ABSTRACT
Use of chemical insecticide with natural enemies could be more effective in mosquito control strategy. This study examined the toxicity of chlorpyrifos insecticide against three field-collected mosquito species, Culex pipiens, Anopheles pharoensis and Ochlerotatus caspius and their associated predators dragonfly (Pantala flavescens) and mayfly (Caenis stephens) naiads as non- target insect for chlorpyrifos. The predation potential of Pa. flavescens and Ca. stephens against tested mosquito larvae was also investigated. Additional biochemical assays were carried out to detect the effect of chlorpyrifos on some detoxifying enzymes of tested mosquito larvae and their associated predators. The results showed that (Pa. flavescens) have higher predation potential than (Ca. stephens). The toxicological results recorded high toxic effect of chlorpyrifos on Ca. stephens followed by An. pharoensis and Oc. caspius with percent mortality 100, 90 and 85% respectively, while Pa. flavescens exhibited high resistance followed by Cx. pipiens with percent mortality 20 and 40% respectively. Moreover, Acetylcholinesterase and glutathione S-transferase were significantly increased only in Cx. pipiens and Pa. flavescens. It could be concluded that, chlorpyrifos have different toxicological effect on the tested mosquito larvae and associated predators. So, the side effect of chlorpyrifos must be taken in consideration before using it in control programs.
Introduction
Mosquitoes was deemed to be one of the most medically and economically important insects. They are predominant, and able to transmit a wide variety of human and animal diseases [Citation1,Citation2]. These diseases cause for millions of deaths yearly [Citation1,Citation3].
Mosquitoes have many natural enemies such as invertebrate predators which play important roles in reducing the mosquito larval populations. Natural predators distinguish by restricted breeding season and prolonged generation period than that of mosquito larvae, so they have a necessary role in mosquito larvae control. Odonata (dragonfly) is one of the common predators for mosquito controls. Dragonflies plays important role in mosquito control for many years ago, also considered as one of the most arthropods for to mosquito control [Citation4].
Some species of mayfly naiads (Ephemeroptera) are carnivorous, but most of the naiads feed on algae, higher plants, and organic material [Citation5]. However four North American mayfly naiads appear to be carnivorous [Citation6]. The carnivorous habit and its adaptive changes appear to have had at least three species among the four genera of North American mayflies [Citation7]. The predation efficiency of mayfly naiads in Egypt weren’t examined on mosquitoes.
Chemical insecticides still considered the most important methods for mosquito population’s depression [Citation8]. Insecticides are threatening the environment; the effect of insecticides on aquatic systems may be more common and more important than direct effects on pest [Citation9]. There are less predictable effects of insecticides in non-targets populations in the same mosquito breeding habitat. The potential effects of insecticides on non-target organisms can vary widely depending on their mode of action and insect species [Citation10].
This study investigated the predation efficacy of dragonfly and mayfly on mosquito larvae and the effect of chlorpyrifos insecticide on target mosquitoes and their non-target predators, as well as the activity of the most important detoxifying enzymes acetylcholinesterase and glutathione S-transferase of both mosquito larvae and their predators were examined.
Materials and methods
Study area
Wild populations of mosquitoes were collected from different mosquito breeding sites; brackish water, water pool, rice field and drainage water during the study from El Fayoum Governorate (a large oasis in Western Desert); El-Galaa, (29°21ʹ22.6”N 30°40ʹ50.7”E), Tameyah city, Tamiyyah district (29°28ʹ19.7”N 30°57ʹ04.1”E) and El Nazlah (29°18ʹ54.6”N 30°38ʹ33.6”E) Ibsheway district.
Larvae collection and identification
To promote mosquito larvae collection, the breeding site was examined for the presence of larvae by dipping using a 250 ml standard mosquito larvae dipper according to WHO, [Citation11) method. The physicochemical parameters of breeding sites were measured such as; salinity, total dissolved solid, pH, conductivity and temperature. The collected larvae were transferred into labeled plugged plastic jars, leaving 3 cm space for larvae breathing and every two hours plug was removed to supply the specimens with fresh air until reaching the lab [Citation11].
