ABSTRACT
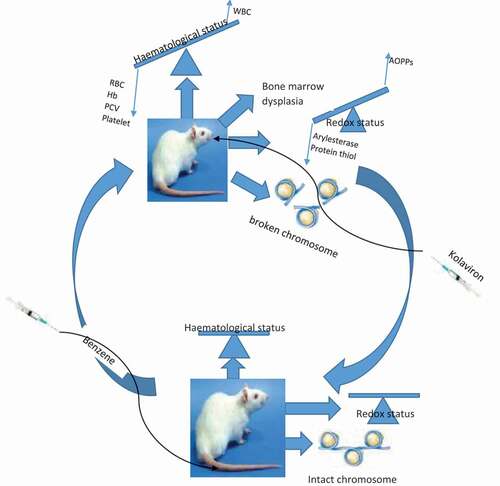
Kolaviron (KV) is a biflavonoid phytoconstituent of defatted Garcinia kola seeds that possessed antiproliferative and pharmacological activities. Benzene is an industrial solvent that, however, constitutes occupation hazard, leading to hematological disturbance and leukemia. Therefore, the potency of kolaviron against benzene-induced hematological and myeloid toxicity leading to leukemia was investigated in a rat model. Preleukemic conditions were induced in Wistar rats by intravenous administration of benzene solution. Following induction, 200 mg/kg kolaviron was administered orally for seven days. Hematological parameters, percentage blast cell occurrence and blood cell morphology were compared between baseline control and leukemic rats with or without kolaviron treatment. Plasma activity of arylesterase of paraoxonase-1, total thiol and advanced oxidation protein products (AOPPs) along with clastogenicity and bone marrow architecture was assessed. Kolaviron restored altered hematology, reduced the occurrence of blasts and improved blood cell morphology. Kolaviron also decreased levels of AOPPs, increased total thiol, improved arylesterase activity and mitigated clastogenicity and dysplasia induced in leukemic rats. In conclusion, kolaviron protected against benzene-induced hematological and myeloid toxicities that are implicated in leukemia.
Graphical Abstract
Introduction
The global impact of hematopoietic malignancy and cancer at large on the health of people and economy of nation is becoming more worrisome [Citation1]. Hematopoietic malignancies are diverse groups of disorders that include plasma cell tumors, lymphomas, myelodysplastic syndromes or myelodysplasia (MDS), mastocytosis and leukemias. The etiology of hematopoietic malignancies such as myelodysplastic syndromes and leukemia includes the exposure to environmental factors such as benzene, radiation and some chemotherapies [Citation2]. Myelodysplasia is a hematopoietic disease that has its origin in the hematopoietic stem and progenitor cell compartment with variable degrees of cytopenias, morphological dysplasia and risk of progression to acute myeloid leukemia [Citation3]. The symptoms of myelodysplasia include anemia, cytopenias, morphological dysplasia of precursor and mature bone marrow blood cells [Citation4,Citation5]. Myelodysplastic syndromes also called myelodysplasia are usually referred to as the premalignant condition as incurement of additional genetic abnormalities may lead to the transformation of MDS into acute myeloid leukemia (AML). Leukemia is majorly a cancer of the white blood cells and bone marrow and present in most common form of cancer in children worldwide where it remains the second leading cause of death in children [Citation6,Citation7]. Currently among males, it is the 10th most commonly diagnosed cancer and 8th leading cause of male cancer mortality in 2020 [Citation1].
