ABSTRACT
Indomethacin (IND) is a non-steroidal anti-inflammatory drug with many pharmacological benefits and multi-organ toxicities. Thymol (THY) is a natural phenolic monoterpenoid with many biological properties. However, there is scarcity of information on its potential on IND-induced hepatorenal damage in rats. This study investigated the effect of THY on IND-induced hepatorenal damage in rats. The four experimental groups contain control, IND alone (5 mg/kg), THY alone (250 mg/kg) and co-treated group that were treated orally for 21 consecutive days. Twenty four hour after treatments, rats were sacrificed and markers of hepatorenal damage (ALT, ALP, AST, G6PDH, GGT, BIL, CHOL, GLDH, LDH, MDH, SDH, creatinine, urea, uric acid, cystatin-C and electrolytes), antioxidant status markers, myeloperoxidase (MPO) activity, nitric oxide (NO), lipid peroxidation (LPO), tumor necrosis factor alpha (TNF-α), interleukin-1β (IL-1β) levels and caspase-3 activity were evaluated. IND increased hepatorenal toxicity, inflammatory and apoptotic markers and decreased antioxidant status. Co-administration of IND with THY significantly decreased hepatorenal damage biomarkers improved the antioxidant status and reduced the MPO activity, LPO, NO, TNF-α, IL-1β levels and caspase-3 activity in the liver and kidney of rats. In conclusion, THY mitigates against IND-induced oxidative stress, inflammation and caspase-3 activation in the liver and kidney of the rats.
Introduction
Drug-induced multi-organ toxicities are common adverse reaction triggered by numerous drugs like indomethacin (IND). Indomethacin is one of the non-steroidal anti-inflammatory drugs used as analgesic and also has antipyretic property. However, its adverse side effects have raised a lot of concern for its continuous use in clinical settings. Indomethacin cause many organ toxicities including liver, kidney and gastrointestinal toxicities in humans and experimental animals [Citation1,Citation2]. In the liver, IND has been associated with hepatocellular enzymes elevation and cholestatic jaundice whereas in the kidney, IND caused acute interstitial nephritis typified by wide spread interstitial edema with infiltration of inflammatory cells [Citation3,Citation4]. The mechanisms by which IND causes its toxicities include prostaglandin synthesis inhibition, generation of reactive oxygen species (ROS) resulting to cellular oxidative stress, inflammation and apoptosis [Citation5].
The liver and kidney are very critical organs in drug metabolism [Citation6]. Liver is the main organ for drug metabolism, detoxifies and eliminates by-products from body and the kidney is responsible mainly for excretion, maintenance of body homeostasis and also aid in drug metabolism. These roles expose these organs to toxic assaults and injuries through oxidative stress, the discharge of pro-inflammatory markers including TNF-α and IL-1β and stimulation of apoptotic process [Citation7]. Several proteomic and genetic biomarkers formed during hepatorenal toxicities including transaminases, alkaline phosphatase, bilirubin, cholesterol, creatinine, triglycerides, urea and uric acid level indicates the severity of hepatorenal damage [Citation8,Citation9].
Thymol (THY) is a monoterpene phenolic compound found in some plants belonging to the Lamiaceae (Thymus, Ocimum, Origanum, and Monarda genera) and many others including those belonging to the Apiaceae, Scrophulariaceae, Verbenaceae, and Ranunculaceae families [Citation10,Citation11]. Thymol has antibacterial, antioxidant, anti-inflammatory, anticarcinogenic, antiapoptotic, anti-hyperlipidemic and anti-hyperglycemic, hepatoprotective, radioprotective anti-ulcerogenic, neuroprotective, renoprotective, activities [Citation12–17].
However, despite the numerous clinical and therapeutic benefits of THY, literature on its hepatorenal potential is scarce. The present study investigates protective potential of thymol against indomethacin-induced hepatorenal oxidative stress, inflammation and inactivation of caspase-3 and the possible mechanisms of action by which thymol abrogates indomethacin-induced hepatorenal dysfunction in adult male rats.
Materials and methods
Animals and treatment
Thirty two adult male Wistar rats (8 weeks old, 172 ± 5 g) were purchased from the Veterinary Anatomy Department animal house of the University of Ibadan, Nigeria, and were kept in the Biochemistry Department animal house of the University throughout the period of this study in polyethylene-walled cages. The rats were acclimatized for 14 days and were kept on a 12-h light: 12-h dark regime at 28°C prior to the experiments and were fed with standard rat’s chow with free access to water. All procedures in this study conformed to the ‘Guide for the Care and Use of Laboratory Animals’ published by the National Institute of Health (NIH publication No. 85–23 revised 1996) and the study carried out according to the US NAS guidelines as approved by the University Ethical Committee. The rats were denied food for 18 hours before the start of the experiment, but have free access to clean drinking water.
Drugs and experimental design
Indomethacin used in this study was manufactured by Fabrique par: Yangzhou No. 3 pharmaceutical Co. Ltd, Jiangsu, China while thymol (99.99%), was from Sigma Aldrich Chemical Co. (St. Louis, MO, USA). All other chemicals were of analytical grade and were obtained from the British Drug Houses (Poole, Dorset, UK) and other standard suppliers.
