, [Citation21]. Sulphadoxine pyremethamine (SP) was administered at a dosage of 10 mg/kg body weight. The dosages were selected following the method of [Citation14]. We, therefore, administered the extracts at a dose that is not toxic in non-pregnant mice. The lowest dose (100 mg/kg body weight) was lower than 10% of the LD50 while the highest Dose (300 mg/kg body weight) was lower than 20% of the LD50.
Estrus synchronization and mating
A method for inducing estrus and proestrus in female mice by exposing them to bedding soaked with male urine (Whitten effect) [Citation22] were used. Sexually mature females (at least 60 days old) were housed for 1 week. Sexually mature male mice (at least 60 days old) were separately housed for 1 week. The females were then exposed for 4 days to bedding soaked with male urine; one male was placed with a pair of naturally synchronized females for a period of 48 h in order to generate timed pregnant females.
Parasites
Malaria parasites P. berghei NK 65 obtained from a stock maintained at the Institute for Advance Medical Research and Training, University College Hospital (UCH) Ibadan were used. These were prepared as an inoculum.
Inoculation of experimental mice
Malaria parasite inoculums were prepared by collecting blood samples from donor mouse. The blood collected from the donor mouse was then diluted with normal saline such that 0.1 ml contained 103 of the parasite.
Part of the mice were infected with the parasites by inoculating them intraperitonially with 0.1 ml of the prepared blood solution.
Assessments of percentage malaria parasitemia and chemosuppression
Each day, blood samples were taken from the caudal vein of each mouse on a clean glass slide, thin films were prepared and stained with 10% Giemsa solution. The parasitemia was estimated by careful examination of the well-stained thin blood film. Parasitemia was determined by dividing the number of parasitized red blood by the total number of red blood cells and then multiplied by 100 to express it as a percentage.
Percentage malaria parasitemia (%) and Day 1 chemo suppression (%) were determined using the formula below:
Percentage malaria parasitemia (%)
Day 1 Malaria chemosuppression
where MP is the percentage malaria parasitemia
Determination of pregnancy outcome
At littering, number of pups from the remaining animals from each groups were counted, and the mean values were calculated. The pups were also weighed using a digital weighing scale (Mettler Tolebo Veritas scale for laboratory with model number ES 422). Within 24 hours of littering, number of dead pups were counted against the total to give percentage mortality. Also, number of days taken to litter from GD 1 were also recorded. Oviduct of mice assumed to be pregnant without fetus and traces of implantations upon dissection were cross-sectioned. The presence of prominent endometrial gland(s) in the section were taken as indication of disrupted pregnancy.
Histological examination of the placenta
The placentas were removed from the animals (on gestational day 18) and fixed in 10% neutral buffered formalin, then dehydrated in an ascending series of ethyl alcohol. The processed placentas were embedded in paraffin wax. The prepared tissue blocks were sectioned on glass slides (5–6 μm thick) using microtome. The paraffin sections were further processed and stained with hematoxylin and eosin [Citation23]. The histological prepared sections were examined, and microphotographed using an Olympus CX31 Digital microscope (Olympus Japan).
Statistical analysis
Raw data obtained from the laboratory were analyzed using one-way analysis of variance (ANOVA) to compare means across groups using Statistical Product and Service Solution (SPSS), version 21.0 (Chicago, IL). The results were presented as mean ± SE in tables. Mean values with p < 0.05 were considered significant.
Results
Body weight changes
There was no significant change in mean body weight of all infected and non-infected pregnant mice treated with the plant extracts compared with the control at gestation days (GD) 1 and 9. However, at GD 17, the mean body weights of infected and non-infected pregnant mice treated with E. chlorantha was significantly (p < 0.001) lowered compared other groups including the control groups ().
Table 2. Body weight changes of infected and non-infected pregnant mice treated with the plant extracts compared with the control at gestation days (GD) 1, 9, and 17
Daily chemosupression in treated and control groups of infected pregnant mice
There was a significant difference in chemosupression between all the plant extracts in the 6-days treatment of infected pregnant mice (p < 0.05). A dose-dependent increase in chemosuppression was observed in infected pregnant mice treated with the plant extract throughout the 6 days of treatment ().
