ABSTRACT
Methomyl (ME) is a carbamate insecticide that is causes a change in biochemical parameters and affects the oxidative state in the body through the production of free radicals. Herbal medicines derived from plant extracts are useful in treating many diseases, as they are considered natural antioxidants that have a protective role against toxins. This study aimed to evaluate the ability of green tea extract (GTE) to treat ME-induced cardiac toxicity in experimental animals. The experimental animals were divided into five groups. Blood and tissue were collected to estimate the biochemical parameters. ME caused significantly elevated levels of AST, LDH, NF-κB, and malondialdehyde. While a significant decrease was observed in levels of superoxide dismutase, a decrease in glutathione and glutathione-S-transferase was observed. Alterations in these biochemical markers were referred to oxidative stress-induced cardiac damage. The administration of GTE at a concentration (1.5%) to the ME treated group caused the tested biochemical parameters to appear close to those of the control group. GTE alleviates ME-induced oxidative stress in female albino mice. A higher concentration of GTE {1.5%} appears to have a better therapeutic effect than a lower concentration of extract {0.75%} in cardiac toxicity ME.
Introduction
Green tea (GT) is a type of tea made from the leaves of Camellia sinensis and has not undergone the oxidation process that is used in the manufacture of black tea. The main ingredients of GT are polyphenols, which account for about 30–40% of the solids that can be extracted from dried GT leaves. Among the most important ones are catechins (Cs) which include epicatechingallate (ECG), epigallocatechin (EGC), epicatechin (EC), and epigallocatechin gallate (EGCG) [Citation1]. Green tea extract (GTE) has been found to have healthy properties such as antioxidants and free radical scavenger properties [Citation2]. Green tea catechins (GTCs) have the ability to induce an increase in the blood level of antioxidant enzymes, leading to protection by scavenging ROS such as superoxide, hydrogen peroxide (H2O2), and hydroxyl radicals. GTCs have proven their ability to overcome free radicals produced by the toxic oxidizing compound in the environment thus reducing cellular damage from toxicity, DNA damage, cancer, and apoptosis [Citation3].
Methomyl (ME) is a synthetic carbamate consisting of the third major group of pesticides used worldwide in agriculture [Citation4], and it has been found to play a vital role in controlling insect pests on various types of field crops such as fruits, vegetables, and grains [Citation5,Citation6]. These compounds have a rapid effect on the target pests and have a relatively short life in the environment [Citation7]. According to the World Health Organization (WHO) [Citation8], ME is classified as a severely hazardous compound (Class 1B) [Citation9]. ME causes adverse effects on human health through the overproduction of ROS that builds up causing lipid peroxidation, and LPO causes the degradation of nucleoprotein, nucleic acid, and DNA fragmentation. Also, lipid peroxide reduces GST [Citation10]. ME activates the inflammatory pathway in the heart through NF-κB phosphorylation [Citation11]. ME causes dangerous effects on human health. It causes cardiac toxicity [Citation12], genotoxicity and teratogenicity [Citation13], reproductive toxicity [Citation14], and hormonal disruption [Citation9].
In this regard, different types of clinical diseases can be treated by herbal medicines isolated from plant extracts, so there is an interest in protecting the role of natural antioxidants from the toxicities they cause chemically [Citation11]. Significant increase in scientific research for the prevention and treatment of many diseases using GT is due to the main Cs polyphenols [Citation15].
Therefore, based on these results, our context was designed to study the effect of GT aqueous extract against cardiac toxicity and oxidative stress induced by the widely used insecticides ME in female Swiss albino mice.
Materials and methods
Chemicals
GT was purchased from the local market for herbs and medicinal plants in the form of dry packages. The chemicals were purchased from Sigma Aldrich & Company. The kits were purchased from Biodiagnostic Company, Cairo, Egypt.
GTE preparation
Aqueous solutions of GTE equivalent to 0.75% and 1.5% (w/v) were used according to Ibrahim et al. [Citation16]. It was adopted as follows. About 15 g of GT leaves was soaked in 1 L of distilled boiled water for 5 min with occasional swirling. The prepared solutions were filtered and distributed in glass flasks, each of which contained 300 ml of liquid. These bottles were placed in the animal cages (one bottle/cage) as the source of drinking fluid. During the experimental period (14 days), the tested liquids were freshly prepared and the actual consumed liquid was measured.
