 ?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
The use of glucocorticoids causes elevation of blood pressure (BP) in man and experimental rats. We evaluated the antihypertensive potential of methanol leaf extract ofStruchium sparganophora (SPA) on dexamethasone-salt-induced hypertension in rats. Rats were assigned randomly to six groups (n=4/group). Group A represented control, while BP elevation (BP≥140/90) was induced in groups B-F via single subcutaneous administration of dexamethasone at a dose of 2 mg/kg with 4% NaCl substituted as drinking water for 5 days. Groups B was left untreated and treatments of other groups twice daily (C-F) for 10 days were as follows: C: nifedipine 3 mg/kg D: nifedipine 3 mg/kg + SPA l300 mg/kg, E: SPA 300 mg/kg and F: SPA 600 mg/kg. BP was read using a noninvasive method, lipid profile and nitric oxide (NO) concentration were determined with appropriate procedures. SPA at 300 and 600 mg/kg markedly decreased the systolic and diastolic BP below suboptimal value of 140/90. Treatment with SPA caused increase (P<0.05) in the levels of HDL-cholesterol and NO as well as concomitant decrease in triglyceride concentration. This study revealed that SPA might lower blood pressure by increasing NO concentration and maintaining lipid homeostasis in hypertensive rats.
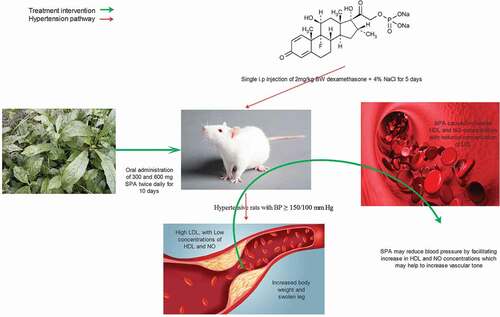
Graphical abstract
Introduction
Hypertension causes persistent disturbance in hemodynamic function characterized by abnormal elevation of systemic blood pressure (BP) [Citation1]. Hypertension, if not detected early or managed appropriately may lead to myocardial infarction, stroke, renal impairment and death [Citation2]. The progression of hypertension is multifaceted, and the pathogenesis of above 90% of the cases of this disease (essential hypertension) is unknown [Citation3]. Management of hypertension is a crucial challenge to the healthcare system. Its complications can be prevented by adequate BP control through lifestyle modification or by the use of antihypertensive drugs or both [Citation4]. Despite the availability of several antihypertensive medicines and increasing marketing of newly formulated drugs, large numbers of studies revealed that substantial or adequate BP control is yet to be achieved in a higher population of hypertensive patients. Besides, the treatment outcome of most antihypertensive drugs produces adverse severe effects either when used individually or concomitantly with other medications even at prescribed doses [Citation5].
The use of alternative medicine has been an effective way to decrease the population of people with high BP globally. Varieties of alternative therapies include diet, exercise, stress, supplements, and herbs. Knowledge about medicinal plants, their composition, and mechanism of action play a vital role in the discovery of new products and therapeutic agents. Various plant species have been explored and said to possess antihypertensive potentials [Citation6]. Some herbal drugs that have been used for the management of hypertension include garlic, ginger, ronwolfia, arjuna, barberry, to mention a few [Citation7]. Struchium sparganophora (Linn.) Ktze belongs to the asteraceae Family. The leaf is commonly referred to as water bitter leaf and locally called ‘ewuro odo’ amongst the Yorubas of South-Western Nigeria. Struchium sparganophora is a culinary herb in several African countries. It is a branched and fibrous water plant that is consumed as a leafy vegetable in soups among the occupants of the rural areas of Nigeria. The nutritive, phytochemical, antioxidant, antimicrobial and antimalarial properties of the leaves have been reported [Citation8–10].