After the two weeks acclimatization, the rat were randomly divided into four groups of eight rats and were orally treated once daily for 21 days consecutively. The rats were fasted for 24 h prior to administration of the test drugs. The drugs were dissolved in corn oil. The rats were grouped into four groups of eight rats each and treated orally by gavage once daily as follows.
Group 1 served as the control and received 1 ml/kg corn oil, group 2 rats received 5 mg/kg IND while group 3 rats were co-treated with 5 mg/kg IND and 250 mg/kg THY and group 4 received 250 mg/kg THY alone. Doses of IND and THY used in this study were based on our preliminary studies and previous studies [Citation18,Citation19].
Sample collection and tissue preparation
Twenty-four hours after the expiration of the experiment, the final weight of the rats were taken and rats were anesthetized with sodium pentobarbital (35 mg/kg, i.p) before blood collection via retro-orbital venous plexus into separate plain tubes with a glass capillary tube from each rat and sacrificed. The collected blood were centrifuged at 3000 g for 10 min at 4°C and kept at −80°C and used for hepatic and renal function tests, and other biochemical analyses.
Liver and kidney samples of the rats were harvested separately, rinsed with ice-cold phosphate buffer saline and homogenized in ice cold 50 mM Tris-HCl buffer (containing 1.15% of potassium chloride; pH 7.4) in a ratio of 1:9 (w/v) using a Teflon glass homogenizer. The homogenate was then centrifuged at 14,000 g for 15 min at 4°C. The supernatant obtained for each rat liver and kidney were collected separately for each rat, stored at −80°C and was used for evaluating the total antioxidant capacity, oxidative stress, inflammation and apoptotic markers as well as arginase activity.
Determination of liver and kidney function markers
The serum activities of sorbitol dehydrogenase (SDH; cat. No. MAK316), malate dehydrogenase (MDH; cat. no. MAK194) (Sigma Chemical Co., St Louis, MO, USA), albumin (ALB; cat. no. AB361), alanine aminotransferase (ALT; cat. no. AL8004), aspartate aminotransferase (AST; cat. no. AS8003), alkaline phosphatase (ALP; cat. no. AP9762), gamma glutamyl transferase (GGT; cat. no.GT3807), glucose-6-phosphate dehydrogenase (G6PDH cat. no. PD409), glutamate dehydrogenase (GLDH; cat. no. GL432), lactate dehydrogenase (LDH; cat. no. LD3832) and concentrations of direct and total bilirubin (dBIL and tBIL; cat. nos. BR4058 and BR9765), creatinine (cat. no.CR2335), cystatin-C (cat. no. CYS4003), uric acid (cat. no.UA8059) and urea (cat. no.UR443) levels were estimated using commercially available kits according to the manufacturer’s instructions (Randox Laboratories Limited, UK). Serum paraoxonase 1 (PON1) activity assay was carried out according to the methods of La Du and Eckerson [Citation20] by measuring arylesterase activity using phenyl acetate as a substrate. Serum purine nucleoside phosphorylase (PNP) activity was measured by using a colorimetric assay as described by Chu et al. [Citation21]. Serum arginase (ARG) activity was determined by the method of Porembska and Kedra [Citation22]. The citrulline (CIT) level was determined according to the method described by Knipp and Vašák [Citation23]. Hydroxyproline (HYP) level was determined according to the method described by Reddy and Enwemeka [Citation24]. Serum electrolytes (sodium, potassium, chloride, and bicarbonate ions) were determined by flame photometry. Serum cystatin C was estimated by immunoturbidimetric method.
Assessment of serum lipid profile
Levels of serum total cholesterol (CHOL; cat. no. CH8016), triglyceride (TRIGS; cat. no. TR9769), low-density lipoprotein (LDL cat. no.CH2645), and high-density lipoprotein (HDL; cat. no.CH2645) were determined according to the manufacturer’s instructions using commercially available diagnostic kits (Randox Laboratories Limited, UK).
Estimation of hepatic and renal antioxidant status and oxidative stress markers
Superoxide dismutase (SOD) activity was estimated as described by Magwere et al. [Citation25], catalase (CAT) activity was determined using H2O2 as a substrate according to the method described by Clairborne [Citation26]. Glutathione peroxidase (GPx) was according to the method of Rotruck et al. [Citation27], reduced GSH level was determined at 412 nm according to the method described by Jollow et al. [Citation28], glutathione-S-transferase (GST) was estimated by the method of Habig et al. [Citation29], lipid peroxidation was quantified as malondialdehyde (MDA) according to method of Nakamura et al. [Citation30] and the result was expressed as micromoles of MDA per milligram protein. Protein concentration was determined according to the method described by Popovic et al. [Citation31]. The total antioxidant capacity (TAC) and total oxidative stress (TOS) concentrations were determined in the liver and the kidney according to the Erel methods [Citation32,Citation33].