Table 3. Daily chemosupression in treated and control groups of pregnant-infected mice
Pregnant mice treated with Morinda lucida plant extract recorded the highest chemosupression (p < 0.05) when compared with other plant extracts at the same concentrations (). A significant difference existed (p < 0.05) in day 1 percentage chemosupression of mice treated with M. lucida at varying concentrations. Morinda lucida extract produced a higher day 1 chemosuppression (16.05%) compared to the group treated with IpT-Sp (Fansidar) (11.37%). However, a total parasite clearance was observed at day 5 in groups treated with 100 mg/kg body weight of fansidar ().
The extracts of Morinda lucida at 100, 200, and 300 mg/kg body weight produced a day 1 chemosuppression of 10.34 ± 0.01%, 10.42 ± 0.03%, and 16.05 ± 1.15%, respectively, which is significantly higher than that of Enatia chlorantha with chemosuppression of 2.94 ± 0.01%, 5.08 ± 1.15% and 10.34 ± 0.01% at 100, 200, and 300 mg/kg body weight, respectively. On the other hand, the least percentage chemosuppression were recorded in mice treated with Cymbopogon citratus plant extracts. There was no significant difference (p > 0.05) in day 1 percentage chemosupression of mice treated with C. citratus at varying concentrations. A percentage chemosupression of 0.17 ± 0.17%, 1.92 ± 0.03% and 1.85 ± 0.01% were recorded in mice treated with 100, 200 and 300 mg/kg body weight of C. citratus extract, respectively ().
The same trend in percentage chemosuppression was observed throughout the 6 days of treatment. Pregnant mice treated with Morinda lucida plant extract recorded the highest level of chemosupression (p < 0.05) at day 6 when compared with other plant extracts at the same concentrations ().
Mice treated with 300 mg/kg body weight of Morinda lucida plant extract recorded the highest level of chemosuppression (77.26%) at the sixth day of treatment when compared with the other plant extract. This was followed by that of Enantia chlorantha (74.32%) administered at 300 mg/kg body weight.
Pregnant mice treated with Cympobogon citratus recorded the least day 6 percentage chemosupression ranging from 44.83% (100 mg/kg body weight) to 55.56% (300 mg/kg body weight). Except for C. citratus, none of the other plant extracts produced chemosupression less than 50% even at the lowest concentration (100 mg/kg body weight) ().
The untreated pregnant group recorded no chemosupression throughout the 6 days of treatment with parasite multiplication rate of 87.89%.
Effect of M. lucida and C. citratus extracts on placenta parasitemia
Placenta of mice treated with C. citratus extracts tested positive to P. berghei infection at different dosages. Furthermore, there was no significant difference (p > 0.05) in placenta parasitemia between mice treated with 100 mg/kg body weight of C. citratus extract and untreated. On the other hand, placenta of mice treated with M. lucida extract only tested positive (with very low parasitemia) at 100 mg/kg body weight ().
Figure 1.: Effect of M. lucida and C. citratus extracts on placenta parasitemia
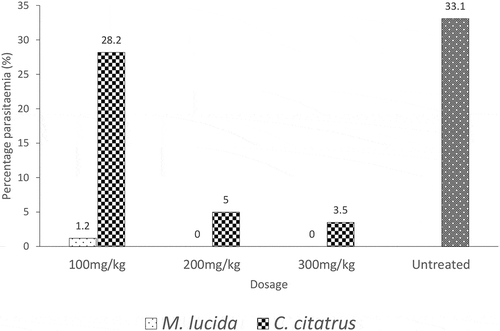
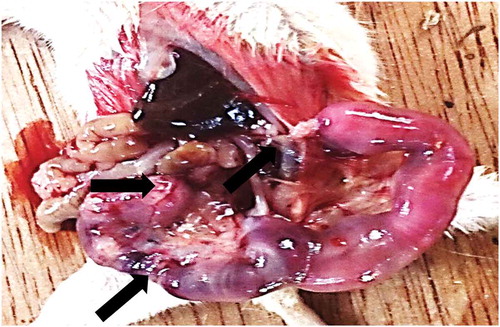
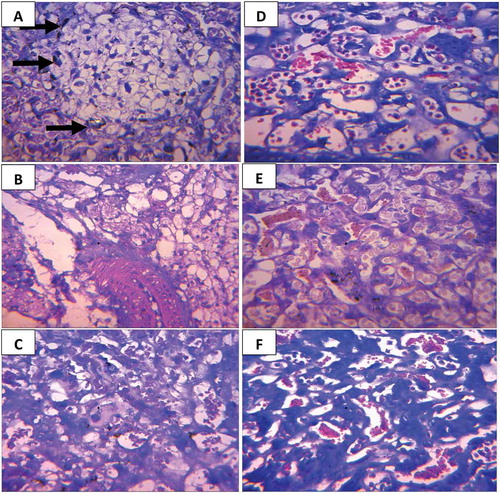
Figure 2. Section of (a) Oviduct of mice treated with E. chlorantha extract: showing well-formed glands (long arrows) in the endometrial submucosa. The epithelia lining is normal (short arrows), H and E × 400 (b) Uterus of mice treated with E. chlorantha extract showing fetal resorption (long arrows) Mag ×1.5