Larvae were inspected and identified according to [Citation12,Citation13]. The most abundant Culex pipiens, Anopheles pharoensis and Ochlerotatus caspius. Sufficient numbers of living larvae were used for predation experiment and larval bioassay. On the other side, treated larvae with chlorpyrifos were stored frozen till biochemical assays.
Collection of mosquito predators
Mosquito predators were collected from the same mosquito larval breeding habitats. The collected insects were listed and transferred to the lab in sampling jars provided with water from the same collected sites. Associated insects to mosquito larvae were identified to family and species according to Bouchard, [Citation14–16]. The two most abundant predator species were then used for the predation experiment and also bioassays and biochemical assays.
Predation potential trial
Predation efficiency of the dragonfly and mayfly naiads was investigated according to [Citation17]. Predators were provided with tested mosquito larvae and pupae as supplementary food. Four replicates of 100 larvae of each instars as well as pupae separately were provided to one dragonfly or mayfly naiads within the beakers of 500 ml capacity with only 300 ml H2O. Monitoring prey mortality was investigated by calculating and recording of live mosquito larvae and pupae at an interval of 6 and 24 h.
Toxicological studies
Bioassay for target mosquitoes and non- target predators
Chlorpyrifos, which recommended for mosquito larval control [Citation18] was used in the present investigation. Chlorpyrifos was bought from the Medical Entomology Institute, Doki; Egypt. Single-concentration (diagnostic dose 0.01 ppm) [Citation19], as conducted against the three mosquito species, as well as their predators (dragonfly and mayfly naiads). The bioassay method was according to WHO procedure [Citation20]. Four replicates with 25 from 3rd instar of each tested mosquito larvae, as well as predator naiads per replicates were put in glass beaker contained 249 ml tap water and 1 ml of the chlorpyrifos diagnostic dose. The mortality was recorded after 24 hours post treatment. Other four replicates were treated with only 1 ml ethanol as control experiment and mortality never exceeded 4%.
Biochemical experiments
Specimens preparation
Biochemical assays were conducted after 24 h after chlorpyrifos exposure to determine the activity of some detoxifying enzymes (acetylcholinesterase and glutathione S-transferase) in the tested species. Specimens were prepared by homogenization of 10 individuals in an ultrasonic homogenizer, and then the homogenates were centrifuged for 15 min in a cooling centrifuge [5°C, 8000 r.p.m). The final supernatants were kept frozen till used [Citation21].
Enzymes assay
Total proteins activity was estimated as stated by [Citation22], by using Bio-Rad protein assay, while Glutathione S-transferase [GST] activity was determined as stated by [Citation23], by using (1-chloro-2, 4dinitrobenzene] as substrate. Finally acetylcholinesterase [AChE) activity was calculated as stated by [Citation24], using acetylcholine bromide (AChBr] as substrate.
Statistical analysis
Mortality data were pooled from four replicates and figured by using Microsoft Excel (Microsoft office, 365). Predation and biochemical assay were gathered from three replicates and determined as (mean± SD & mean ± SE, respectively). The data were statistically analyzed by ANOVA using SPSS V. 20 [Citation25].
Results
Survey of mosquito species
Mosquito larvae were found in different breeding habitats; drainage water, brackish water, rice field and water pool. The results showed that the occurrence of four mosquito species in the study area: two Culicines, Culex pipiens (Linnaeus), Cx. antennatus (Becker), while Culex pipiens was the most abundant one. As well as there was one Anopheline, An. pharoensis and one Aedine, Oc. Caspius ().
Table 1. Predominance of mosquito larvae from different breeding sites in El-fayoum
Survey of associated mosquito predators
The two collected naiads; dragonfly: Pantala flavescens (Odonata: Libellulidae) and mayfly: Caenis stephens (Ephemeroptera: Caenidae), were inhibited the shallow, brackish and highly polluted water which contain high dissolved oxygen, alkaline pH, high organic materials, high dissolved solid and high conductivity. They were also associated with mosquito larvae in breeding places studied. Moreover, mosquito larval predators prefer polluted water than fresh water. In general dragonfly naiads were collected in large number than mayfly naiads ().