Assessment of hepatic and renal pro-inflammatory markers and caspase-3 activity
Liver and kidney myeloperoxidase activity (MPO) was evaluated by the method of Granell [Citation34] and nitric oxide (NO) level was estimated by the method of Li et al. [Citation35]. Briefly, equal volume of the liver or kidney supernatant and Griess reagent were mixed and incubated at 37oC for 15 min and absorbance read at 540 nm. The level of NO was extrapolated from the standard curve prepared by different concentration of sodium nitrite and result expressed as Unit/mg protein. The levels of TNF-α (cat. no. CSB-E11967r), IL-1β (cat. no. CSB-E08045r) and caspase-3 activity (cat. no. CSB-E08854r) were determined using commercially available ELISA kits (Cusabio Biotech Co., Ltd, Wuhan, China) and the final colored product was measured at 450 nm by a microplate reader (Cytation 5, BioTek Instrument, USA). All the experiments were performed in triplicate, and the results were presented in pg/ml.
Histopathological analysis
The liver and kidney tissues specimens were fixed in 10% neutral phosphate-buffered formalin solution for at least 24 h. After dehydration procedures, the samples were blocked in paraffin. Sections of 4–5 μm were cut by a microtome, deparaffinized, hydrated and stained with hematoxylin and eosin (H & E). All slides were coded before examination with light microscope (Olympus BX-41 microscope, Hamburg, Germany) and photographed using a digital camera by pathologists who were blinded to control and drug-treated groups.
Statistical analyses
The statistical analyses were performed using GRAPHPAD PRISM 5 software (Version 5; GraphPad Software, La Jolla, California, USA), and data were presented as mean ± SEM. Statistical significant differences were determined using one-way analysis of variance (ANOVA) followed by Turkey’s post-hoc test, where p < 0.05 was considered significant.
Results
Effect of Thymol on relative organ weight and body weight in IND-treated rats
The results of the body weight gain and the relative liver and kidney weights of rats treated for 21 days are depicted in . Rats treated with IND alone showed a significant (p < 0.05) decrease in the body weight but increased liver and kidney weights compared to the control rats, but co-administration of IND and THY showed an increased in body weight of the rats and decreased liver and kidney weights when compared with the IND alone treated rats. However, rats treated with THY alone showed values of both the body weight and relative organ weights similar to those of the control rats.
Table 1. Body weight gain and relative organ weights of Control, IND alone, IND + THY and THY alone rats for 21 days
THY improves hepatic and renal function markers in indomethacin-treated rats
The serum activities and levels of hepatic and renal function biomarkers of the control, IND alone, IND + THY and THY alone were shown in
Table 2. Hepatic function biomarkers in rats treated with IND alone, IND with THY and THY alone for 21 days
Table 3. Renal function biomarkers in rats treated with IND alone, IND with THY and THY alone for 21 days
Influence of THY on indomethacin-induced serum lipid profile
The effect of THY on serum lipid profile is represented in . Oral administration of IND caused a marked increase in the levels of CHOL, TRIGS and LDL with simultaneous decrease in HDL level as evidenced in the IND alone treated rats compared to the control rats. But co-treatment of IND with THY deceased significantly (p < 0.05) the levels of CHOL, TRIGS and LDL and concomitantly increased the HDL level. The rats given THY alone showed values that were similar to that of control.
Table 4. Serum lipid profile of rats treated with IND alone, IND with THY and THY alone for 21 days
Thymol enhances hepatic and renal antioxidant status in IND-treated rats
The results in showed the effect of IND, IND with THY and THY alone on antioxidant biomarkers in the liver and kidney of rats. Administration of IND alone for 21 consecutive days revealed significant (p < 0.05) decrease in SOD, CAT, GPx, GST activities and GSH level with concurrent increased LPO level in the liver and kidney of rats compared to the control rats. Co-administration of IND with THY however, significantly (p < 0.05) elevated the activities and the level of the antioxidant markers accompanied with decreased LPO level compared with the IND alone rats. Rats given THY alone on the other hand yielded values that were similar to that of the control in both the liver and kidney antioxidant status.
Table 5. Hepatic and renal antioxidant status in rats treated with IND alone, IND with THY and THY alone for 21 days
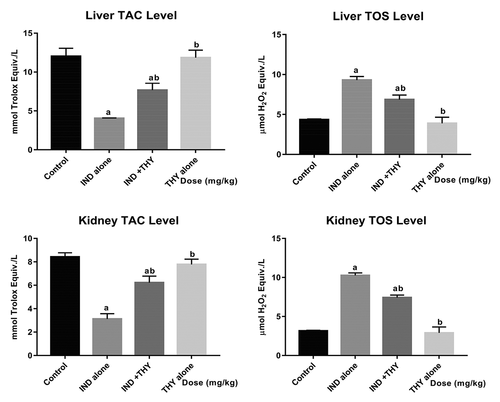
Effect of thymol on hepatic and renal TAC and TOS in indomethacin-treated rats
The effect of THY on total antioxidant capacity and total oxidative stress in IND-treated rats’ liver and kidney are shown in . Indomethacin administration significantly (p < 0.05) decreased the TAC level and markedly increased the TOS level compared to the control group. Treatment with THY and IND however, significantly (p < 0.05) increased TAC level with concomitant decrease in TOS level. Rats administered with THY alone also produced levels of TAC and TOS similar to those of the control rats.