Histological changes of the placenta of infected-untreated mice and mice treated with fansidar (Control)
Few trophoblastic cells that appear pyknotic were observed in the placenta of infected-untreated mice. Degeneration and coagulative necrosis (small arrow) of the labyrinth as well as parasitized red blood cells and malaria parasite pigments were also observed in the placenta of infected-untreated mice ().
On the other hand, no visible lesion was observed in placenta of both infected and non-infected mice treated with fansidar ().
Histological changes of the placenta of mice treated with C. citratus extract
Transverse section of the placenta from infected mice treated with C. citratus showed few trophoblastic cells that appear pyknotic at all concentrations. Furthermore, parasitized red blood cells were observed in placenta of infected mice treated with 100 mg/kg body weight of C. citratus extract (). All the non-infected pregnant mice treated with different concentrations of C. citratus extracts showed no visible lesions ().
Histological changes of the placenta of mice treated with M. lucida extract
Transverse section of the placenta from infected mice treated with M. lucida showed few trophoblastic cells that appear pyknotic at 100 mg/kg body weight (). All the non-infected pregnant mice treated with different concentrations of M. lucida extracts showed no visible lesions ().
Figure 3. (a-d): Photomicrograph of cross histological section of the placenta from mice showing; A (infected not treated), B (not infected not treated) C (infected treated with fansidar) D (not infected treated with fansidar) (a) Trophoblastic cells that appear pyknotic (long arrow) in the infected mouse, degeneration and coagulative necrosis (small arrow) of the labyrinth, parasitized red blood cells and malaria parasite pigments). (b-d) No visible lesions in both infected (treated) and non-infected groups. H and E × 400
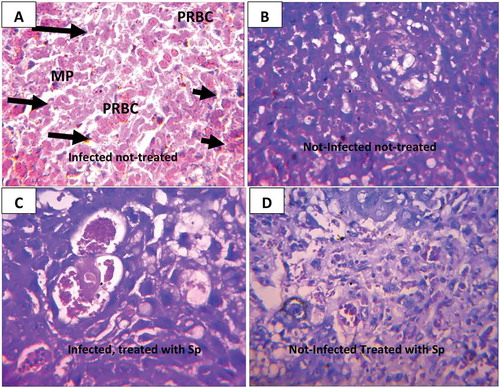
Figure 4. Photomicrograph of cross histological section of the placenta from mice treated with different concentrations of C. citratus; (a-c) Infected mice treated with 100, 200 and 300 mg/kg body weight respectively. (d-f) Non-infected mice treated with 100, 200 and 300 mg/kg body weight, respectively. (a-c) few trophoblastic cells that appear pyknotic in the infected-treated group (Long arrow). (d-f) No visible lesions in non-infected groups. Parasitized red blood cells (PRBC). H and E × 400
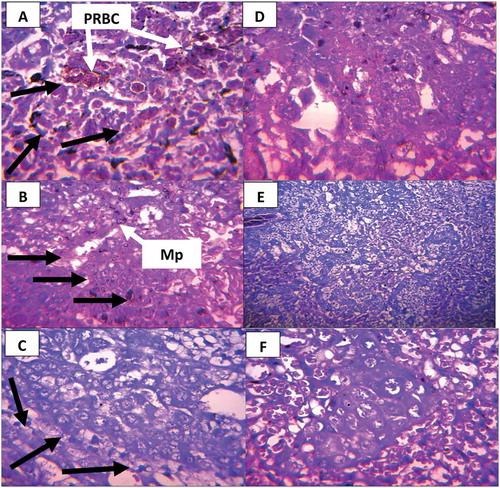
Figure 5. (a-f): Photomicrograph of cross histological section of the placenta from mice treated with different concentrations of M. lucida; (a-c) Infected mice treated with 100, 200 and 300 mg/kg body weight respectively. (d-f) Non-infected mice treated with 100, 200, and 300 mg/kg body weight, respectively. (a) Few trophoblastic cells that appear pyknotic in the infected-treated group (Long arrow). (b-f) No visible lesions in both infected and non-infected group, H and E × 400
Effect of plant extracts on pregnancy outcome of (infected and non-infected) pregnant mice
At littering, a significant difference in pup’s weight, litter’s size and pup’s mortality existed between infected (treated) mice and non-infected-treated mice (). Pup’s weight and litter’s size were significantly higher in the non-infected groups compared to the infected groups ().