Predation potential
Measurement of predation potency of dragonfly and mayfly naiads on mosquito immature stages of three tested species, (Cx. pipiens. An. pharoensis and Oc. caspius) indicates that, dragonfly naiads have greater predation efficiency than mayfly naiads. The result showed also that tested predators appeared to like better the youngest larval instars of mosquito ().
Table 2. Predatory potency of caenis stephens (mayfly) naiads over the mosquito larvae and pupae of three mosquito species after 24 h
Table 3. Predatory potency of pantala flavescens (dragonfly) naiads over the mosquito larvae and pupae of three mosquito species after 6 hrs
Table 4. Predatory potency of pantala flavescens (dragonfly) naiads over the mosquito larvae and pupae of three mosquito species after 24 h
The results indicate that, mayfly naiads prefer mainly An. pharoensis larvae followed by Oc. caspius, while Cx. pipiens was showed the least predation rate. There wasn’t any predation of mayfly naiads at the first 6 hours on all instars of tested mosquitoes predator potency equal zero (didn’t be represented in table), while at 24 hours the maximum predation of mayfly naiads was on the 1st instar larvae only (18, 15.3 & 13.5 for An. pharoensis, Oc. caspius Cx. pipiens, respectively) followed by the 2nd and 3rd larval instar (11.8 & 8.5) and (12 & 9.3), (11.5& 7.8), for An. pharoensis, Oc. Caspius and Cx. pipiens, respectively. The minimum predation was on the 4th larval instar (1, 2 & 0, for An. pharoensis and Oc. Caspius and Cx. pipiens, respectively, while there wasn’t any predation in pupal stage of the three tested mosquito species preys ().
The predation rate of dragonfly naiads showed that naiads prefer mainly Cx. pipiens followed by An. pharoensis then Oc. Caspius at 6 h, while at 24 h An. pharoensis recorded the higher predation rate. The feeding data of dragonfly after 6 h (), showed that, the 3rd, 2nd and 1st recorded the highest predation ratio (7.8, 9.5 &6), (8, 7 & 8) and (6.8, 3.5 & 4) for Cx. pipiens, An. pharoensis and Oc. caspius, respectively. On the other hand, the 4th larval instar was the lowest favorable prey recorded (3.5, 3.5 and 5.8,) for Cx. pipiens, An. pharoensis and Oc. caspius, respectively. Again there wasn’t any predation in pupal stage of three mosquito species. The results also indicate that, at 24 hours the maximum predation of dragonfly naiads was on the 3rd followed by 2nd then 1st larval instar (50, 37.8 &32.5), (46.8, 42 & 40.3) and (39.5, 30.3 & 33.3) for Cx. pipiens, An. pharoensis and Oc. caspius, respectively. Again the 4th recorded the lowest predation ratio followed by pupal stage (17.5 & 9.8), (21 & 6.5) and (14.5 &8.3) for Cx. pipiens. An. pharoensis and Oc. caspius, respectively ().
Susceptibility of mosquito larvae and associated aquatic predators to chlorpyrifos insecticides
Data of tested of mosquito larval species; Cx. pipiens. An. pharoensis and Oc. caspius as target for chlorpyrifos in showed that An. pharoensis larvae were more susceptible to chlorpyrifos diagnostic dose with percent mortality + SE (90%+ 2), followed by Oc. caspius with percent mortality + SE (85% + 2). While Cx. pipiens larvae have high resistance to chlorpyrifos with percent mortality + SE (40% + 1.8).
Figure 1. Toxicity of the chlorpyrifos insecticide against mosquito larvae and associated aquatic predators.
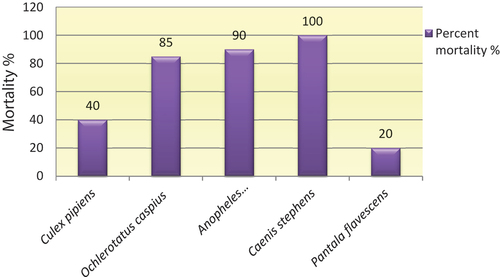
Whereas susceptibility to chlorpyrifos in its non-target predators varied depending on the species (). Caenis stephens (mayfly naiads) showed high susceptibility to chlorpyrifos with percent mortality 100%, while Pa. flavescens (dragonfly naiads) recorded high resistance to chlorpyrifos with percent mortality + SE (20% +1).