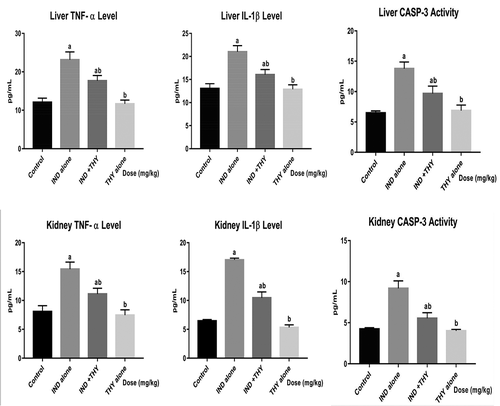
Thymol inhibits hepatic and renal pro-inflammatory biomarker and activation of caspase-3 in indomethacin-treated rats
The results of the effect of THY and IND co-treatment on MPO activity and NO level are depicted in while showed the levels of TNF-α, IL-1β and caspase-3 activity. Administration of IND to rats significantly (p < 0.05) increased the hepatic and renal MPO activity, NO, TNF-α and IL-1β levels when compared to the control. Additionally, IND alone increased hepatic and renal caspase-3 activity when compared with the control. But co-treatment of IND with THY reduced these biomarkers of inflammation and apoptosis significantly (p < 0.05) in the liver and the kidney of rats when compared with IND alone treated group. Rat administered with THY alone showed values and activity that were similar to that of the control rats.
Figure 2. Influence of thymol on hepatic and renal MPO activity and NO level in indomethacin-treated rats. n = 8.Each bar represents mean ± SEM of 8 rats. ap < 0.05 versus control; bp < 0.05 versus IND alone; cp < 0.05 versus THY alone. IND: 5 mg/kg indomethacin; THY: 250 mg/kg thymol.
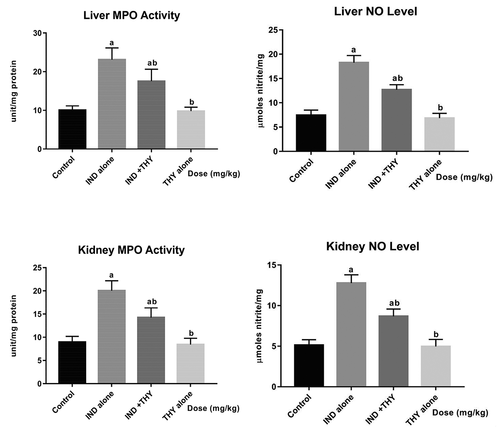
Figure 3. Effect of thymol on hepatic and renal levels of TNF-α, IL-1β and caspase-3 activity in indomethacin-treated rats. n = 8. Each bar represents mean ± SEM of 8 rats in triplicates. ap < 0.05 versus control; bp < 0.05 versus IND alone; cp < 0.05 versus THY alone. IND: 5 mg/kg indomethacin; THY: 250 mg/kg thymol.
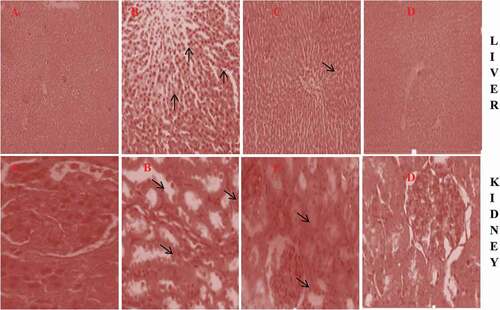
Thymol improves histological damage in the liver and kidney of IND-treated rats
The photomicrographs of the representative liver and kidney of control and rats treated with IND alone and IND co-treated with THY and THY alone are shown in . Histological examination of the tissues of the control group showed an intact structural arrangement of the liver and the kidney. The liver of rats treated with IND alone showed degeneration, fibrotic necrosis in the focal area and infiltration of inflammatory cells (red arrows), Liver of rats co-treated with IND and THY showed fewer inflammatory cells, in THY alone treated rats liver showed arrangements of all the components similar to the control rats liver. Also, kidney of rats treated with IND alone showed noticeable degeneration and inflammatory cell infiltration in the proximal and congestion of vessels, kidney of rats co-treated with IND and THY showed mild tubular degeneration and few inflammatory cell infiltration and rats treated with THY alone showed no lesion.
Figure 4. Photomicrograph of representatives liver and kidney of (A) Control, (B) IND alone, (C) IND + THY and (D) THY alone. Control liver and kidney showed intact architectural arrangement. Liver of IND alone rats showed infiltration of inflammatory cells, dispution of hepatocytes arrangement and enlargement of the sinusoids; Liver of rat co-treated with IND + THY showing few inflammatory cells while THY alone treated group showing near to normal architecture. The kidney of IND alone showed vacuolation and renal tubular necrosis, disintegration of medullary tubules with edema (arrow) at interstitium and inflammatory cellular infiltration. The kidney of rats co-treated with IND + THY showed mild tubular degeneration and infiltration of inflammatory cells whereas THY alone treated group showing near to normal architecture (H & E X 400).
Discussion
The liver and kidney are two major organs in that play vital role in the metabolism and excretion of drugs and their metabolites. In this present study, IND administration to rats caused a significant decrease in the body weights but increase relative liver and kidney weights. This signifies obvious common organ toxicities in rats and our result conformed to the previous report [Citation36, Citation37]. This observation was improved in rats co-treated with IND and THY.