Most cancer chemotherapy regimens and even the remission-induction therapy for the treatment of leukemia are accompanied by severe side effects besides their chemotherapeutic efficacy [Citation8,Citation9]. There is a need for modified treatment and intensive assessment of cytotoxic agents in the field of oncology [Citation10] because many cytotoxic agents conferred severe side effects during the course of treatment [Citation11,Citation12]. Most commonly, some cancer chemotherapeutic agents are radiomimetic in nature especially alkylating agents affecting hematology, bone marrow cellularity and effective dysplasia formation in myeloid tissue, which may ultimately result in therapy-related myelodysplasia or acute myelogenous leukemia [Citation13]. Therefore, leukemia burden has led to increased research in isolation and identification of more cytotoxic agents [Citation14,Citation15]. Considerably, herbal medicine that presents natural compounds of sufficient chemotherapeutic effect with little or no side effects may be investigated for cancer chemotherapy. One such natural compound is kolaviron, which is a biflavonoid isolate of the seed of Garcinia kola extract. It is a defatted fraction of Garcinia kola seed with valuable major constituents such as Garcinia biflavonoids GB1 and GB2 and kolaflavone [Citation16,Citation17]. It has organ protective capability [Citation18], improved hematological indices and offered immunity boosting effects [Citation19]. The safety profile of kolaviron, its antioxidant properties and antiproliferative capacity have been extensively studied in vitro and in vivo [Citation20–21]. Moreover, it is known to offer protection against xenobiotic and chemical-induced oxidative stress-mediated toxicities in experimental murine models [Citation22,Citation23]. Therefore, the present work investigated the myeloprotective effect of kolaviron on benzene-induced bone marrow dysplasia in Wistar rats.
Materials and methods
Extraction and isolation of kolaviron from Garcinia kola
Kolaviron was extracted and isolated from the seeds of Garcinia kola following the procedure of Iwu et al. with slight modification [Citation16]. The seeds of Garcinia kola were peeled then sliced, air-dried and ground into powder. The powdered seeds were extracted with n-hexane to obtain a defatted marc, which was dried and subsequently extracted with methanol. The KV fraction, which gives a golden yellow solid, was obtained from methanol extract by the twin purification process of dilution using chloroform.
Experimental animals
Twenty four adult male Wistar strain rats of weight range 90–100 g were used for this study. The animals were obtained from the Department of Physiology University of Ibadan and acclimatized for 14 days in the animal house of the Department of Chemical Sciences, Ajayi Crowther University, Oyo. They were housed in plastic cages and fed with standard rat feed and clean tap water ad libitum. The designed work was conducted with the approval of the Faculty of Natural Sciences Ethical review of Ajayi Crowther University, Oyo with approval code: Fns/Erc/2,019,003 and the protocol conformed to the guidelines of the National Research Council for laboratory animal care and use [Citation24].
Animal treatments and groupings
After 14 days of acclimatization, preleukemic conditions were induced in 90–100 g rats following the procedure of Akanni et al. [Citation25] by intravenous injection of 0.2 ml of benzene solution (1:5:5 of benzene/2-propanol/distilled water v/v) every 2 days for 4 consecutive weeks. Following induction, 24 rats, which comprise 12 leukemic rats with traces of appearance of cell blasts in peripheral blood film () and 12 normal baseline control, were assigned into four experimental groups of 6 animals each as follows: Group CTRL, normal baseline rats; Group KV, normal baseline rats that received kolaviron (200 mg/kg) for 7 days, Group LKM, rats with benzene-induced preleukemic condition that received 0.2 ml intravenous injection of benzene:2-propanol:water mixture (1:5:5 v/v) for four consecutive weeks and Group LKM + KV, preleukemic rats that received kolaviron (200 mg/kg) for 7 days.
Collection of blood and bone marrow
After 24 hours of final treatment, blood samples were collected from each animal through retro-orbitals plexus into lithium heparinized tubes for biochemical assays and ethylene diaminetetraacetic acid (EDTA) bottles for hematological parameters such as hematocrit, total white blood cell (WBC) counts, red blood cell (RBC) counts, hemoglobin (Hb) and platelet counts estimation using the automated blood analyzer (SYSMEX KX21) and thereafter sacrificed. The femur bones were excised to obtain bone marrow for micronucleus assay and hematoxylin and eosin staining for histopathological examination.