Biochemical assay
In order to realize the base on which resistance of the mosquito larvae and their tested natural enemies to chlorpyrifos and activities of two detoxified enzymes involved in resistance mechanism; acetylcholinesterase (main target sit of chlorpyrifos) and glutathione S-transferase were evaluated. The comparison of the two enzymes levels between treated and untreated (control) populations were investigated. The total protein was measured in all tested species; the values were used to standardize the activity of AChE and GST enzymes. Caenis stephens showed mortality 100%, so the biochemical assay was carried only for the other tested species (Cx. pipiens, Oc. caspius, An. pharoensis and Pa. flavescens). The results of AChE and GST were represented in . In general, there is great elevation in the levels of AChE than those of GST after treatment of chlorpyrifos as compare with control ones. Represented data showed that, there is significant increase in the activity of AChE in Cx. pipiens larvae and Pa. flavescens naiads after treatment with chlorpyrifos (6.1 ± 0.15 & 5.4 ± 0. 3 μg AChBr/min/mg protein, respectively), as compared to control which recorded (2.94 ± 0.11 & 3.6 ± 0.1 μg AChBr/min/mg protein, respectively). While there is no significant increase in the levels of AChE in Oc. caspius, An. pharoensis after treatment with chlorpyrifos (2.2 ± 0.2 & 1.3 ± 0.15 μg AChBr/min/mg protein, respectively) in compared with control which recorded (1.38 ± 0.1 & 0.8 ± 0.1 μg AChBr/min/mg protein, respectively) ().
Table 5. Effect of chlorpyrifos on aChE level in mosquito larvae and associated aquatic predators
Table 6. Effect of chlorpyrifos on GST level in target and non-target species
There is a significant increase in the levels of GST (), in Cx. pipiens larvae and Pa. flavescens naiads after treatment with chlorpyrifos (3 ± 0.11 & 3.62 ± 0.1 mmol sub. conjugated/min/mg protein, respectively), as compared to control which recorded (2 ± 0.12 & 2.7 ± 0.13 mmol sub. conjugated/min/mg protein, respectively), while there is no significant increase in the level of GST and in Oc. caspius, An. pharoensis after treatment with chlorpyrifos (2 ± 0.1 & 1.78 ± 0.1 mmol sub. conjugated/min/mg protein, respectively) in compared with control which recorded (1.8 ± 0.1 & 1.72 ± 0.3 mmol sub. conjugated/min/mg protein, respectively).
Discussion
The chemical insecticides considered an effective master plan in preventing the mosquito-borne diseases. There are very little insecticides that recommended for mosquito control from WHO. Unfortunately, mosquito species have advanced resistance mechanisms to most insecticide groups [Citation18,Citation26].
The mosquito control strategy required alternative methods that are ecofriendly [Citation27]. Biological control strategies considered one of the most ecofriendly, prospective and targeting mosquito species [Citation28].
The present study detects gluttonous and intensive predation of dragonfly naiads (Pantala flavescens) on all tested mosquito species, while mayfly (Caenis stephens) recorded lower predation efficiency than dragonfly naiads, also the two tested predators preferred the youngest stages. The present results agree with [Citation29] who reported that the predation potency of dragonfly naiads caused 50 reductions in Culiseta longiareolata breading site. Odonata naiads are large that requires large space and might polyphagous and might be in efficient for mosquito larvae. The dragonfly naiads were active and strong predators for mosquito larvae, especially An. pharoensis [Citation30]. As well as mayfly naiads consider herbivores, which feeds on algae, or detritivores – robbing waste of flooded leaves and stones. A few species can prey on minute animals [Citation31]. Also [Citation32], estimated that the dragonfly naiads (Labellula spp.) prefer to consume all stages of Aedes aegypti larvae and pupae but prefer the smaller one [Citation33], reported that Pa. flavescens naiads are excellent predator and able to consume Ae. aegypti larvae in large quantities in lab conditions.