Table 4. Effect of plant extracts on pregnancy outcome of infected and non-infected pregnant mice
At littering, infected pregnant mice from the control groups (Fansidar and untreated) recorded higher (p < 0.05) pup weight compared with those treated with plant extracts (). However, there was no significant difference in litter’s size between the extract and untreated group (p > 0.05). On the other hand, a significant difference existed in litter’s size (p < 0.05) between the extract and groups treated with fansidar ().
Non-infected pregnant mice from the control group (untreated) recorded significantly higher (p < 0.05) pup weight compared with plant extracts groups (). However, there was no significant difference in pup’s weight between the Morinda lucida extract groups and fansidar groups (p > 0.05). On the other hand, a significant difference existed in pup’s weight (p < 0.05) between non-infected pregnant mice administered with 200 and 300 mg/kg body weight of C. citratus extract and groups treated with fansidar (). There were no pup’s mortality in all the non-infected pregnant groups.
Discussion
The fact that malaria parasites are currently showing resistance to currently available drugs has led researcher into intensive search for a new source of safer and effective antimalarial agent. In this case, there are no other best candidate apart from our ancient medicinal plants. Apart from that, a study have revealed that many pregnant women who can even afford the cost of hospital for antenatal care still preferred to seek health care in traditional birth homes where management of malaria and other ailments solely depends on herbal concoctions [Citation6].
This could have been a great progress toward development of newer and safer drugs against malaria in pregnancy if there were no complications associated with the use of these substances. Moreover, it is very important that, if any substance was to be used (either natural or synthetic) by pregnant women, there should be a consideration for the risk-benefit ratio. However, researches toward evaluation of herbs used in pregnancy against malaria and other infections have been neglected for so long.
We therefore evaluated the efficacy of three most commonly used plants by pregnant women in Nigeria, on placental malaria and pregnancy outcome in murine model.
Leaf extract of M. lucida in this study demonstrated a chemo-suppression up to 77% in pregnant and 87% in non-pregnant mice in a six-day treatment. Moreover, M. lucida extract administered at 300 mg/kg recorded a higher day 1 chemosuppression compared with fansidar groups. This is an indication that M. lucida leaf extract was able to respond quickly as an antimalarial agent.
This is consistent with the findings of [Citation24] and Idowu et al. [Citation20] who reported that the in vivo anti-plasmodial activity of aqueous leaf extract of M. lucida carried out in P. berghei NK-65 parasitized mice showed a significant chemosuppression of up to 85.05% and 84.7%, respectively.
Studies from the phytochemical screening of the aqueous leaf extract of M. lucida revealed the presence of alkaloids and flavonoid as the predominant secondary metabolite [Citation24]. Therefore, the observed antimalarial activity in the group treated with M. lucida may be attributed to its high alkaloid and flavonoid contents. Previous works also showed the antimalarial activity of alkaloids and flavonoids in plants [Citation25,Citation26].