Assay for oxidative stress markers in the plasma
Plasma AOPP was determined by the method described by Witko et al. [Citation26] as modified by Zhang et al. [Citation27]. Briefly, plasma (100 μl) was added to 400 μl of phosphate buffer saline (PBS) solution and 25 μl of 1.16 M potassium iodide was then added followed 2 min later by 50 μl of acetic acid. The absorbance of the reaction mixture was immediately read at 340 nm against a blank containing 500 μl of PBS, 25 μl of 1.16 M potassium iodide and 50 μl of acetic acid. Plasma total thiol was measured spectrophotometrically using DTNB (2,2’-dinitro-5,5’-dithiodibenzoic acid) [Citation28]. Arylesterase activity was determined using phenylacetate as the substrate following the procedure described by Erdem et al. [Citation29].
Micronucleus assay
Clastogenicity in preleukemic rats was evaluated in the bone marrow of the rats employing the micronucleus assay techniques as described by Heddle and Salmone [Citation30], with modification by Heddle, et al. [Citation31]. Briefly, bone marrow from femurs of rats was used for preparation of slides using the standard procedure by Matter and Schmid [Citation32].
Statistical analysis
Data are presented as the mean ± standard deviation (SD) of six replicates. Statistical significance was determined by one-way analysis of variance (ANOVA) followed by Duncan’s multiple comparison between control and treated rats in all groups using SigmaPlot® statistical package (Systat Software Inc., San Jose, CA, USA). P-values less than 0.05 (P< 0.05) were considered statistically significant.
Results
Effect of kolaviron on hematological parameters and blood morphology of benzene-induced leukemic rats
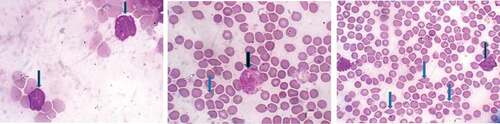
The effect of kolaviron on hematological parameters and blood morphology of benzene-induced leukemic rats is shown in . There were a decrease in the packed cell volume (PCV), erythrocyte counts and hemoglobin contents and a significant increase in WBC counts in leukemic rats when compared to the baseline control. Also, the peripheral blood film of the leukemic rats shows the presence of poikilocytosis, anisocytosis and appearance of blast. Upon treatment with kolaviron, the alteration in hematological parameters was normalized and the blast population was reduced when compared to untreated leukemia control.
Table 1. Effect of kolaviron on hematological parameters and blood morphology of benzene-induced leukemia in rats
Influence of kolaviron on bone marrow architecture of Benzene-induced leukemia in Wistar rats
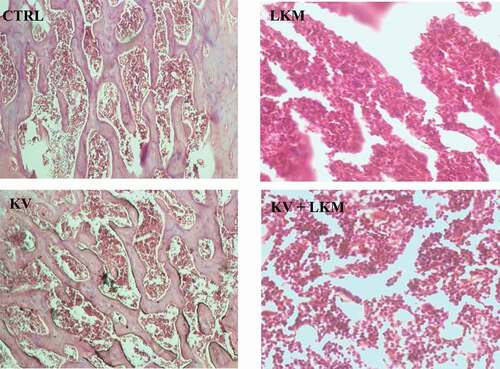
The influence of kolaviron on bone marrow architecture of benzene-induced leukemia in Wistar rats is shown in . Leukemic rats (LKM group) showed a hypercellularity in bone marrow tissue with severe dysplasia. However, in leukemic rats supplemented with kolaviron, the effect of benzene was alleviated, resulting in mild dysplasia observed in (KV + LKM) group
Effect of kolaviron on the formation of Micronucleated polychromatic erythrocytes (mPCEs) in Benzene-induced Leukemia in Wistar rats
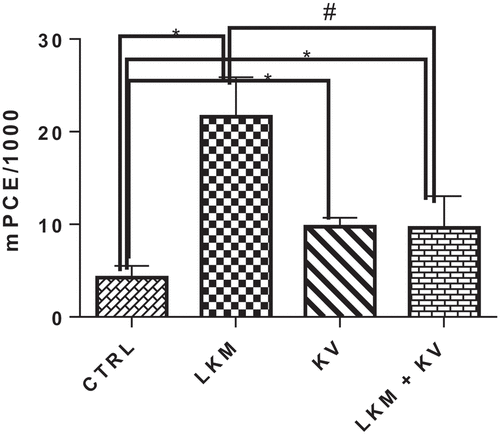
The influence of kolaviron on bone marrow formation of micronucleus on Benzene-induced leukemia in Wistar rats is shown in . Leukemic rats showed a significant increase in the frequency of micronucleus present in the bone marrow by 408.24% when compared to the control group. However, kolaviron supplementation in leukemic rats significantly alleviated the effect of benzene-induced genotoxicity by reducing the bone marrow micronucleus occurrence by 55.55% when compared with the leukemia control (LKM) group