Animals
Adult female albino mice (20–25 g) were obtained from the Animal Breeding House, National Research Center, Dokki, Cairo, Egypt. Mice were housed for 1 week to acclimatize and kept in clean cages (five in each), with free access to water ad libitum. The mice were kept in appropriate environmental and food conditions during the experiment period.
All the experiments were conducted in agreement with the ‘Institutional Animal Ethics Committee at Mansoura University, Mansoura, Egypt’ regulation, which is in accordance with the ‘Handbook for the Care and Use of Laboratory Animals’ issued by the ‘National Academy of Sciences’ (Code number: SCi-Ch-P-2021-72).
Experimental design
Thirty adult female albino mice were assigned to one of the six groups, five mice each. Group one (G1) served as negative control and received water. Groups G2 and G3 were treated with GTE orally, at a dose of 0.75% (w/v) and 1.5% (w/v), respectively [Citation17]. Groups G4, G5, and G6 were injected intraperitoneally with a sublethal dose of ME (5 mg/kg body weight. i.p.) [Citation11]. The next day, G4 and G5 were treated orally with GTE at a dose of 0.75% (w/v) and 1.5% (w/v), respectively, for 14 days.
Sample collection
After 14 days of the experiment, all animals were sacrificed under light anesthesia with diethyl ether. Blood samples were collected in centrifuge tubes for serum separation. They were kept at 4 °C and centrifuged at 2500 rpm for 5 min. The sera obtained were used to investigate biochemical parameters, aspartate aminotransferase (AST), and lactate dehydrogenase (LDH) as a test of cardiac function.
Hearts were dissected and washed with physiological saline. Small pieces of the heart were preserved in the formalin at 10% concentration for histopathology studies. Heart homogenization was applied to quantify parameters, superoxide dismutase (SOD), glutathione S-transferase (GST), reduced glutathione (GSH), and malondialdehyde (MDA) as markers for lipid peroxidation, and the NF-κB assay.
Preparation of cardiac homogenates
Fresh tissue specimens were used to prepare the homogenate. Tissue samples were washed with isotonin Tris EDTA solution, 3.029 g of 0.1 M Tris (hydroxy methylamine methane, 1.022 g of 0.07 M sodium chloride (ADWIC) and 0.47 g of 0.005 M EDTA. Then, dissolved in 250 ml of purified water. The pH was adjusted at 7.5 with 1 N HCl and then the cellular suspension was centrifuged at 1800 rpm for 10 minutes. If the supernatants were contaminated with blood, they were subjected to hemolysis with filtered tap water for 10 minutes. After centrifugation and suction of the supernatant, the supernatant was removed. Fix the cell in ice-cold 96–100% ethanol (BDH) at approximately 1 ml per sample, and store it indefinitely in the refrigerator.
Biochemical analyzes
Aspartate Aminotransferase (U/L) and Lactate Dehydrogenase (U/L) activities were measured in sera at 505 nm according to Reitman and Frankel [Citation18]. LPO (ng/mg) was determined in terms of MDA, an LPO marker at 534 nm according to Satoh [Citation19]. SOD activity (U/mg) was measured at 560 nm according to the method by Nishikimi et al. [Citation20]. GST activity (U/mg) was measured according to Habig et al. [Citation21]. The GSH level (ng/mg) was estimated using a colorimetric technique as reported by Jollow et al. [Citation22]. Nuclear factor NF-kappa-B (NF-κB) concentration (ng/mg) was measured in tissue homogenate at 450 nm using ELISA Kit (Cat No. MBS260718) according to the method of Gilmore [Citation23]. The concentration of protein was determined according to Lawry et al. [Citation24].
Histological studies
Heart samples were taken from female Swiss albino mice of different groups and fixed in formalin at 10% concentration for 24 h. Samples were washed in tap water and then dried in progressing degrees of ethyl alcohol. Samples were wiped in xylene and incorporated into paraffin bees at 56 °C in a hot air oven for 24 h. Paraffin blocks were prepared for slicing with a thickness of 4 μm by a slide microtome. The obtained histological sections were collected on glass slides and stained with hematoxylin for 20 min and eosin (1%) for 10 min. Slides were examined under a light microscope according to Banchroft and Stevens [Citation25]. The histopathology was performed in the Pathology Department, Faculty of Veterinary Medicine, Mansoura, Egypt.