In the current study, the toxicity of chlorpyrifos insecticide was tested against three mosquitoes (Culex pipiens. Anopheles pharoensis and Ochlerotatus caspius) species as well as their predators [Caenis stephens and Pantala flavescens), the result showed that, An. pharoensis larvae were more susceptible to chlorpyrifos followed by Oc. caspius with percent mortality 90%, and 85%, respectively. On the other hand, Cx. pipiens larvae have high resistance to chlorpyrifos with percent mortality 40%. Effects of chlorpyrifos on mosquito associated predators also have been tested. The result evaluated that, chlorpyrifos is a slightly toxic to dragonfly naiads Pa. flavescens. In contrast, chlorpyrifos is a highly toxic to mayfly naiads Ca. stephens with percent mortality 100%.
The effect of chlorpyrifos in target mosquitoes agrees with [Citation34], who examined the susceptibility of Cx. quinquefasciatus larvae to chlorpyrifos, he found that Cx. quinquefasciatus larvae are more resistant to chlorpyrifos. Also, [Citation35], observed that An. gambiae s.l was fully susceptible to chlorpyrifos and fenitrothion but resistant to DDT and pyrethroids. As well as [Citation36], who evaluated the effect of chlorpyrifos on six strains of Aedes aegypti. Relative to LC50 and LC90 three strains were highly resistant to chlorpyrifos, one strain was sparingly resistant, and two strains had high susceptibility.
Effect of chlorpyrifos on mosquito predators agree with [Citation37], who observed that susceptibility to spinosad differed between predator insects; Caenis spp. was the most susceptible, while Ischnura sp. and Pa. longipennis showed low susceptibility. [Citation38], found that toxicity of most insecticides to aquatic organisms is due to share the same mode of action as insects, and also to lack proper detoxification systems. [Citation39], expected that pyrethroids high toxicity to mayfly, dragonfly naiads, followed by neonicotinoids, as well as aquatic insects are more sensitive to organophosphorus than carbamates.
To gain satisfactory explanation for effect of chlorpyrifos on mosquito larvae and its predators, a biochemical assay was investigated for two enzymes which are known to be involved in insect resistance. The enzymes are acetylcholine esterase AChE and glutathione S- transferase GST.
Chlorpyrifos is an organophosphorus insecticide, which inhibiting the breakdown of acetylcholine. When insects are exposed, chlorpyrifos interacts to the active site of the AChE, which inhibiting breakdown of acetylcholine in the synapse [Citation40]. Elevation of AChE in resistant insects results in a decreased sensitivity [Citation41].
Glutathione S-transferase (GST) which is dimeric multifunctional enzymes involved in detoxification of a large range of xenobiotics. Elevated GST activity leads to increase resistance of insect to different classes of insecticides [Citation42].
The results of biochemical assays detected high elevation in the activity of AChE and GST especially in Cx. pipiens and Pa. flavescens is supported the increase chlorpyrifos resistance in tested population.
The results agree with [Citation43], who suggested that occurrence of OP have great risk to all aquatic environments. Inhibition (AChE) is a specific target site to both OP and carbamate insecticides, like OP pesticide chlorpyrifos, aldicarb also affects acetylcholinesterase activity [Citation44]. As well as, GST are most important detoxifying enzymes [Citation45], so the mode of action of insecticides is responsible the toxicity degree to all organisms. Aquatic insects usually have high susceptibility to most types of insecticides because they have many physiological features share with the target insects [Citation39].
Conclusions
t could be concluded that, dragonfly naiads have high predation efficiency against all tested mosquito species, and also mayfly naiads can considered as predators for mosquito larvae. Chlorpyrifos insecticide has different degrees of toxicity on non-target mosquito natural enemies. So, we must be taken in consideration the side effect of insecticides before used it in control programs.
Acknowledgments
The authors are grateful to Dr. Azza Abdelfatah Mostafa professor of pesticides, for her help and cooperation. The work was supported by the Medical Entomology Institute, Doki; Egypt.
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- Roth D, Henry B, Mak S, et al. Members of the british columbia west nile virus surveillance team. West nile virus range expansion into British Columbia. Emerg Infect Dis. 2010;16(8):1251–1258.
- Weaver SC, Reisen WK. Present and future arboviral threats. Antivir. Res. 2010;85:328–345.