Indomethacin administration has been reported to generate ROS that has been linked to lipid peroxidation with attendant disruption of cellular membranes resulting in leakage of resident enzymes and subsequent multi-organ toxicities [] through oxidative stress induction, inflammation and apoptosis [Citation5]. Liver damage is usually assessed by ALT, AST, ALP, ALB, GGT, total BIL and LDH [Citation38–40]. Elevated levels of serum ALT, AST, ALP, GGT and total BIL with low ALB level are sensitive markers of hepatic injury, cholestasis and hepatobiliary injury [Citation41]. G6PDH protects the cellular redox potential in the course of oxidative stress [Citation42]. Though these hepatic markers are used to assess liver overall damage, some of them are not specific to hepatic damage and are released into the blood stream after damage had occurred, thus, we evaluated more specific and early detected hepatic injury markers. GLDH, MDH SDH and PNP are enzymes whose serum activities are elevated during hepatocellular damage and are involved in the metabolic role of the liver [Citation43–45] whereas PON1 is an ester hydrolase with both aryl esterase and paraoxonase activities and protects both LDL and HDL from oxidation [Citation46]. ARG converts arginine to ornithine and urea. But, L-arginine metabolism is usually diverted to citrulline and nitrate during inflammation while hydroxyproline, a component of collagen whose presence in the serum could indicate the rates and progression of liver fibrogenesis [Citation47]. In this study, elevated levels of ALT, AST, ALP, GGT, G6PDH, LDH, tBIL and dBIL GLDH, MDH, SDH, PNP, ARG, CIT, HYP with concomitant decrease in ALB and PON1 levels connotes hepatic toxicity that affects the integrity, metabolic, excretory, transport, synthetic and other liver functions. Our results conformed with previous reports [Citation18,Citation48]. The decreased levels of these biomarkers with the exception of ALB and PON1 that were increased following co-administration of IND with THY suggests that THY protects against IND-induced hepatic damage probably due to its ability to prevent hepatocytes membrane disruption and thus prevents leakage of hepatocytes constituents.
The role of the liver in lipid and lipoprotein secretion, synthesis and metabolism is vital in maintain the energy level of the cell [Citation49]. In this present study, the significant increase in the levels of CHOL, TRIGS and LDL with concomitant decrease in the level of HDL in the serum of rats given IND alone suggest destruction of the hepatocytes membrane, release of membrane lipids and subsequent lipid peroxidation. Our results are similar to the previous results [Citation50]. However, rats co-treated with IND and THY showed reduced levels of CHOL, TRIGS and LDL and increased level of HDL suggesting the anti-hyperlipidemic potential of THY in rats by preventing lipid peroxidation. The THY alone rats showed results that were similar to the control group.
Excretion is one of the kidney’s functions. Creatinine, cystatin-C, urea and uric acid are waste products excreted by the kidney [Citation51] and measurement of serum cystatin-C, urea and creatinine levels gives an assessment of kidney function especially glomerular filtration rate and decrease in reabsorption [Citation51]. In addition, electrolyte disturbances and dehydration has also been associated with renal toxicity. Increase serum levels of Na+, K+, HCO3−, and Cl− may connote an unpleasant effect on the ion-dependent processes. Thus, in this study, the elevated levels of these renal function indices in rats following administration of IND suggest kidney’s excretory dysfunction, reduction of GFR and glomerular filtration. The result of this study is similar to the previous reports [Citation48,Citation51]. The diminished levels of these kidney function indices in IND and THY co-treated rats revealed the nephroprotective effect of THY on IND-induced renal toxicity.
Reactive oxygen species and reactive nitrogen species (RNS) are parts of normal physiological process in the cell and are balanced by the antioxidant system [Citation52]. However, disturbance of the homeostasis resulting from excessive generation of these products can cause various pathophysiological processes associated with injuries or diseases via oxidative stress [Citation53,Citation54]. IND-induced organ damage involves oxidative stress and cellular redox homeostasis disruption via lipid peroxidation, DNA damage, protein oxidation, inflammation and induction of apoptosis [Citation55]. The SOD, CAT, GPx and GSH serve as the main antioxidant and provide the first line of defense against ROS [Citation56] while GST complements the neutralization of xenobiotics [Citation57]. The reduction in the activities and level of this antioxidant system in this study suggest overwhelming generation of ROS and exhaustion of these antioxidant cascades to cope with their detoxification resulting in their accumulation in the liver and kidney of rats. Our result is similar to the previous [Citation58,Citation59]. The elevated activities and level of the antioxidant system observed in the hepatic and renal tissues of rats co-treated with IND and THY in this study, indicates that THY possesses scavenging ability against ROS generated by IND biotransformation and antioxidant boosting capacities probably due to the presence of the phenolic hydroxyl groups in its structure that confer redox characteristics on it to produce transient phenoxyl radicals. Elevated MDA and TOS with low TAS levels indicate oxidative damage and act as oxidative stress indicators [Citation60,Citation61]. The increased MDA and TOS levels with decreased TAS level in the liver and kidney of rats treated with IND confirmed oxidative stress. This result agreed with the report of Geyikoglu et al. [Citation62]. However, co-treatment of IND with THY effectively decreased the MDA level and restored the oxidant/antioxidant balances by enhancing TAC and attenuating the increased TOS, probably due to THY redox characteristics highlighted previously.