Statistical analysis
The data obtained were analyzed with the Statistical Package for Social Sciences, version 23 (SPSS Software, SPSS Inc., Chicago, USA) and expressed as a mean ± standard error (SE). The normality test was analyzed using the Kolmogorov–Smirnov test. For data with a Gaussian distribution with homogeneity of differences, statistical analysis was performed using analysis of variance (One Way ANOVA) followed by a Tukey’s multi-test. For data with a Gaussian distribution with heterogeneity of differences, the statistical analysis was performed using an analysis of variance (One Way ANOVA) followed by Tamhane's T2 test. For parameters with a non-Gaussian distribution, the Kruskal–Wallis test followed by the Mann–Whitney U-test was used for multiple comparisons. The differences were considered significant at p < 0.05.
Results
shows that mice exposed to ME had a significant increase (P < 0.05) in AST activity in the blood compared to the control group. However, when a 0.75% or 1.5% concentration of GTE was administered to the ME-treated mice, a decrease in AST activity was observed when compared to the ME group. This decrease was significant (P < 0.05) at 1.5% GTE while this decrease showed no significant change (P > 0.05) at 0.75% GTE. Moreover, when comparing the ME group with the normal treated group (G2, G3), AST activity in serum showed no significant changes (P > 0.05). Also, the administration of 0.75% or 1.5% GTE showed no significant changes in the AST activity compared to the control group (P > 0.05).
Figure 1. Serum AST activity [U/L] of control and different treated mice groups. Each value represents the mean ± SE (n = 5), values superscripts with different letters (a-c) were significantly different at P ≤ 0.05
![Figure 1. Serum AST activity [U/L] of control and different treated mice groups. Each value represents the mean ± SE (n = 5), values superscripts with different letters (a-c) were significantly different at P ≤ 0.05](/cms/asset/8c66900d-b967-4f59-bb35-25096e15a186/teba_a_1914401_f0001_oc.jpg)
The administration of ME caused a significant increase (P < 0.05) in LDH activity compared to the control group. The administration of a concentration of 0.75% or 1.5% of GTE in the ME group caused a significant decrease (P < 0.05) in LDH activity compared to the ME-treated group. When comparing the G5 and G6 groups with the G2 and G3 groups, respectively, the LDH activity showed a significant increase (P < 0.05) in any of the GTE concentrations. Also, the administration of 0.75% or 1.5% GTE showed no significant changes in the LDH activity compared to the control group (P > 0.05) ().
Figure 2. Serum LDH activity [U/L] of control and different treated mice groups. Each value represents the mean ± SE (n = 5), values superscripts with different letters (a-d) were significantly different at P ≤ 0.05
![Figure 2. Serum LDH activity [U/L] of control and different treated mice groups. Each value represents the mean ± SE (n = 5), values superscripts with different letters (a-d) were significantly different at P ≤ 0.05](/cms/asset/7e53664b-d027-411c-9606-e7528945f4c1/teba_a_1914401_f0002_oc.jpg)
As shown in , the administration of ME caused a significant increase (P < 0.05) in MDA concentration and a significant decrease (P < 0.05) in SOD and GST activities as well as a significant decrease (P < 0.05) in GSH concentration in cardiac tissue compared to a control group. The administration of either a concentration of 0.75% or 1.5% of GTE to the ME treatment groups resulted in a significant decrease (P < 0.05) in the MDA concentration and a significant increase (P < 0.05) in SOD and GST activities with a significant decrease (P < 0.05) in GSH concentration compared with the ME group. By comparing the MDA concentration of the fifth group with that of the second group, a significant increase (P < 0.05) was found with a decrease in SOD and GST activities as well as the GSH concentration but it was considered not statistically significant (P > 0.05). By comparing the MDA concentration of the sixth group with that of the third group, a significant decrease (P < 0.05) was found with an increase in SOD and GSH activities, while there was a non-significant increase (P > 0.05) in terms of GSH. When comparing the G2 group with the G1 group, a significant increase (P < 0.05) in MDA concentration and SOD activity was observed, while a significant decrease (P < 0.05) in GST activity was observed. Also, there is no significant change (P > 0.05) in the GSH concentration. By comparing the concentrations of MDA, SOD, GSH, and GST of the third group with the first group, significant increases were found (P < 0.05), respectively
Table 1. Mean ± SE of MDA, SOD, GSH, and GST in cardiac tissue homogenates of control and different treated mice groups
The results of show that the mice exposed to ME had a significantly (P < 0.05) higher NF-κB concentration compared to the control group, whereas the administration of either 0.75% or 1.5% of GTE to the ME-treated mice resulted in a significant (P < 0.05) decrease in NF-κB concentration compared to the ME-treated groups. When comparing groups G5 and G6 with groups G2 and G3, respectively, the NF-κB concentration showed no significant changes (P > 0.05) at 1.5% GTE, while the NF-κB concentration at 0.75% GTE showed a significant increase (P < 0.05). Also, neither concentration showed any significant changes in NF-κB concentration compared to the control group (P > 0.05).