Struchium sparganophora is used medicinally in many countries, the decoction of the leaves of the plant is used in the treatment of diabetes mellitus, headaches, gonorrhea [Citation11]. It is also used in treating malaria and measles in the Sao Tome and Principe Island of the Gulf of Guinea [Citation12–14]. Ethnomedicinal surveys around southern Nigeria revealed different extracts and decoction of water bitter leaf is used in the management of sexually transmitted infections, gastrointestinal disorders, diabetes and hypertension. We lack information on the possible pharmacological mechanisms of action on the use of this plant in the management of hypertension to justify the underlying principle behind folklore applications. Our study, therefore, focused on BP lowering, antidyslipidemia and nitric oxide modulatory efficacy of methanol extract of the leaves of Struchium sparganophora in Dexamethasone-salt-induced hypertensive rats.
Materials and methods
Chemicals and drugs
Reagents used for this study were of analytical grade. Biochemical assay kits were products of RANDOX®. Nifedipine was purchased from Unicure Pharmaceuticals, Nigeria, dexamethasone from Hubel Tianyao Pharmaceuticals, China.
Plant sample preparation
S. sparganophora was collected from Badeku farm in Ibadan, Oyo State, Nigeria, and it was authenticated and deposited in herbarium with voucher UI 22,655. The leaves were detached from the stems, dried, and milled to a powder. Three hundred gram of the powdered leaf was weighed and extracted with absolute methanol (1:8, w/v). The solvent was evaporated at 40°C on a rotary evaporator to obtain the crude methanol extract.
Assay for active drug content
Nifedipine and Dexamethasone actual contents were assayed using standard procedure before use British pharmacopoeia, 2009 [Citation15,Citation16].
Experimental design
Twenty-four adult male rats of Wistar strain with weights between 160 and 165 g were purchased from the Department of Anatomy, University of Ibadan. These rats were brought to Lead City University and were kept in clean and well-ventilated plastic cages maintained in 12 hours light and 12 hours dark cycle. The rats were acclimatized for two weeks before the commencement of the study. Management of the rats was done following the protocol of the Animal Research Ethics committee of the Lead City Ethical Review Board (LCU/ERB) which is in line with the Canadian Council on Animal Care (CCAC) guidelines [Citation17]. The animals were fed pellets throughout the study and were given clean drinking water. The rats were divided into six groups (n = 4/group). Group (A) was the control group that was administered vehicle (distilled water, 0.3 mL), groups B, C, D, E and F were administered single dose, 2 mg/kg body weight (bw) dexamethasone, and had their drinking water substituted with 4% NaCl solution for five days to induce hypertension. After high BP (≥140/90) was noticed, the rats were treated orally twice daily for ten days as follows: B; untreated (DEX), C; nifedipine 3 mg/kg bw(DEX +NIF), D; nifedipine 3 mg/kg bw + SPA 300 mg/kg bw (DEX+NIF+SPA 300), E; SPA 300 mg/kg bw(DEX+SPA 300) and F; SPA 600 mg/kg bw (DEX+SPA 600).
Blood pressure determination
The systolic (SBP) and Diastolic (DBP) BP of the rats were monitored from the hind leg using a noninvasive BP monitoring device (CONTEC 0.8A) with a veterinary cuff 3–5 cm as described in previous study [Citation18]. Record of measurements taken before induction of hypertension (Baseline), prior treatment, and after completing treatment was used for this study.
Lipid profile determination
After the 10th day of treatment, the rats were fasted 8 hours overnight, and blood samples were collected from each rat into heparinized vials and centrifuged at 3000 rpm for 10 minutes to obtain plasma, which was used for biochemical assays. Total cholesterol was determined using the method of Allain [Citation19], HDL-C was determined with the method of Lopez-Virella et al. [Citation20], triglyceride was determined with the method of Fossati and Prencipe [Citation21] and LDL-C and vLDL were calculated from the obtained lipid parameters using the formula by Friedewald et al. [Citation22].
Determination of cholesterol index and atherogenic index
Cholesterol ratio (CR) was calculated with the formula:
Atherogenic index (AI) was calculated using the following formula:
Plasma nitric oxide determination
Nitric oxide was analyzed using the method described by Grisham et al. [Citation23]. Briefly, 200 µL of Griess reagent containing 0.05% N-(1-naphthyl)ethylenediamine dihydrochloride, 0.5% sulfanilic acid, and 2.5% phosphoric acid was reacted with the same volume of serum, and color developed was measured at 548 nm. Varying concentration of nitric oxide from sodium nitrite was used as a standard.