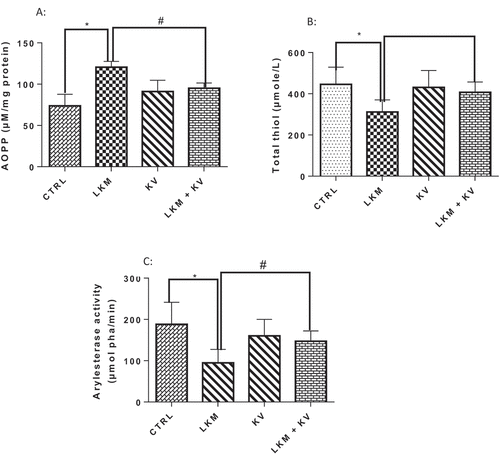
Effect of kolaviron on plasma oxidative stress status in Leukemic rats
As presented in , a significant decrease by 49.59% and 30.06% in plasma activity of arylesterase and total thiol content were observed, respectively, following leukemia induction with corresponding significantly elevated advanced oxidation protein products present in the blood plasma by 63.32% when compared to control. However, administration of kolaviron significantly ameliorated the decrease in plasma activity of arylesterase and total thiol content and increase in AOPPs ().
Figure 4. Effect of kolaviron on plasma oxidative stress markers: Advanced Oxidation Protein Products—AOPPs (A), total thiol (B) and plasma activity of arylesterase (C) on benzene-induced leukemia in Wistar rats.
Discussion
Routine hematological assessment is a diagnostic tool for monitoring the clinical status of blood-related conditions such as leukemia. The leukemogenic effect of benzene has been linked to hematological imbalance in human and animal models where reduced red blood cells, hemoglobin contents, packed cell volumes and platelets counts are common pictures [Citation33,Citation34].
Reductions in PCV, RBC count and Hb level were observed in rats induced with leukemic conditiona by benzene when compared to baseline rats. Abnormal occurrences of poikilocytosis and anisocytosis were also noted in leukemic rats relative to the baseline animals. The decrease in PCV, RBC count, Hb level and observable deformability in erythrocytes are reliable indications of anemia in animals [Citation35]. A study has shown that hydroquinone impairs the maturation of granulocytes [Citation36]. Moreover, exposure to hydroquinone was reported to induce neutrophilia, which may probably be due to intense mobilization of segmented cells from the bone marrow, leading to increased numbers of neutrophils at the peripheral compartment [Citation37]. The increase in white blood cell counts was observed in leukemic rats in this study, which supported the earlier finding that related leukocytosis to the leukemic condition [Citation25]. The occurrence of blasts in the peripheral blood film of leukemic rats in the present study is an indication of undifferentiated blood-forming cells in the blood mobilized from the marrow that had been associated with leukemia [Citation38]. Kolaviron has been shown to cause an increase in red blood cell (RBC) counts and hemoglobin and hematocrit concentrations [Citation39,Citation40] due to its effectiveness as antioxidants in red cell survival and viability [Citation41]. This is reflected in this present study where treatment with kolaviron offered a restoration of reduced Hb level, total WBC and RBC in benzene-exposed rats toward the normal level of control with concomitant reduction in the frequency of the blasts in the leukemic rat model when compared to leukemia control. This study also presented cross sectional examination of bone marrow severe dysplasia and hypercellularity, which were observed in the leukemic rats. Dysplasia occurrence in bone marrow is a morphological observation that has been linked to newly diagnosed acute myelogenous leukemia [Citation42,Citation43]. However, the supplementation of leukemic rats with kolaviron isolate reduced the dysplasia occurrences in the bone marrow tissue of the treated rats.