Inflammation is one of the processes that occurs in drug-induced multi-organ toxicities and has been linked to ROS generation [Citation63]. Damage to these organs promotes neutrophil infiltration and subsequent release of inflammatory cytokines such as TNF-α and IL-1β and MPO [Citation64]. TNF-α has been linked to increase nitric oxide formation via inducible nitric oxide synthase. The increase in the hepatic and renal MPO activity and NO, TNF-α and IL-1β levels in rats signifies induction of inflammatory process in the rats. The result of this study agreed with the previous reports [Citation18]. The reduced MPO activity and NO, TNF-α and IL-1β levels in the liver and kidney tissues of rats co-treated with IND and THY showed the anti-inflammatory property of THY on IND-mediated inflammatory process in the rats probably due to its inhibitory effect on expression of genes that codes for the pro-inflammatory markers.
Cellular redox capacity depends heavily on the GSH and it depletion due to ROS generation can trigger the apoptotic process via the activation of caspases (cysteinyl aspartate proteases) [Citation65]. Caspases are stimulated by protein breakdown and dimerization that can result to the cleavage of their substrates and cell death. Thus, increased level of caspase-3 in the liver and kidney of IND treated rats in this study, shows involvement of apoptosis in IND-induced hepatorenal damage. This finding is similar to the report of Peng et al. [66]. Interestingly, co-treatment with THY resulted in a remarkable inhibition of Caspase-3 activities, suggesting that THY has a potential antiapoptotic effect on liver and kidney.
In conclusion, the results of the present study show that, oral administration of thymol to rats improved the indomethacin-induced hepatorenal injury via mechanisms involving suppression of oxidative stress, inflammation and activation of caspase-3 in the liver and kidney of rats. Thus, suggesting that thymol can be a potential therapeutic agent for hepatorenal damage induced by indomethacin.
Acknowledgments
The authors would like to thank Mr. Olayinka Olaleye for his assistance in histopathology work.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Majeed RK, Muhammed HF, Rahim HM. Acute toxicity study of indomethacin and oxytetracycline in rabbits. Med J Babylon. 2018;15:218–221.
- Khan S, Yusufi FNK, Yusufi ANK. Comparative effect of indomethacin (IndoM) on the enzymes of carbohydrate metabolism, brush border membrane and oxidative stress in the kidney, small intestine and liver of rats. Toxicol Rep. 2019;6:283–394.
- Rofaeil RR, Gaber SS. Gastroprotective effect of memantine in indomethacin-induced peptic ulcer in rats, a possible role for potassium channels. Life Sci. 2019;217:164–168.
- Al-Mayali ZK, Jaffat HS, Mohammed JA. Effect of the indomethacin drug on kidney histology in male albino rats. Intern J Pharm Qual Assur. 2019;10(3):162–167.
- Hilal AM, Fatima M, Hossain MM, et al. Determination of potential oxidative damage, hepatotoxicity, and cytogenotoxicity in male Wistar rats: role of indomethacin. J Biochem Mol Toxicol. 2018;32(12):e22226.
- El-Megid MHM A, Abdul Azeem AM, El-Shahat AN, et al. Identification of the therapeutic effects of gamma-irradiated chamomile aqueous extract against alcohol induced hepatonephrotoxicity in rats. Egypt J Radiat Sci Appl. 2017;30(1):53–61.
- Abdel-Daim MM, Abdeen A. Protective effects of rosuvastatin and vitamin E against fipronil mediated oxidative damage and apoptosis in rat liver and kidney. Food Chem Toxicol. 2018;114:69–77.
- Kotsampasakou E, Montanari F, Ecker GF. Predicting drug-induced liver injury: the importance of data curation. Toxicol. 2017;389:139–145.
- Dhama K, Latheef SK, Dadar M, et al. Biomarkers in stress related diseases/disorders: diagnostic, prognostic, and therapeutic values. Front Mol Biosci. 2019;6:91.
- Marchese A, Orhan IE, Daglia M, et al. Antibacterial and antifungal activities of thymol: a brief review of the literature. Food Chem. 2016;210:402‐414.
- Xue J, Davidson PM, Zhong Q. Inhibition of Escherichia coli O157:H7 and Listeria monocytognes growth in milk and cantaloupe juice by thymol nanoemulsions prepared with gelatin and lecithin. Food Control. 2016;73:1499‐1506.
- Aljabeili HS, Barakat H, Abdel-Rahman HA. Chemical composition, antibacterial and antioxidant activities of thyme essential oil (Thymus vulgaris). Food Nutri Sci. 2018;9(5):433–446.
- El-Sayed EM, Abd-Allah AR, Mansour AM. Thymol and carvacrol prevent cisplatin-induced nephrotoxicity by abrogation of oxidative stress, inflammation, and apoptosis in rats. J Biochem Mol Toxic. 2015;29:165–172.
- Yu YM, Chao TY, Chang WC, et al. Thymol reduces oxidative stress, aortic intimal thickening, and inflammation-related gene expression in hyperlipidemic rabbits. J Food Drug Anal. 2016;24(3):556–563.
- Jafarin A, Rasmi Y, Hajaghazadeh M, et al. Hepatoprotective effect of thymol against subchronic toxicity of titanium dioxide nanoparticles: biochemical and histological evidences. Environ Toxicol Pharmacol. 2018;58:29–36.