Ethics approval
Ethical permission (Reference Number: LCUERB205) was obtained from Lead City University Ethical Review Board (LCU/ERB) to perform this animal research.
Data analyses
Parameters were calculated as mean ± standard deviation of animals in each group. Two-factor analysis of variance was used to compare changes in BP among groups with time. One-way analysis of variance coupled with Tukey’s multiple comparisons test was used to study differences in biochemical parameters among groups. A p-value of less than 0.05 was considered significant.
Results
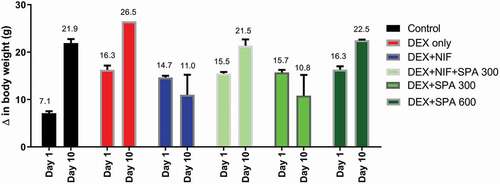
SPA extract administration at low dose (300 mg/kg) for 10 days, comparably to nifedipine, markedly reduced the bodyweight of dexamethasone-hypertensive rats. The treatment with low dose extract plus nifedipine or with 600 mg/kg extract caused an increase in body weight slightly lower than dexamethasone alone ().
Figure 1. Effect of Struchium sparganophora leaf methanol extract on changes in body weights of dexamethasone-induced hypertensive rats
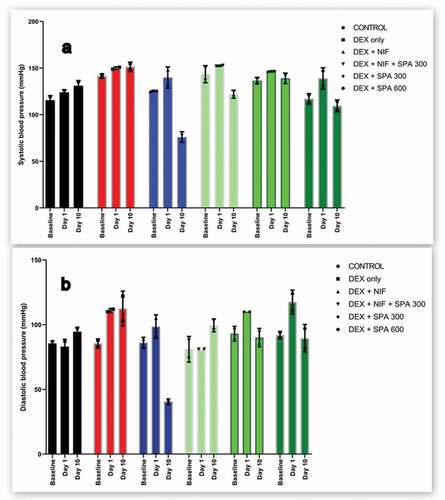
Administration of dexamethasone-salt caused an increase in systolic BP by an average of 9.50 mmHg which was maintained unchanged during the next 10 days. However, treatment with the extract at 300 mg/kg and 600 mg/kg decreased systolic BP by an average of 7.5 mmHg (0.75/day) and 29.5 (2.95/day) mmHg in hypertensive rats respectively, nifedipine markedly lowered systolic BP by an average of 64 mmHg (6.4/day) in dexamethasone-salt hypertensive rats (). Dexamethasone-salt caused a marked increase in diastolic BP. Treatment of hypertensive rats with 300 mg/kg and 600 mg/kg extracts decreased diastolic BP by an average of 20 mmHg (2.0/day) and 28 mmHg (2.8/day), respectively ().
Figure 2. Effect of methanol extract of Struchium sparganophora leaves on (a) systolic and (b) diastolic blood pressure in dexamethasone induced hypertensive rats
There was a significant increase in the level of total cholesterol in rats administered dexamethasone-salt alone. Rats treated with low dose extract and in combination with nifedipine also showed a similar increase in the level of total cholesterol, while total cholesterol in rats treated with high dose extract was more elevated compared to others (P < 0.05). Dexamethasone caused a significant decrease in the concentration of HDL-cholesterol. Low dose extract, its combination with nifedipine, and the higher dose extract were noticed to cause a marked increase in HDL-cholesterol concentration in hypertensive rats. LDL-cholesterol was observed to increase significantly (P < 0.05) in rats that were treated with SPA and its low dose combination with nifedipine. However, there was marked decline in the concentration of triglyceride and VLDL in rats treated with SPA when compared to control, nifedipine and hypertensive untreated rats ().