Myelodysplasia has been suggested to be a significant step in the generation of leukemia by benzene [Citation36]. Benzene metabolite hydroquinone was earlier reported to promote proliferation and differentiation of the myeloblast into the myelocyte stage but inhibited the maturation of myelocyte into neutrophil [Citation44]. The mutation of the clone of myelocytes without subsequent DNA repair may be further proliferated and promote the development of leukemia [Citation45]. Reactive metabolites generated during benzene biotransformation can induce genotoxicity and cytotoxicity through diverse mechanisms [Citation46–48]. The study had reported involvement of benzoquinones and other benzene reactive oxygen metabolites in the induction of oxidative DNA damage, lipid peroxidation and strand breaks in the DNA of bone marrow cells in benzene-induced toxicity [Citation49–51]. The result of this study indicated the significant induction of clastogenicity in the marrow of the rats exposed to benzene as shown by generation of significantly high occurrence of micronucleated polychromatic erythrocyte in the marrow of leukemic rats. However, administration of kolaviron significantly ameliorated the benzene-induced clastogenicity in the leukemic rats.
The induction of oxidative stress by benzene and its subsequent hematotoxicity had been reviewed [Citation52]. The present work assesses the plasma redox status markers such as arylesterase activity, total sulfhydryl and advanced oxidation protein products of leukemic rats to investigate a link between onco-hematological diseases and oxidative stress. Prolonged oxidative stress has been associated with the occurrence of tumors [Citation53]. The result showed a decrease in the plasma total thiol content with a concomitant increase in advanced oxidation protein products in leukemic rats relative to normal control. Advanced oxidation protein products (AOPPs) are oxidized protein products of oxidative stress, which resulted from chlorinated oxidants on plasma proteins [Citation26,Citation54], and its elevated level has been reported in some pathological conditions such as chronic kidney disease, diabetes, uremia and rheumatoid arthritis [Citation55–57]. The thiol group present on protein especially over albumin constitutes the major in vivo antioxidant and reducing group in the body fluid [Citation58]. Therefore, antioxidant status is indicated by the level of total thiol where the low protein thiol content correlates with an increase in peroxides and advanced oxidation protein products [Citation58,Citation59]. The increase in AOPP and low protein content in this study suggests the induction of oxidative stress due to benzene reactive metabolites that consequently led to oxidant-mediated protein damage [Citation60]. However, supplementation of kolaviron in this study significantly alleviated the increased AOPP level and restored the total thiol status when compared to the leukemia control group. Moreover, in this study, there was a significant reduction in the plasma activity of arylesterase in the leukemic rats when compared to control. Arylesterase of paraoxonase 1 (PON 1) is an esterase enzyme that possesses lipophilic antioxidant characteristics [Citation61]. The susceptibility and occurrence of some cancers like prostate, breast and hematologic cancers have been linked to PON1 polymorphism [Citation62,Citation63]. However, the reduction in arylesterase activity was mitigated on treatment with kolaviron when compared with the leukemia control group. Therefore, kolaviron may reduce the tendency of the development of the leukemia through enhanced activity of arylesterase of paraoxonase 1.
Overall, kolaviron of Garcinia kola protected against hematological and myeloid assault in benzene-induced bone marrow dysplasia in Wistar rats through improvement on plasma antioxidant status.
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- Sung H, Ferlay J, Siegel RL, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71:209–249.
- Wong O, Harris F, Armstrong TW, et al. Hospital based case-control study of acute myeloid leukemia in Shanghai: analysis of environmental and occupational risk factors by subtypes of the WHO classification. Chem Biol Interact. 2010;184:112–128.
- Hellström-Lindberg E, Tobiasson M, Greenberg P. Myelodysplastic syndromes: moving towards personalized management. Haematologica. 2020;105(7):1765–1779.
- Arber DA, Orazi A, Hasserjian R, et al. The 2016 revision to the World Health Organization classification of myeloid neoplasms and acute leukemia. Blood. 2016;127(20):2391–2405.