Figure 3. NF-κB in cardiac tissue homogenate [ng/mg protein] of control and different treated mice groups. Each value represents the mean ± SE (n = 5), values superscripts with different letters (a-d) were significantly different at P ≤ 0.05
![Figure 3. NF-κB in cardiac tissue homogenate [ng/mg protein] of control and different treated mice groups. Each value represents the mean ± SE (n = 5), values superscripts with different letters (a-d) were significantly different at P ≤ 0.05](/cms/asset/659a8cf8-866e-4aa5-954c-1bc2cc255702/teba_a_1914401_f0003_oc.jpg)
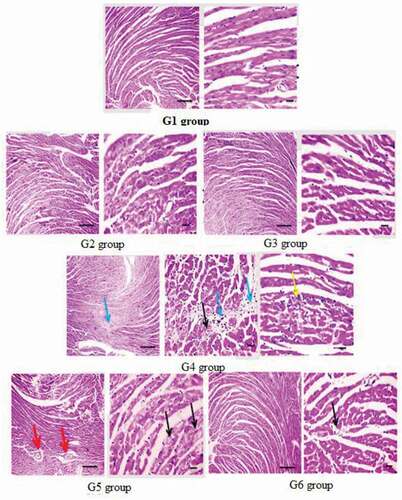
Microscopic examination of the histopathological study of cardiac sections of the G1, G2, and G3 groups showed that the myocardial cells are normal as shown in . The heart of ME-treated mice (G4) showed significantly degraded cardiomyocytes, interstitial edema, and few leukocytic cell infiltrations, whereas the heart of the mice treated with 0.75% GTE (G5) showed mild congestion with perivascular edema and slightly degraded cardiomyocytes. Moreover, the mice heart treated with 1.5% GTE (G6) showed mild vacuoles in cardiomyocytes.
Figure 4. Microscopic photomicrographs of histological sections of cardiac tissue of mice in different groups. G1, G2, and G3 groups showing normal structured cardiomyocytes, G4 group; ME-treated group; showing markedly degenerated cardiomyocytes (black arrows), interstitial edema (blue arrow), few leukocytic cell infiltration (yellow arrow). G5; ME-treated group with 0.75% GTE; showing mild congestion with perivascular edema (red arrows), mildly degenerated cardiomyocytes (black arrows), G6; ME-treated group with 1.5% GTE; showing mild vacuolations in cardiomyocytes (black arrows). X: 400 bar 50
Discussion
This study aimed to evaluate the therapeutic effect of two concentrations of GT leaf extracts on the cardiac toxicity of ME in female albino mice.
Treating the mice with ME caused cardiac tissue damage as indicated by a significant rise in AST and LDH activities. These results are in agreement with Chabane et al. [Citation26]. These ME-induced elevations may be due to increased ROS production, leading to oxidative stress in tissues and thus chronic permanent damage [Citation27]. Administration of any of the GTE concentrations (0.75% and 1.5%) to ME-treated group resulted in a significant decrease in AST and LDH activities in the ME-treated group compared to the control group. This GTE-induced reduction is consistent with Diao et al. [Citation28]. It may be the result of reduced production of reactive oxygen species as catechins exhibit the strong property of scavenging reactive oxygen species [Citation29].