Table 1. Effect of Struchium sparganophora leaf methanol extract on lipid profile in dexamethasone-induced hypertensive rats
Dexamethasone caused an increase in cholesterol ratio and the atherogenic index, these effects were partially reversed in all the treatment groups especially in hypertensive rats treated with the 300 mg/kg of the extract ().
Table 2. Effect of Struchium sparganophora leaf methanol extract on cholesterol ratio and atherogenic index in dexamethasone-induced hypertensive rats
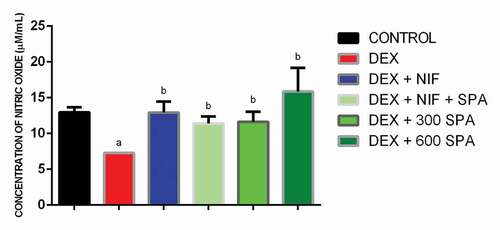
Dexamethasone significantly decreased plasma nitric oxide levels. Treatment with nifedipine, combination of low dose extract and nifedipine, 300 mg/kg extract, and 600 mg/kg extract effectively reversed the effect by increasing plasma nitric oxide level. The highest concentration of plasma nitric oxide was observed in rats administered high dose extract ().
Figure 3. Effect of Struchium sparganophora leaf methanol extract on nitric oxide level in dexamethasone-induced hypertensive rats. a Significant when compared to control p < 0.05. b Significant when compared to dexamethasone p < 0.05. c Significant when compared to standard drug (Nifedipine) p < 0.05
Discussion
The use of dexamethasone had been reported to cause hypertension in man and animal models by several studies [Citation24]. In this study, the antihypertensive effect of methanol extract of Struchium sparganophora leaves on dexamethasone-salt induced hypertensive rats was evaluated. From our findings, the administration of dexamethasone-salt to rats increased both systolic and diastolic BP. This effect was ameliorated by methanol extract of Struchium sparganophora leaves. Bodyweight increase was noted in rats administered dexamethasone-salt only. Our outcome on the effect of dexamethasone on bodyweight disagrees with the study by Dubey et al. [Citation25] reporting a reduction of body weight upon administration of 10 µg/kg/day dexamethasone. The extract caused bodyweight reduction in hypertensive rats, an effect that may contribute a positive impact on BP lowering. The dietary approach to stop hypertension (DASH) program suggested an eating plan that is rich in vegetables for hypertensive patients [Citation2], consisting of low fats diets and low-energy foods that help to reduce body weight.
SPA extracts at 300 and 600 mg/kg exhibit a considerable ability to reduce both systolic and diastolic BP, an effect that may be attributed to some bioactive compounds reported to be present in the green leaf extract. Terpenoids (e.g. triterpenes, oleanolic), alkaloid, and some other classes of phytochemicals found in this plant extract have been reported to possess an antihypertensive effect [Citation26–28]. The financial burden and high incidence of adverse effects associated with formulated antihypertensive drugs have intensified the need for researchers to search for medicinal plants that will possess an antihypertensive effect as they are cheaper and may possess a multifaceted mechanism of action that makes them more efficient. For example, St John’s wort and garlic have been in use for centuries in the management of hypertension [Citation7].
Nifedipine belongs to the dihydropyridine class of calcium channel blockers (CCBs), antihypertensive therapies that reduce cardiovascular events associated with hypertension [Citation3]. From our results, rats administered nifedipine alone at 3 mg/kg were hypotensive, and the hypotensive effects were minimized when combined with 300 mg/kg SPA. The hypotensive effect of nifedipine observed in dexamethasone-salt hypertensive rats agrees with a study by Watcher [Citation29] who used nifedipine to induce symptomatic hypotension in hypertensive patients. Mitigation of nifedipine’s effects, when combined with 300 mg/kg SPA, may be a result of interaction between nifedipine and compounds present in the plant extract. CCBs, especially the dihydropyridines, have been noticed to inhibit the elimination of plant’s bioactive substances, especially the cardiac glycosides by blocking their transporters, the p-glycoprotein transporters [Citation30]. This effect may be one of the results of the interaction between nifedipine and the plant bioactive component, which could favor moderation of BP.