- Ryden J, Edgren G, Karimi M, et al. Male sex and the pattern of recurrent myeloid mutations are strong independent predictors of blood transfusion intensity in patients with myelodysplastic syndromes. Leukemia. 2019;33(2):522–527.
- Bray F, Jemal A, Grey N, et al. Global cancer transitions according to the human development index (2008-2030): a population-based study. Lancet Oncol. 2012;13:790–801.
- American cancer society cancer facts & figures. Childhood leukemia Atlanta, Georgia; 2015.
- Shati AA. Sub-chronic administration of vincristine sulfate induces renal damage and apoptosis in rats via induction of oxidative stress and activation of Raf1-MEK1/2- Erk1/2 signal transduction. Int J Morphol. 2019;37(1):273–283.
- de Lima MB, Gama LA, Hauschildt AT, et al. Gastrointestinal motility, mucosal mast cell, and intestinal histology in rats: effect of prednisone. Biomed Res Int. 2017;2017(3):1–8.
- Onodera T, Poe JC, Tedder TF, et al. CD22 regulates time course of both B cell division and antibody response. J Immunol. 2008;180:907–913.
- Omole JG, Ayoka OA, Alabi QK, et al. Protective effect of kolaviron on cyclophosphamide-induced cardiac toxicity in rats. J Evid Based Integr Med. 2018;23:1–11.
- Kwatra M, Kumar V, Jangra A, et al. Ameliorative effect of naringin against doxorubicin-induced acute cardiac toxicity in rats. Pharm Biol. 2016;54(4):637–647.
- Goldberg H, Lusk E, Moore J, et al. Survey of exposure to genotoxic agents in primary myelodysplastic syndrome: correlation with chromosome patterns and data on patients without hematological disease. Cancer Res. 1990;50:6876–6881.
- Omosa LK, Midiwo JO, Masila VM, et al. Cytotoxicity of 91 Kenyan indigenous medicinal plants towards human CCRF-CEM leukemia cells. J Ethnopharmacol. 2016;179:177–196.
- Zaini R, Clench MR, Le Maitre CL. Bioactive chemicals from carrot (Daucus carota) juice extracts for the treatment of Leukemia. J Med Food. 2011;14(11):1303–1312.
- Iwu MM. Antihepatotoxic constituents of garcinia-kola seeds. Experimentia. 1985;41:699–700.
- Farombi EO, Akanni OO, Emerole GO. Antioxidant and scavenging activities of flavonoid extract (kolaviron) of Garcinia kola seeds. Pharm Biol. 2002;40(2):107–116.
- Okoko T, Oruambo FI. Garcinia kola extract reduced lipopolysaccharide activation of macrophages using U937 cells as a model. Afr J Biotechnol. 2008;7(6):792–795.
- Alabi QK, Akomolafe RO, Olukiran OS, et al. Assessment of haematological and biochemical effects of Kolaviron in Male Wistar Rats. Br J Pharm Res. 2017;16(3):1–14.
- Farombi E, Awogbindin I, Farombi T, et al. Neuroprotective role of kolaviron in striatal redoinflammation associated with rotenone model of Parkinson’s disease. Neurotoxicology. 2019;73:132–141.
- Oyagbemi AA, Omobowale TO, Adedapo AA, et al. Kolaviron, Biflavonoid complex from the seed of Garcinia kola attenuated angiotensin II- and Lypopolysaccharide-induced vascular smooth muscle cell proliferation and nitric oxide production. Pharmacognosy Res. 2016;8(S1):50–55.
- Olayinka E, Ore A. Kolaviron and L-ascorbic acid attenuate chlorambucil-induced testicular oxidative stress in rats. J Toxicol. 2014;2014:1–9.
- Adedara A, Mathur PP, Farombi EO. Kolaviron prevents ethylene glycol monoethyl ether-induced testicular apoptosis via down-regulation of stress proteins, Fas/Fas-L and caspases expressions in rats. Toxicol Mech Methods. 2013;23(9):689–696.
- Research Council N. Guide for the care and use of laboratory animals. 8th ed. Washington, DC, USA: National Research; The National Academies Press; 2011.