Bark extract of E. chlorantha in this study showed a significant chemo-suppression up to 74.32% in pregnant and 74.81% in non-pregnant mice in a six-day treatment. Other studies has reported similar findings in in-vivo evaluation of E. chlorantha against P. berghei-infected mice [Citation27,Citation28].
The leaf extract of C. citratus recorded the least chemo-suppression in this study with chemo-suppression of up to 55.56% in pregnant and 49.14% in non-pregnant mice in a six day treatment. This implies that C. citratus leaf extract possesses minimal antiplasmodial activity hence might not be a good candidate for an alternative source of anti-malaria. This result confirmed earlier findings on in-vivo and in-vitro activities of C. citratus extract against Plasmodium parasites [Citation29,Citation30].
All the extracts showed chemo-suppression property which was significantly different when compared to the infected non-treated group. A complete clearance was observed in the positive control group treated with Fansidar, in the six-day treatment. While the plant extracts showed ability to suppress parasitemia, none was able to achieve complete parasite clearance in the six-day treatment with extracts. This might be due to the fact that the plant extracts are in their crude forms with the active ingredients not having been isolated and compressed into active drugs [Citation20,Citation24,Citation31].
In this study, placental malaria-related histological damages were observed in all mice treated with leaf extract of C. citratus even though placental parasite density reduced with dosage. This findings might be related to the poor antiplasmodial property shown by the extract in the peripheral blood, hereby facilitating the infectivity of the parasite in the placenta. However, pathological damages to the placenta was more pronounced in the infected non-treated group. It has been suggested that malaria parasites are able to selectively infect the placenta due to their ability to adhere to the chondroitin sulfate A (CS-A) receptor on the syncytiotrophoblast in the placenta [Citation32,Citation33]. By so doing, they elicit immune responses by the host causing pathological lesions in infected placenta [Citation32].
M. lucida seems to be a promising anti-malarial plant, apart from its popularity for use in virtually all African countries, its strong efficacy has been widely reported. One of the additional information that our study is contributing to existing literature on the use of M. lucida is it’s efficacy against placenta malaria and potentially unsafe use in pregnancy. It is interesting that malaria-related pathological damages were reduced to the barest minimum in mice treated with extracts of M. lucida.
However, M.lucida and C. citatrus caused miscarriage in both infected and non-infected pregnant mice (though occurred less in M. lucida treatment groups). A recent study also mentioned Morinda citrifolia (Linn.) belonging to the same family (Rubiaceae) with M. lucida as one of the herbs with abortifacient property [Citation34]. One major factor that could be responsible for this is the alkaloid content of M. lucida. Studies have shown that M. lucida is highly rich in alkaloid [Citation20,Citation24]. It is however established that alkaloids containing plants could impair progestogenic activities [Citation34,Citation35–36], thereby facilitating abortion and/or miscarriage. The results also suggested that these plants possessed embryotoxic (death of fetus in the uterous) properties..
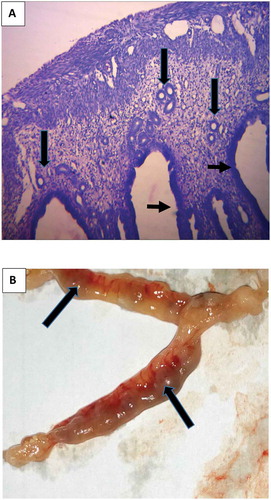
The fact that extract of E. chlorantha induced abortion (determined jointly by drastic loss of weight, fetal absorption and presence of prominent endometrial glands in the oviduct) is an indication that this plant possessed abortifacient properties.
It is however worrisome that there is dearth of information on the effect of these commonly used herbs against malaria on pregnancy in Nigeria. Few studies on plants with abortifacient properties did not mention these plants [Citation35–37]. In fact, a recent study on traditional and medicinal uses of Morinda lucida from Nigeria claimed that M. lucida posed no risk to pregnant women without any study or experiment to proof this [Citation38].
All the mice treated with the plant extracts showed poor pregnancy outcome (pups weight, litter size and pups mortality) when compared with the control. The findings of the study is pointing to the fact that use of herbs in management of pregnancy-associated malaria should be given a thorough consideration in research.