From this study, dexamethasone causes a notable decrease in serum HDL-cholesterol concentration, with no significant elevation in total cholesterol, triglycerides and LDL-cholesterol. The extract, at selected doses, and in combination with nifedipine attenuates this effect by increasing HDL-cholesterol and reducing triglycerides concentrations significantly. The BP-lowering mechanism of the extract may involve reducing storage fat in the form of triglycerides. SPA extract could favor the increased level of HDL-cholesterol, and this is in agreement with Chait [Citation31] who linked increased VLDL-cholesterol and triglycerides with a decreased level of HDL-cholesterol and increased level of LDL-cholesterol.
SPA reversed the effect of dexamethasone by increasing plasma nitric oxide. One of the mechanisms suggested to be involved in the progression of hypertension through the use of dexamethasone is the blockade of nitric oxide secretion pathway by directly interfering with nitric oxide synthase, and inhibiting L-arginine transport and tetrahydrobiopterin synthesis, especially the endothelial isozymes [Citation32,Citation33]. Increasing nitric oxide (a potent vasodilator) concentration helps to replenish vascular tone, and this may be one of the mechanisms employed by this plant in producing the antihypertensive effect.
In conclusion, the methanol extract of Struchium sparganophora leaves produced a considerable BP-lowering effect. The effect could be linked to the extract’s ability to increase plasma nitric oxide concentration, increased HDL-cholesterol concentration, and reduced triglycerides concentration, whose combined effects may help to reduce total peripheral resistance, an essential factor that is crucial in controlling high BP.
Acknowledgments
We acknowledge the Laboratory technologist of the Department of Biochemistry, Lead City University, Ibadan, Nigeria.
Disclosure statement
We have no competing interest to declare.
Additional information
Funding
References
- Van Der Feen DE, Bartelds B, de Boer RA, Berger RM. Assessment of reversibility in pulmonary arterial hypertension and congenital heart disease. Heart. 2019;105(4):276–82.
- James PA, Oparil S, Carter BL, et al. 2014 evidence-based guideline for the management of high blood pressure in adults: report from the panel members appointed to the Eighth Joint National Committee (JNC 8). Jama. 2014;311(5):507–520.
- Chobanian AV, Bakris GL, Black HR, et al. The seventh report of the joint national committee on prevention, detection, evaluation, and treatment of high blood pressure: the JNC 7 report. Jama. 2003;289(19):2560–2571.
- Beckett NS, Peters R, Fletcher AE, et al. Anderson C Treatment of hypertension in patients 80 years of age or older. N Engl J Med. 2008;358(18):1887–1898.
- Adeosun AM, Adedapo AD, Adedeji WA. Adverse drug reactions report among hospitalized patients with hypertension in a Nigerian tertiary healthcare centre: a retrospective study. Medicine (Baltimore). 2017;6(2):276–279.
- Verma T, Sinha M, Bansal N, et al. Plants Used as Antihypertensive. Nat Prod Bioprospect. 2021;11(2):155–184.
- James PB, Kamara H, Bah AJ, Steel A, Wardle J. Herbal medicine use among hypertensive patients attending public and private health facilities in Freetown Sierra Leone. Complementary therapies in clinical practice. 2018; 31:7–15.
- Akah P, Ekekwe R. Ethnopharmacology of some asteraceae family used in Nigerian traditional medicine. Fitoterapia. 1995;66(4):351–355.
- Adeosun AM, Asejeje FO, Ighodaro OM, et al. Hypoglycemic, antidyslipidemic, and antioxidant activities of methanol extract of Struchium sparganophora leaves in alloxan-induced oxidative stress-mediated diabetes in rats. Futur J Pharm Sci. 2020;6(59). DOI:https://doi.org/10.1186/s43094-020-00078-2
- de Madureira M, Martins AP, Gomes M, et al. Antimalarial activity of medicinal plants used in traditional medicine in S. Tomé and prıncipe islands. J Ethnopharmacol. 2002;81(1):23–29.
- Heywood,VH, Harborne, JB, Tuner, BL. Thebiology and chemistry of the compo- site. New York: Academic Press Inc. 1977;2: 851–941.