- Akanni EO, Alli OAT, Oloke JK. Anti-leukemic and immunomodulatory effects of fungal metabolites of Pleurotus pulmonarius and Pleurotus ostreatus on benzene-induced leukemia in Wister rats. Korean J Hematol. 2012;47:67–73.
- Witko-Sarsat V, Friedlander M, Capeillère-Blandin C, et al. Advanced oxidation protein products as a novel marker of oxidative stress in uremia. Kidney Int. 1996;49:1304–1313.
- Zhang G, Skorokhod OA, Khoo S. Plasma advanced oxidative protein products are associated with anti-oxidative stress pathway genes and malaria in a longitudinal cohort”. Malar J. 2014;13:134.
- Motchnik P, Frei B, Ames B. Measurement of antioxidants in human blood plasma. Methods Enzym. 1994;234:269–279.
- Erdem FH, Karatay S, Yildirim K, et al. Evaluation of serum paraoxonase and arylesterase activities in ankylosing spondylitis patients. Clinics. 2010;65(2):175–179.
- Heddle JA, Salmone MF. The micronucleus assay I: in vivo. In: Stich HF, San RHC, editors. Topics in environmental physiology and medicine. Short term tests for chemical carcinogens. New York Heidelberg, Berlin: Springer-Verlag; 1981. p. 243–249.
- Heddle JA, Sudharsan RA, Krepinsky AB. The micronucleus assay II: in vitro. In: Stich HF, San RHC, editors. Topics in environmental physiology and medicine. Short term tests for chemical carcinogens. New York, Heidelberg, Berlin: Springer-Verlag; 1981. p. 250–254.
- Matter B, Schmid W. Bone marrow toxicity. Mutat Res. 1971;12:417‑425.
- Ola OS, Sofolahan TA. A monoterpene antioxidant, linalool, mitigates benzene-induced oxidative toxicities on hematology and liver of male rats. Egypt J Basic Appl Sci. 2021;8(1):39–53.
- Akanni EO, Faremi A, Akanni RA, et al. African polyherbal formulation possesses chemopreventive and chemotherapeutic effects on Benzene- induced Leukemia in Wistar rats. Ann Res Rev Biol. 2017;16(2):1–11.
- Berger J. Review: phenylhydrazine haematotoxicity. J Appl Biomed. 2007;5:125–130.
- Snyder R. Benzene and leukemia. Critical review toxicol. 2002;32(3):155–210.
- Macedo SMD, Lourenco ELB, Borelli P, et al. Effects of in vivo phenol or hydroquinone exposure on events related to neutrophil delivery during an inflammatory response. Toxicology. 2006;220:126–135.
- Kabeel MM, Ghoneim AM, Mansy SE. Anti-leukemic activity of a four-plant mixture in a leukemic rat model. J Basic Appl Zool. 2018;79:7.
- Alabi QK, Akomolafe RO. Kolaviron diminishes diclofenac-induced liver and kidney toxicity in Wistar rats via suppressing inflammatory events, upregulating antioxidant defenses, and improving hematological indices. Dose-Response: Int J. 2020;18(1):1–12.
- Ahumibe AA, Braide BV. Effect of gavage treatment with pulverized Garcinia kola seeds on erythrocyte membrane integrity and selected haematological indices in male albino Wistar rats. Nigerian J Physiol Sci. 2009;24(1):47–52.
- Atolaiye BO, Adebayo MA, Jagha OO, et al. Evaluation of the potency of certain substances as antioxidants in the assessment of red cell viability. J Med Plants Res. 2009;3(6):485–492.
- Islam A, Catovsky D, Goldman JM, et al. Bone marrow biopsy changes in acute myeloid leukemia: i. observations before chemotherapy. Histopathology. 1985;9:939–957.
- Ballen KK, Gilliland DG, Kalish LA, et al. Bone marrow dysplasia in patients with newly diagnosed acute myelogenous leukemia does not correlate with history of myelodysplasia or with remission rate and survival. Cancer. 1993;73(2):314–321.