- Abedi SM, Yarmand F, Motallebnejad M, et al. Radioprotective effect of thymol against salivary glands dysfunction induced by ionizing radiation in rats. Iran J Pharm Res IJPR. 2016;15(4):861–866.
- Salehi B, Mishra AP, Shukla I, et al. Thymol, thyme, and other plant sources: health and potential uses. Phytother Res. 2018;32(9):1688–1706.
- Entedhar SR, Wadee SA, Sedeeq BI, et al. Biochemical and histological evaluation of indomethacin-induced hepatotoxicity in rats. Sci Trans Med. 2019;12(109):23–35.[.
- Marin LD, Sanchez-Borzone M, Garcia DA. Comparative antioxidant properties of some GABAergic phenols and related compounds, determined for homogeneous and membrane systems. Med Chem. 2011;7(4):317–324.
- La Du BN, Eckerson HW. The polymorphic paraoxonase/arylesterase isozymes of human serum. Fed Proc. 1984;43:2338–2341.
- Chu SY, Cashion P, Jiang M. A new colorimetric assay for purine nucleoside phosphorylase. Clin Biochem. 1989;22:357–362.
- Porembska Z, Kedra M. Early diagnosis of myocardial infarction by arginase activity determination. Clin Chim Acta. 1975;60:355–361.
- Knipp M, Vašák M. A colorimetric 96-well microtiter plate assay for the determination of enzymatically formed citrulline. Anal Biochem. 2000;286:257–264.
- Reddy GK, Enwemeka CS. A simplified method for the analysis of hydroxyproline in biological tissues. Clin Biochem. 1996;29:225–229.
- Magwere T, Naiks YS, Hasler JA. Effects of chloroquine treatment on antioxidant enzymes in rat liver and kidney. Free Radic Biol Med. 1997;22:321–327.
- Clairborne A. Catalase activity. In: Greewald AR, editor. Handbook of methods for oxygen radical research. Boca Raton: CRC Press; 1995. p. 237–242.
- Rotruck JT, Pope AL, Ganther HE, et al. Selenium: biochemical role as a component of glutathione peroxidase. Science. 1973;179:588–590.
- Jollow DJ, Michelle JR, Zampaglione N, et al. Bromobenzene induced liver necrosis protective role of GSH and evidence for 3,4-Bromobenzene oxideas the Hepatotoxic metabolite. Pharmacol. 1974;11:151–169.
- Habig WH, Pabst MJ, Jakoby WB. Glutathione S-transferase. The first enzymatic step in mercapturic acid formation. J Biol Chem. 1974;249(22):7130–7139.
- Nakamura T, Ohta Y, Ikeno K, et al. Protective effect of repeatedly pre-administered Brazilian Propolis ethanol extract against stress-induced gastric mucosal lesions in rats. J Evid Based Complementary Altern Med. 2014;2014:383482.
- Popovic D, Kocic G, Katic V, et al. Protective effects of anthocyanins from bilberry extract in rats exposed to nephrotoxic effects of carbon tetrachloride. Chem Biol Interact. 2019;304:61–72.
- Erel O. A novel automated direct measurement method for total antioxidant capacity using a new generation, more stable ABTS radical cation. Clin Biochem. 2004;37:277–285.
- Erel O. A new automated colorimetric method for measuring total oxidant status. Clin Biochem. 2005;38:1103–1111.
- Granell S, Gironella M, Bulbena O, et al. Heparin mobilizes xanthine oxidase and induces lung inflammation in acute pancreatitis. Crit Care Med. 2003;31:525–530.
- Li WF, Hao DJ, Fan T, et al. Protective effect of chelerythrine against ethanol-induced gastric ulcer in mice. Chem Biol Interact. 2014;208:18–27.
- Eljafari MM, Dugani AM, Treesh SA, et al. The effect of tamoxifen on indomethacin induced hepato-nephrotoxicity in rats. J Clin Exp Pharmacol. 2020;10:269.
- Karimi-Khouzani O, Heidarian E, Amini SA, et al. Anti-inflammatory and ameliorative effects of gallic acid on fluoxetine-induced oxidative stress and liver damage in rats. Pharmacol Rep. 2017;69:830–835.
- Adedara IA, Anao OO, Forcado GE, et al. Low doses of multi-walled carbon nanotubes elicit hepatotoxicity in rats with markers of oxidative stress and induction of pro-inflammatory cytokines. Biochem Biophys Res Commun. 2018;503(4):3167–3173.
- Gounden V, Vashisht R, Jialal I. Hypoalbuminemia. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2020 http://www.ncbi.nlm.nih.gov/books/NBK526080/. PMID:30252336.
- McGrowder DA, Miller F, Anderson CM, et al. Abnormal liver biochemistry tests and acute liver injury in COVID-19 patients: current evidence and potential pathogenesis. Diseases. 2021;9:50.
- Luzzatto L, Arese P, Longo DL. Favism and glucose-6-phosphate dehydrogenase deficiency. N Engl J Med. 2018;378:60–71.
- Spanaki C, Plaitakis A. The role of glutamate dehydrogenase in mammalian ammonia metabolism. Neurotox Res. 2012;21(1):117–127.
- Li W, Chen S, Mei Z, et al. Polymorphisms in sorbitol-aldose reductase (Polyol) pathway genes and their influence on risk of diabetic retinopathy among Han Chinese. Med Sci Monit. 2019;25:7073.