- Eko M, Eteng M, Eyong E. Phytochemical composition and effect of aqueous extract of Struchium sparganophora (L) on cockroach crude extract–induced airway inflammatory responses in wistar rats. Global Journal of Pure and Applied Sciences. 2008;14(4):417–422.
- Oboh G. Nutritive value, antioxidant and antimicrobial properties of Struchium sparganophora leaves. J Med Food. 2006;9(2):276–280.
- Oboh G, Raddatz H, Henle T. Antioxidant properties of polar and non‐polar extracts of some tropical green leafy vegetables. J Sci Food Agric. 2008;88(14):2486–2492.
- British Pharmacopoeia Nifedipine. Medicinal and Pharmaceutical substances, vol II. 2009
- British Pharmacopoeia Dexamethasone Sodium Phosphate Injection. Formulated Preparations: Specific Monographs, vol III. 2009
- Griffin G. Establishing a three Rs programme at the Canadian council on animal care. Alternatives to Laboratory Animals. 2009;37(2_suppl):63–67.
- Adeosun AM, Ighodaro OM, Akinloye OA New method for determining blood pressure in unanaesthesize rats using non invasive CONTEC 08A device with small cuff: a path to antihypertensive drug development in developing countries. In: 22nd International Conference on New Horizons in Cardiology and Cardiologists Education 2019: Journal of Heart and Cardiovascular Research, Berlin, 2019. Euroscicon, p 43. doi:https://doi.org/10.21767/2576-1455-C1-003
- Allain CC, Poon LS, Chan CSG, et al. Enzymatic determination of total serum cholesterol. Clin Chem. 1974;20(4):470–475.
- Lopez-Virella MF, Stone P, Ellis S, et al. Cholesterol determination in high-density lipoproteins separated by three different methods. Clin Chem. 1977;23(5):882–884.
- Fossati P, Prencipe L. Serum triglycerides determined colorimetrically with an enzyme that produces hydrogen peroxide. Clin Chem. 1982;28(10):2077–2080.
- Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem. 1972;18(6):499–502.
- Grisham MB, Johnson GG, Lancaster J. Quantitation of nitrate and nitrite in extracellular fluids. Methods Enzymol. 1996;268(A):237–246.
- Wallwork CJ, Parks DA, Schmid-Schönbein GW. Xanthine oxidase activity in the dexamethasone-induced hypertensive rat. Microvasc Res. 2003;66(1):30–37.
- Dubey H, Singh A, Patole AM, et al. Antihypertensive effect of allicin in dexamethasone-induced hypertensive rats. Integr Med Res. 2017;6(1):60–65.
- Sporn MB, Liby KT, Yore MM, et al. New synthetic triterpenoids: potent agents for prevention and treatment of tissue injury caused by inflammatory and oxidative stress. J Nat Prod. 2011;74(3):537–545.
- Agrawal M, Nandini D, Sharma V, et al. Herbal remedies for treatment of hypertension. Int J Pharm Sci and Res. 2010;1(5):1–21.
- Hargreaves R, Johnson R, Millington D, et al. Alkaloids of American species of Erythrina. Lloydia. 1974;37(4):569.
- Wachter RM. Symptomatic hypotension induced by nifedipine in the acute treatment of severe hypertension. Arch Internal Med. 1987;147(3):556–558.
- Zhou S, Lim LY, Chowbay B. Herbal modulation of P‐glycoprotein. Drug Metab Rev. 2004;36(1):57–104.
- Chait A. Diabetes, lipids and atherosclerosis. Diabetes Mellitus A Fundamental and Clinical Text. 1996; 772–780..
- Behrendt D, Ganz P. Endothelial function: from vascular biology to clinical applications. Am J Cardiol. 2002;90(10):L40–L48.
- Lou Y-K, Wen C, Li M, et al. Decreased renal expression of nitric oxide synthase isoforms in adrenocorticotropin-induced and corticosterone-induced hypertension. Hypertension. 2001;37(4):1164–1170.