- Hazel BA, Kalf GF. Induction of granulocytic differentiation in myeloblast by hydroquinone, a metabolite of benzene, involves the leukotriene D4 receptor. Recept Signal Transduct. 1996;6(1):1–12.
- Spalding JW, French JE, Tice RR, et al. Development of transgenic mouse model for carcinogenesis bioassays: evaluation of chemistry induced skin tumors in TG.AC mice. Toxicol Sci. 1999;49:241–254.
- Wan J, Badham HJ, Winn L. The role of c-MYB in benzene- initiated toxicity. Chemico-Biologocal Interact. 2005;153-154:171–178.
- Sun R, Zhang J, Yin L, et al. Investigation into variation of endogenous metabolites in bone marrow cells and plasma in C3H/He mice exposed to benzene. Int J Mol Sci. 2014;15(3):4994–5010.
- French JE, Gatti DM, Morgan DL, et al. Diversity outbred mice identify population-based exposure thresholds and genetic factors that influence benzene-induced genotoxicity. Environ Health Perspect. 2015;123(3):237–245.
- Zhang L, Eastmond DA, Smith MT. The nature of chromosomal aberrations detected in human exposed to benzene. Critical review toxicol. 2002;32(1):1–42.
- Roma-Torres J, Teixeira JP, Silva S, et al. Evaluation of genotoxicity in a group of workers from apetroleum refinery aromatics plant. Mutat Res. 2006;604(1–2):19–27.
- Zhu J, Wang H, Yang S, et al. Comparison of toxicity of benzene metabolite hydroquinone in hematopoietic stem cells derived from murine embryonic yolk sac and adult bone marrow. PLoS One. 2013;8(8):e71153.
- Elsayed ASI. Hematotoxicity and oxidative stress caused by Benzene. Pyrex J Biomed Res. 2015;1(6):074–080.
- Farinati F, Piciocchi M, Lavezzo E, et al. Oxidative stress and inducible nitric oxide synthase induction in carcinogenesis. Dig Dis. 2010;28(5):579–580.
- Witko-Sarsat V, Friedlander M, Nguyen-Khoa T, et al. Advanced oxidation protein products as novel mediators of inflammation and monocyte activation in chronic renal failure1, 2. J Immunol. 1998;161:2524–2532.
- Krzystek-Korpacka M, Neubauer K, Berdowska I, et al. Enhanced formation of advanced oxidation protein products in IBD. Inflamm Bowel Dis. 2008;14:794–802.
- Baskol G, Demir H, Baskol M, et al. Investigation of protein oxidation and lipid peroxidation in patients with rheumatoid arthritis. Cell Biochem Funct. 2006;24:307–311.
- Piwowar A, Knapik-Kordecka M, Warwas M. AOPP and its relations with selected markers of oxidative/antioxidative system in type 2 diabetes mellitus. Diabetes Res Clin Pract. 2007;77:188–192.
- Himmelfarb J, McMonagle E, McMenamin E. Plasma protein thiol oxidation and carbonyl formation in chronic renal failure. Kidney Int. 2000;57:2571–2578.
- Prakash M, Upadhya S, Prabhu R. Protein thiol oxidation and lipid peroxidation in patients with uremia. Scand J Clin Lab Invest. 2004;64:599–604.
- Alderman C, Shah S, Foreman JC. The role of advanced oxidation protein products in regulation of dendritic cell function. Free Radic Biol Med. 2002;32:377–385.
- Serdar Z, Aslan K, Dirican M, et al. Lipid and protein oxidation and antioxidant status in patients with angiographically proven coronary artery disease. Clin Biochem. 2006;39:794–803.
- Hussein YM, Gharib AF, Etewa RL, et al. Association of L55M and Q192R polymorphisms in paraoxonase 1 (PON1) gene with breast cancer risk and their clinical significance. Mol Cell Biochem. 2011;351(1–2):117–123.
- Pan X, Huang L, Li M, et al. The Association between PON1 (Q192R and L55M) gene polymorphisms and risk of cancer: a meta-analysis based on 43 studies. Biomed Res Int. 2019;1–14.