In conclusion
All the 3 plants has antiplasmodial activities. However, extract of E. chlorantha casued arbortion at all concentrations. Out of all the three most frequently used plant by pregnant women against malaria in Nigeria, M. lucida seems to be promising against placental malaria. However, it seems to be embryotoxic in mice model. We will try and isolate the active ingredient present in M. lucida in our future study with a view to come up with a new compound that will be safe for use by pregnant women.
Acknowledgments
The authors would like to express the deepest sense of Gratitude and appreciation to Pharmacology Department for preparation of all plant extract.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Perrault SD, Hajek J, Zhong K, et al. Human immunodeficiency virus co-infection increases placental parasite density and transplacental malaria transmission in Western Kenya. Am J Trop Med Hyg. 2009;80:119–125.
- Lesi FE, Mukhtar MY, Iroha EU, et al. Clinical presentation of congenital malaria at the Lagos University Teaching Hospital. NigerJ Clin Pract. 2010;13:134–138.
- Kamwendo D, Dzinjalamala F, Snounou G, et al. Plasmodium falciparum: PCR detection and genotyping of isolates from peripheral, placental, and cord blood of pregnant Malawian women and their infants. Trans Roy Trop Med Hyg. 2002;96:145–149.
- Mwangoka GW, Kimera SI, Mboera LEG. Congenital Plasmodium falciparum infection in neonates in Muheza District, Tanzania. Malar J. 2008;7:117.
- Dike IP, Obembe OO, Adebiyi FE. Ethnobotanical survey for potential anti-malarial plants in south-western Nigeria. J Ethnopharmacol. 2012;144(3):618–626.
- Idowu OA, Mafiana CF, Dapo S. Traditional birth home attendance and its implications for malaria control during pregnancy in Nigeria. Trans Roy Trop Med Hyg. 2008;102(7):679–684.
- Oshowon FE, Nwakwo GC, Ekiyor CP. Traditional birth attendants and women’s health practices: A case study of Patani in Southern Nigeria. J Public Health Epidemiol. 2014;6(8):252–261.
- Unyime IE, Idongesi LJ, Akpabio EA. High patronage of traditional birth homes: a report from Akwa Ibom, Southern Nigeria. AJHR. 2016;1(1):1–6.
- Adeniran A, Goodman OO, Olatona FA, et al. Malaria prevention in pregnancy among traditional birth attendants in Rural Lagos, Nigeria. J Community Med Primary Health Care. 2016;28(1):8–16.
- Bello FA, Morhason-Bello IO, Olayemi O, et al. Patterns and predictors of self-medication amongst antenatal clients in Ibadan, Nigeria. Niger Med J. 2011;52(3):153–158.
- Nergard CS, Than Ho TP, Diallo D, et al. Attitudes and use of medicinal plants during pregnancy among women at health care centres in three regions of Mali, West-Africa. J Ethnobio Ethnomed. 2015;11:73.
- Fakaye TO, Adisa R, Musa IE. Attitude and use of herbal medicines among pregnant women in Nigeria. BMC Complem Altern M. 2009. DOI:10.1186/1472-6882-9-53. [PMC free article] [PubMed]
- Avwioro G. Effectiveness of some medicinal plant decoction in the treatment of malaria in Nigeria. Ann Biol Res. 2010;1(2):230–237.
- Pakrashi A, Bhattacharya N. Abortifacient principle of Achyranthes aspera Linn. Indian J Exp Biol. 1977;15(10):856–858.
- Seely D, Dugoua JJ, Perri D, et al. Safety and efficacy of panax ginseng during pregnancy and lactation. Can J Clin Pharmacol. 2008;15(1):87–94.
- Rogerson SJ, Hviid L, Duffy PE, et al. Malaria in pregnancy: pathogenesis and immunity. Lancet Infect Dis. 2007;7:105–117.
- Rossant J, Cross JC. Placental development: lessons from mouse mutants. Nat Rev Genet. 2001;2:538–548.
- Adamson SL, Lu Y, Whiteley KJ, et al. Interactions between trophoblast cells and the maternal and fetal circulation in the mouse placenta. Dev Biol. 2002;250(2):358–373.