- Fasullo M, Endres L. Nucleotide salvage deficiencies, DNA damage and neurodegeneration. Int J Mol Sci. 2015;16(5):9431–9449.
- Entedhar SR, Wadi SA, Mahmood AR. Effect of ethanolic extraction of moringa oleifera on paraoxonase and arylesterase enzyme activity in high fat diet-induced obesity in rats. Res J Pharm Tech. 2018;11(10):4601–4604.
- Gabr SA, Alghadir AH, Sherif YE, et al. Hydroxyproline as a biomarker in liver disease. In: Preedy VR, editor. Biomarkers in liver disease. Biomarkers in disease: methods, discoveries and applications. Dordrecht: Springer; 2016. p. 1–21.
- Tijani AS, Olori OD, Farombi EO. Manganese inhibits indomethacin-induced hepatorenal oxidative stress in Wistar rats. Int J Biochem Res Rev. 2020;29(9):79–90.
- Lucky LN, Raphael ET. Hot aqueous leaf extract of Lasianthera Africana (Icacinaceae) attenuates rifampicin-isoniazid-induced hepatotoxicity. J Integr Med. 2018;16(4):263–272.
- Akinlolu AA, Ghazali KO, Ameen OM, et al. Spondias mombin promotes gastric mucosa and lipid profile status in gastric ulceration. J Morphol Sci. 2014;31(2):82–88.
- Wadi SA, Entedhar SR, Sedeeq BI, et al. Some Biochemical and histological changes of rats kidneys (males) post indomethacin administration. Sci Adv. 2020;15(15):22–33.
- Gunata M, Parlakpinar H. A review of myocardial ischaemia/reperfusion injury: pathophysiology, experimental models, biomarkers, genetics and pharmacological treatment. Cell Biochem Funct. 2020;39(4):1–28.
- Hussain Z, Khan JA, Arshad A, et al. Protective effects of Cinnamomum zeylanicum L.(Darchini) in Acetaminophen-induced oxidative stress, hepatotoxicity and nephrotoxicity in mouse model. Biomed Pharmacother. 2019;109:2285–2292.
- Kapucu A. Crocin ameliorates oxidative stress and suppresses renal damage in streptozotocin induced diabetic male rats. Biotech Histochem. 2021;96:153–160.
- Shu R, Wang C, Meng Q, et al. Resveratrol enhances the protective effects of JBP485 against indomethacin-induced rat intestinal damage in vivo and vitro through up-regulating oligopeptide transporter 1 (Pept1). Biomed Pharmacother. 2019:251–261. DOI:10.1016/j.biopha.2018.12.084.
- Abdel-Daim MM, Ahmed A, Ijaz H, et al. Influence of Spirulina platensis and ascorbic acid on amikacin-induced nephrotoxicity in rabbits. Environ Sci Pollut Res Int. 2019;26:8080–8086.
- Zhang L, Wang X, Cueto R, et al. Biochemical basis and metabolic interplay of redox regulation. Redox Biol. 2019;26:101284.
- Nagappan AS, Varghese J, Pranesh GT, et al. Indomethacin inhibits activation of endothelial nitric oxide synthase in the rat kidney: possible role of this effect in the pathogenesis of indomethacin-induced renal damage. Chem Biol Interact. 2014;221:77–87.
- Ozcan A, Ogun M. Biochemistry of reactive oxygen and nitrogen species. In: Gowder SJT, editor. Basic principles and clinical significance of oxidative stress. London: InTech; 2015. p. 37–58.
- Zahran R, Ghozy A, Elkholy SS, et al. Combination therapy with melatonin, stem cells and extracellular vesicles is effective in limiting renal ischemia–reperfusion injury in a rat model. Int J Urol. 2020;27(11):1039–1049.
- Soylu KO, Pinar N, Özcan O, et al. Protective effect of alpha-lipoic acid in methotrexate-induced ovarian oxidative injury and decreased ovarian reserve in rats. Gynecol Endocrinol. 2017;33(8):653–659.
- Geyikoglu F, Yilmaz EG, Erol HS, et al. Hepatoprotective role of thymol in drug-induced gastric ulcer model. Ann Hepatol. 2018;17(6):980–991.
- El-Boshy M, BaSalamah MA, Ahmad J, et al. Vitamin D protects against oxidative stress, inflammation and hepatorenal damage induced by acute paracetamol toxicity in rat. Free Radic Biol Med. 2019;141:310–321.
- Liang J, Dou Y, Wu X, et al. Prophylactic efficacy of patchoulene epoxide against ethanol-induced gastric ulcer in rats: influence on oxidative stress, inflammation and apoptosis. Chem Biol Interact. 2018;283:30–37.
- Fan TF, Wu TF, Bu LL, et al. Dihydromyricetin promotes autophagy and apoptosis through ROS-STAT3 signaling in head and neck squamous cell carcinoma. Oncotarget. 2016;7(37):59691–59703.
- Chen P, Chen C, Hu M, et al. S-allyl-L-cysteine protects hepatocytes from indomethacin-induced apoptosis by attenuating endoplasmic reticulum stress. FEBS Open Bio. 2020;10(9):1900–1911.