Administration of ME increases ROS production and oxidative stress, leading to lipid peroxidation and depletion of antioxidant capacity [Citation30,Citation31]. The results of the present study showed an increase in MDA concentration and decreased activity of the level of SOD and GSH. The increase in MDA level in the ME-treated mice may be due to conjugation of the insecticide or its metabolites with the polyunsaturated fatty acid, which may lead to elevated ROS production and free radical buildup. This accumulation leads to disruption of the antioxidant state, thus enhancing lipid peroxidation [Citation10,Citation14,Citation32]. Administration of any of the GTE concentrations to ME-treated mice resulted in a significant decrease in MDA and thus a significant elevation of SOD and GSH state activity. These results are consistent with previous studies [Citation9,Citation10]. This may be due to the presence of polyphenol components that have scavenging properties [Citation33].
GST is a cytoplasmic enzyme that belongs to a family of phase II detoxifying enzymes that work to protect biological systems from damage caused by oxidative stress [Citation34]. The result of the present study show a significant decrease in GST activity in the ME group compared to the control group, and this may be due to reduced GSH, glutathione-dependent enzyme systems, and antioxidant enzymes that protect against oxidative stress [Citation35]. This may be due to the increase in the level of GSH which provides significant protection against ME [Citation11]. Different concentrations of GTE extract were able to treat ME-induced cardiac toxicity.
The transcription factor NF-κB regulates multiple aspects of innate and adaptive immune functions and acts as an axial mediator of inflammatory responses [Citation36]. The present work showed that treatment with ME increased the concentration of NF-κB. This finding is consistent with previous studies [Citation11,Citation37] and this finding may be due to stimulation of the apoptotic pathway through NF-κB activation due to the overproduction of reactive oxygen species [Citation38] induced by ME. Administration of 0.75% or 1.5% of GTE concentration to the ME-treated mice resulted in a significant decrease in NF-κB concentration in the treated group compared to the control group. The reduction of NF-κB induced by GTE is consistent with Nusair et al. [Citation12], and maybe the result of inactivation of NF-κB by catechins [Citation39].
The observed histopathological changes were confirmed in the ME-treated groups with biochemical modifications and were in agreement with those previously reported for the same insecticide attributed to ROS generation [Citation40]. The administration of the tested GTE, and in particular, the 1.5% concentration, improved the cell structure of the investigated tissues to a significant extent. This may be due to GT’s antioxidant capacity [Citation9,Citation41,Citation42].
Conclusion
The ME insecticide caused cardiac dysfunction, oxidative stress, and pathophysiological changes in the cardiac tissues of experimental animals. The observed changes occurred and were referred to as ME-induced oxidative stress. Administration of GTE to ME-treated mice brought the tested biochemical parameters to their control levels. The ability of the tested GTE to scavenge the ROS, which might be generated by exposure to ME, was indicated. Therefore, GTE administration could be effective against ME-induced cardiac toxicity induced by oxidative stress. A higher concentration of GTE (1.5%) appears to have a greater therapeutic effect than a lower concentration of GTE (0.75%) in ME cardiac toxicity.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Khan N, Mukhtar H (2013). ”Tea and health: studies in humans“. Current Pharmaceutical Design 19(34):61417.
- Chunjian Z, Chunying L, Shuaihua L, et al. The galloyl catechins contributing to main antioxidant capacity of tea made from Camellia sinensis in China. ScientificWorldJournal. 2014;2014:863984. 2014 Aug 28.
- Chen L, Mo H, Zhao L, et al. Therapeutic properties of green tea against environmental insults. J Nutr Biochem. 2017;40:1–13.
- West JD, Marnett LJ (2006). Endogenous reactive intermediates as modulators of cell signaling and cell death. Chem Res Toxico 19:173–194.
- Kidd H, James DR. The agrochemicals handbook. 3rd ed. Cambridge, UK: Royal Society of Chemistry Information Services;; 1991. p. 3–11.