- Babalola AS, Idowu OA, Ademolu KO, et al. Antiplasmodial activities and abortifacient properties of three commonly used African indigenous anti-malarial plants in Plasmodium berghei infected pregnant mice: implication for maternal and fetal health. Bull Natl Res Cent. 2020;44:153.
- Idowu OA, Babalola AS, Adenubi OT, et al. Antiplasmodial activities of combined extracts of Morinda morindiodes, Morinda lucida and Vernonia amygdalina in Plasmodium berghei infected mice. Zoologist. 2014;11:40–45.
- Abolaji AO, Eteng MU, Ebong PE, et al. A safety assessment of the antimalarial herb Artemisia annua during pregnancy in wistar rats. Phytother Res. 2012;12:1–8.
- Dalal SJ, Estep JS, Valentin-Bon IE, et al. Standardization of the whitten effect to induce susceptibility to Neisseria gonorrhoeae in female mice. Contemp Top Lab Anim Sci. 2001;40(2):13–17.
- Kim SK, Layton C, Bancroft JD. Bancroft’s theory and practice of histological techniques. 8th ed. UK: Elsevier Health Sciences; 2019.
- Ebiloma GU, Omale J, Aminu RO. Suppressive,curative and prophylactic potentials of Morinda lucida (Benth) against erythrocytic stage of mice infective Plasmodium berghei NK-65. Br J Appl Sci Technol. 2011;1(3):131–140.
- Balogun EA, Adebayo JO, Zailani AH, et al. Activity of ethanolic extract of Clerodendrum violaceum leaves against Plasmodium berghei in mice. ABJNA. 2009;1:307–312.
- Okokon JE, Ofodum KC, Ajibesin KK, et al. Pharmacological screening and evaluation of antiplasmodial activity of Croton zambesicus against Plasmodium berghei berghei infection in mice. Indian J Pharmacol. 2005;37:243–246.
- Agbaje EO, Onabanjo AO. The effects of extracts of Enantia chlorantha in malaria. Ann Trop Med Parasitol. 1991;85:585–590.
- Ogbonna DN, Sokari TG, Agomoh AA. Antimalarial activities of some selected traditional herbs from South Eastern Nigeria against Plasmodium species. Parasitol Res. 2008;3(1):25–31.
- Idowu OA, Ajana OO, Soniran ST, et al. ethnobotanical survey of antimalarial plants used in Ogun state Southwest-Nigeria. Afr J Pharm Pharmaco. 2010;4(2):55–60.
- Kimbi HK, Fagbenro-Beyioku AF. Efficacy of Cymbopogon giganteus and Enantia chrantha against chloroquine resistant Plasmodium yoelii nigeriensis. East Afr Med J. 1996;73(10):636–637.
- Adzu B, Haruna A. Studies on the use of Zizyphus Spina-Christi against pain in rats and mice. Afr J Biol. 2007; 6:1317–1324.
- Guyatt HL, Snow RW. Impact of malaria during pregnancy on low birth weight in sub-Saharan africa. Clin Microbiol. 2004;17(4):760–769.
- Bako BG, Audu BM, Geidam AD, et al. Prevalence, risk factors and effects of placental malaria in the UMTH Maiduguri, north-eastern Nigeria: a cross-sectional study. Obstet Gynaecol. 2009;29:307–310.
- Singh R, Kakar S, Shah M, et al. Some medicinal plants with anti-fertility potential: a current status. J Basic Clin Reprod Sci. 2018. DOI:10.4103/2278-960X.194512.
- Ramya R, Sivasakthi R, Kumar CS. Preliminary phytochemical and antifertility studies on Dodonea viscosa Linn. Asian J Pharm Sci. 2011;1(3):77–79.
- Ahmed HMM, Yeh J, Tang Y, et al. Molecular screening of Chinese medicinal plants for progestogenic and anti-progestogenic activity. J Biosci. 2014;39:453–461.
- D’Alessandro U, Langerock P, Bennett S, et al. The impact of a national impregnated bed net programme on the outcome of pregnancy in primigravidae in The Gambia. Trans Roy Trop Med Hyg. 1996;90(5):487–492.
- Adeleye OO, Ayeni OJ, Ajamu MA. Traditional and medicinal uses of Morinda lucida. J Med Plants Stud. 2018;6(2):249–254.