- World Health Organization (2016): World malaria report 2015. World Health Organization.
- Quiroz-Martínez H, Rodríguez-Castro A. Aquatic insects as predators of mosquito larvae. J Am Mosq Control Assoc. 2007;23(sp2):110–117.
- Elliott, J. M., & Humpesch, U. H. Mayfly larvae (Ephemeroptera) of Britain and Ireland: keys and a review of their ecology. Freshwater Biological Association (FBA). 2010.
- Domínguez, E., Molineri, C., Pescador, M., Hubbard, M. D., & Nieto, C. Ephemeroptera of South-America. Aquatic Biodiversity in Latin America (ABLA) (Vol. 2). Sofia-Moscow: Pensoft. 2006.
- Sartori M, Brittain JE. Order Ephemeroptera. In: Thorp and Covich’s freshwater invertebrates. Academic Press; 2015. Ecology and General Biology Edition: FourthEditionChapter: Order EphemeropteraPublisher: Academic Press, New YorkEditors: J. Thorp, D.C. Rodgers. 2015; (pp.873–891)
- Hemingway J, Ranson H. Insecticide resistance in insect vectors of human disease. Annu Rev Entomol. 2000;45(1):371–391.
- Fleeger JW, Carman KR, Nisbet RM. Indirect effects of contaminants in aquatic ecosystems. SciTotal Environ. 2003;317(1–3):207–233.
- Wolansky MJ, Harrill JA. Neurobehavioral toxicology of pyrethroid insecticides in adult animals: a critical review. Neurotoxicol Teratol. 2008;30(2):55–78.
- World Health Organization. Guide for participants. Malaria entomology and vector control. Methods and techniques. Geneva: World Health Organization; 2012.
- Gillies MT, De Meillon B. The Anophelinae of Africa South of the Sahara (Ethiopian zoogeographical region) 2nd. J. SAM Institute. 1968:1–343. Pp.
- Harbach RE. The mosquitoes of the subgenus Culex in southwestern Asia and Egypt (Diptera: culicidae). Contrib. Amer. Ent. Inst. 1988;24:1–237.
- Bouchard, R. W. Guide to aquatic invertebrates of the Upper Midwest: identification manual for students, citizen monitors, and aquatic resource professionals. University of Minnesota, Water Resources Research Center. 2004.
- Badawy RM, El Hoseny I, Talal M. Biodiversity and seasonal fluctuation of aquatic and semiaquatic insects in Rashid stream, kafr El Zayat (Gharbyia governorate). Egyptian Academic Journal of Biological Sciences. A, Entomology. 2013;6(1):47–66.
- Fonseka TD. Dragonflies of Sri Lanka. Sri Lanka: WHT Publications; 2000.
- Singh RK, Dhiman RC, Singh SP. Laboratory studies on the predatory potential of dragon-fly nymphs on mosquito larvae. The Journal of communicable diseases. 2003;35(2):96–101.
- World Health Organization (2006): Pesticides and their applications, for the control of vectors and pests of public health importance. WHO/ CDS/ NTD/ WHOPES/ GCDPP/1.
- World Health Organization. (1981): Instructions for determining the susceptibility or resistance of mosquito larvae to insecticides. WHO/ VBC 81. 807.
- World Health Organization . Guidelines for laboratory and field testing of mosquito larvicides. World Health Organization. 2005. WHO/CDS/WHOPES/GCDPP/2005.13-39 p.
- Amin TR (1998): Biochemical and physiological studies of some insect growth regulators on the cotton leafworm, Spodoptera littoralis (Boisd.) ( Doctoral dissertation, Ph. D. thesis, Faculty of science, Cairo Univ).
- Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72(1–2):248–254.
- Habig WH, Pabst MJ, Jakoby WB. Glutathione S-transferases the first enzymatic step in mercapturic acid formation. J Biol Chem. 1974;249(22):7130–7139.
- Simpson DR, Bulland DL, Linquist DA. A semimicro technique for estimation of cholinesterase activity in boll weevils. Entomological Society of American. 1964;57:367–371.
- Barrett KC, Morgan GA, Leech NL, et al. IBM SPSS for introductory statistics: use and interpretation. Routledge; 2012.