- Meng SL, Liu T, Chen X, et al. Effect of chronic exposure to methomyl on tissue damage and apoptosis in testis of tilapia (Oreochromis niloticus) and recovery pattern. Bull Environ Contam Toxicol. 2019;102(3):371–376.
- Kaur M, Sandhir R. Comparative effects of acute and chronic carbofuran exposure on oxidative stress and drug-metabolizing enzymes in liver. Drug Chem Toxicol. 2006;29(4):415–421.
- WHO. The WHO recommended classification of pesticides by hazard and guidelines to classification. Geneva, Switzerland: NLM Classification; 2005.
- Mansour SA, Abbassy M, Shaldam H, et al. Zinc ameliorate oxidative stress and hormonal disturbance induced by methomyl, abamectin, and their mixture in male rats. Toxics. 2017;5(4):37. PMID: 29207507
- Heikal TM, et al. Protective effects of vitamin C against methomyl-induced injures on the testicular antioxidant status and apoptosis-related gene expression in rat. J Environ Anal Toxicol. 2014;5(2):2.
- Manar RA. Evaluation the effect of the aqueous extract of green tea on renal toxicity induced by methomyl in experimental animals model. J. Agric Chem Biotechnol. 2020;11(6):197–203.
- Nusair SD, Joukhan AN, Rashaid AB, et al. Methomyl induced effect on fortilin and S100A1 in serum and cardiac tissue: potential biomarkers of toxicity. Hum Exp Toxicol. 2019Mar;383:371–377. 2018 Nov 25 PMID: 30472887
- Do Nascimento Marinho KS, Lapa NCJC, Idd DSC, et al. Genotoxic and mutagenic evaluation of the protective effect of exogenous melatonin in adult rats and their offspring exposed to the insecticides methomyl and cypermethrin during pregnancy. Mutat Res. 2019 Dec;848:503107. Epub 2019 Oct 19.
- Sakr S, Hassanien H, Bester MJ, et al. Beneficial effects of folic acid on the kidneys and testes of adult albino rats after exposure to methomyl. Toxicol Res (Camb). 2018;7(3):480–491. . PMID: 30090598
- Reygaert WC. Green tea catechins: their use in treating and preventing infectious diseases. Biomed Res Int. 2018 Jul 17;2018:9105261. . eCollection 2018.PMID: 30105263.
- Ibrahim MA, Khalaf AA, Galal MK, et al. Ameliorative influence of green tea extract on copper nanoparticle-induced hepatotoxicity in rats. Nanoscale Res Lett. 2015;10(1):363.
- Mansour SA, Ali AR, Mohamd RI. Ameliorating effect of green tea, sage, and their mixture against methomyl-induced physiological, biochemical, and histopathological alterations in male rats. Egypt Pharmaceut J. 2018;17:223–236.
- Reitman A, Frankel S. A colorimetric method for the determination of serum glutamic oxalacetic and glutamic pyruvic transaminases. J Clin Path. 1957;28(1):56.
- Satoh K. Serum lipid peroxide in cerebrovascular disorder determined by a new colorimetric method. Clin Chim Acta. 1978;90(1):37–43.
- Nishikimi M, Roa NA, Yogi K. The occurrence of superoxide anion in the reaction of reduced phenazinemethosulphate and molecular oxygen. Biochem Biophysiol Res Commun. 1972;46(2):849–853.
- Habig WH, Pabst MJ, Jakoby WB. Glutathione-S-transferase the first step in mercapturic acid formation. J Biol Chem. 1974;249(22):7130–7139.
- Jollow DJ, Mitchell JR, Zamppaglione Z, et al. Bromobenzene induced liver necrosis. Protective role of glutathione and evidence for 3,4-bromobenzene oxide as the hepatotoxic metabolites. Pharmacology. 1974;11(3):51–157.
- Gilmore TD. The Rel/NF-κB signal transduction pathway: introduction. Oncogene18. 1999;18(49):6842–6844.
- Lowry, O. H.Rosebrough, N. J.Farr, A. L. and Randall, R. J. (1951). Protein measurement with the Folin phenol reagent. J. Biol. Chem. 193:265–275.