- World Health Organization . Test procedures for insecticide resistance monitoring in malaria vector mosquitoes. Second edition, Global Malaria Programme, World Health Organization. 2013.
- Ghosh A, Chowdhury N, Chandra G. Plant extracts as potential mosquito larvicides. Indian J Med Res. 2012;135(5):581.
- Benelli G, Jeffries CL, Walker T. Biological control of mosquito vectors: past, present, and future. Insects. 2016;7(4):52.
- Stav G, Blaustein L, Margalith J. Experimental evidence for predation risk sensitive oviposition by a mosquito, Culiseta longiareolata. Ecol Entomol. 1999;24(2):202–207.
- Moirangthem BD, Singh SN, Singh DC. Comparative studies of three potent bioagent against mosquito larvae. Int. J Mosq. Res. 2018;5:10–14.
- Hadlington PW, Johnston JA. An introduction to Australian insects. UNSW Press. Kensington, N.S.W. : New South Wales University Press, 1998; viii, 116 p. : ill. (some col.); 27 cm.
- Alahmed AM, Alamr SA, Kheir SM. Seasonal activity and predatory efficacy of the water bug Sigara hoggarica poisson (Hemiptera: corixidae) against the mosquito larvae Culex quinquefasciatus (Diptera: culicidae) in Riyadh City, Saudi Arabia. Journal of Entomology. 2009;6(2):90–95.
- Samanmali C, Udayanga L, Ranathunge T, et al., Larvicidal potential of five selected dragonfly nymphs in Sri Lanka over aedes aegypti (Linnaeus) larvae under laboratory settings. BioMed research international. 2018;2018:1–10.
- Saha P (2003): effectiveness and residual action of five selected organophophate larvicides against third instar larvae of Cx. Quinquefasciatus. Unpublished M. Sc. Thesis. Dept. Zool. Jahangirnagar University, Savar Dhaka Bangladesh. 84 pp.
- Ahadji-Dabla KM, Amoudji AD, Dery DB, et al. Susceptibility to carbamate and organophosphate, and ace-1 allele in Anopheles gambiae sl from pyrethroid resistance areas in the city of Lomé, Togo, West Africa. Int. J. Biol. Med. Res. 2017;6:5843–5847.
- Lopez B, Ponce G, Gonzalez JA, et al. Susceptibility to chlorpyrifos in pyrethroid-resistant populations of Aedes aegypti (Diptera: culicidae) from Mexico. J Med Entomol. 2014;51(3):644–649.
- Jones OM (2012). The effects of spinosad on Culex quinquefasciatus (Diptera: culicidae) and non-target insect species, LSU digital commons, LSU Master’s Theses. 2619.
- Walker CH. Organic pollutants: an ecotoxicological perspective. CRC press.;2008.
- Sanchez-Bayo FP. Insecticides mode of action in relation to their toxicity to non-target organisms. Journal of Environmental & Analytical Toxicology. 2012;S(4):S4-002.
- Karanth S, Pope C. Carboxylesterase and A-esterase activities during maturation and aging: relationship to the toxicity of chlorpyrifos and parathion in rats. Toxicol Sci. 2000;58(2):282–289.
- El- Kady GAE, Kamel NH, Mosleh YY, et al. Comparative toxicity of two bio-insecticides (spinotoram and vertemic) compared with methomyl against Culex pipiens and Anopheles multicolor. World J Agric Sci. 2008;4(2):198–205.
- Karunaratne P, De Silva P, Weeraratne T, et al. Insecticide resistance in mosquitoes: development, mechanisms and monitoring. Ceylon Journal of Science. 2018;47(4):299–309.
- Belden JB, Lydy MJ. Joint toxicity of chlorpyrifos and esfenvalerate to fathead minnows and midge larvae. Environ Toxicol Chem Int J. 2006;25(2):623–629.
- Chow WS. (2009). Effects of Pesticides on Biomarker Gene Expressions in Zebrafish Embryo-larvae (Doctoral dissertation, Chinese University of Hong Kong).
- Jokanović M. Biotransformation of organophosphorus compounds. Toxicology. 2001;166(3):139–160.