- Banchroft JD, Steven A, Turner DR. Theory and practice of histological techniques. 6th ed. Edinburgh: Churchill Livingstone; 2008. p. 725.
- Chabane K, Khene MA, Zaida F, et al., Subacute and subchronic methomyl exposure induced toxic effects on intestines via oxidative stress in male albino rats: biochemical and histopathological study. Drug Chem Toxicol. 2020 Feb 17:1–14. Online ahead of print.PMID: 32063051. https://doi.org/10.1080/01480545.2020.1727496.
- Mansour SA, Abbassy MA, Shaldam HA. Hepato-renal toxicity induced by chlorpyrifos, diazinon and their mixture to male rats with special concern to the effect of zinc supplementation. J Toxicol Pharmacol. 2017C;1:015.
- Diao JX, Ou J-Y, Dai H, et al. Antioxidant and antiapoptotic polyphenols from green tea extract ameliorate CCl4-induced acute liver injury in mice. Chin J Integr Med. 2020;26(10):736–744. PMID: 31768871
- Lee SB, Choi E-H, Jeong K-H, et al. Effect of catechins and high-temperature-processed green tea extract on scavenging reactive oxygen species and preventing Aβ 1–42 fibrils’ formation in brain microvascular endothelium. Nutr Neurosci. 2020;23(5):363–373. . PMID: 3011127
- Djeffal A, Messarah M, Boumendjel A, et al. Protective effects of vitamin C and selenium supplementation on methomyl-induced tissue oxidative stress in adult rats. Toxicol Ind Health. 2015;31(1):31–43.
- Trachantong W, Saenphet S, Saenphet K, et al. Lethal and sublethal effects of a methomyl-based insecticide in Hoplobatrachus rugulosus. J Toxicol Pathol. 2017;30(1):15–24.
- Gutteridge JM, Halliwell B. Free radicals and antioxidants in the year 2000: a historical look to the future. Ann NY Acad Sci. 2000;899(1):136–147.
- Zokti JA, Sham Baharin B, Mohammed AS, et al. Green tea leaves extract: microencapsulation, physicochemical and storage stability study. Molecules. 2016;21(8):940.
- Bhattacharjee R, Sil PC. The protein fraction of Phyllanthus niruri plays a protective role against acetaminophen induced hepatic disorder via its antioxidant properties. Phytother Res. 2006;20(7):595–601.
- Ashour MB, Ahmed OM, Asran AA, et al. Assessment of the preventive effects of Salvia officinalis and Ruta graveolens ethanolic leaf extracts on chlorpyrifos- and methomyl-induced renal toxicity and oxidative stress in albino rats. International Journal of Prevention and Treatment. 2017;6(2):34.
- Liu T, Zhang L, Joo D, et al. NF-κB signaling in inflammation. Signal Transduct Target Ther. 2017;2(1):e17023.
- Liang Y, Msm I, Jcw M, (-)-Epigallocatechin-3-gallate suppresses cigarette smoke-induced inflammation in human cardiomyocytes via ROS-mediated MAPK and NF-κB pathways. Phytomedicine. 2019;58(May):152768. [2018 Nov 20]. PMID: 31005721.
- Perkins ND, Gilmore TD. Good cop, bad cop: the different faces of NF-kappaB. Cell Death Differ. 2006;13(5):759–772. . [PubMed] [Google Scholar]
- Lorenzo CD, Dell’Agli M, Sangiovanni E, et al. Correlation between catechin content and NF-κB inhibition by infusions of green and black tea. Plant Foods Hum Nutr. 2013 Jun;68(2):149–154.
- Mansour SA, Ali AR, Mohamed RI. Toxicity of different doses of methomyl on male rats and the protective effect of zinc especially at high lethal doses. Curr Top Toxicol. 2017a;13:81–93.
- Haque AM, Hashimoto M, Katakura M, et al. Green tea catechins prevent cognitive deficits caused by Abeta1-40 in rats. J Nutr Biochem. 2008;19(9):619–626.
- Coimbra S, Castro E, Rocha-Pereira P, et al. The effect of green tea in oxidative stress. Clin Nutr. 2006;25(5):790–796.
