ABSTRACT
Atherosclerosis is a chronic inflammatory disease of the inner wall of large- and medium-sized arteries. The histopathological lesion of atherosclerosis is the atherosclerotic plaque. This study aimed to investigate the vascular protective effects of trihoney against the development and progression of atherosclerotic plaques. Thirty male New Zealand white (NZW) rabbits were grouped into six groups, comprising five rabbits per group as follows: normal diet (C), normal diet with 0.6 g/kg/day of trihoney (C + H), 1% cholesterol diet (HCD), 1% cholesterol diet with 0.3 g/kg/day of trihoney (HCD+H1), 1% cholesterol diet with 0.6 g/kg/day of trihoney (HCD+H2) and 1% cholesterol diet with 2 mg/kg/day of atorvastatin (HCD+At). Animals were sacrificed after 12 weeks of treatment. Atherosclerotic plaques were quantified, measured and examined using Sudan IV, Hematoxylin and Eosin (H&E), Verhoeff-van Gieson (VGS) and Masson’s trichrome staining techniques. Trihoney resulted in a significant (p < 0.05) reduction in the number of atherosclerotic lesions, tunica intima thickness, and tunica intima/tunica media thickness ratio. Trihoney preserved the orientation of medial elastic tissues and exhibited a plaque stability effect in the form of subendothelial smooth muscle cells (SMCs) recruitment and reduction of collagen deposition in tunica media. Trihoney could be recommended as a protective natural product and a future adjuvant to statins for the management of atherosclerotic plaques.
GRAPHICAL ABSTRACT
KEYWORDS:
Introduction
Atherosclerosis is defined as a chronic inflammatory disease of the inner wall of large- and medium-sized arteries [Citation1]. This condition may lead to several vascular complications such as heart attack, acute coronary syndrome (ACS), stroke, peripheral arterial disease, or even death [Citation2,Citation3]. The earliest histopathological lesion of atherosclerosis is the fatty streaks which further progresses to atherosclerotic plaque in the vascular wall at predilection points such as arterial branching sites where the laminar blood flow is disturbed [Citation4,Citation5].
Complex multiple processes of inflammation and oxidation, especially low-density lipoprotein cholesterol (LDL-c) metabolism, are implicated in the development of fatty streaks [Citation6]. The possibility of vascular complications in the setting of atherosclerosis largely depends on plaque characteristics [Citation7,Citation8]. Further, the consequences of the complications are determined by various histological characteristics of the plaque, which include the severity of vascular narrowing, lipid core, fibrosis, hemorrhage, calcification, and fibrous cap rupture [Citation9]. The most fatal complication of atherosclerotic plaque is its rupture and luminal thrombosis – a common mechanism responsible for ACS and sudden death [Citation10]. Plaque rupture is attributed to continued inflammation, thinning of the fibrous cap, and collagen degradation [Citation11,Citation12].
Honey has been demonstrated to possess anti-inflammatory effects [Citation13] and antiatherogenic properties [Citation14]. Honey was also proven to function as an antioxidant, by inhibiting oxidative stress in addition to its radical scavenging ability [Citation15]. Moreover, honey can suppress lipid peroxidation [Citation16] and reduce malondialdehyde (MDA) concentration [Citation17,Citation18].
Trihoney is a combination of trigona, mellifera, and dorsata honey. Trigona and dorsata honey are local Malaysian honey with proven high antioxidant capacities. People may not tolerate trigona due to its sour taste. Nevertheless, it has a very high phenolic content which makes it a potent antioxidant. Dorsata (Tualang) honey was also proven to have a profound antioxidant function. Meanwhile, mellifera honey has lower moisture than dorsata honey. In the current study, the combined ratio was 45, 15, and 10 of the mentioned honey, respectively. The ratio was determined by Design Expert Version 6.0 software and response surface methodology (RSM) for a combination formula having the maximum total phenolic content (TPC) [Citation19]. Moreover, trihoney contains high concentrations of phenolic compounds such as quercetin, kaempferol, rutin, catechin, maleic acid, caffeic acid, cinnamic acid, and coumaric acid, gallic acid, p-hydroxybenzoic acid, salicylic acid, sinapic acid, and vanillic acid. In addition, it has high antioxidant properties such as ferric reducing ability of plasma analysis (FRAP) and DPPH free radical scavenging activity analysis [Citation20].
Interestingly, trihoney in a previous work depicted cardiovascular protective functions in the form of hypocholesterolemic effects and suppressing adhesion molecules namely: intercellular adhesion molecule-1 (ICAM-1) and vascular cell adhesion molecule-1 (VCAM-1) [Citation19]. Likewise, trihoney exhibited anti-inflammatory effects against pro-atherogenic cytokines such as interleukin-1β (IL-1β), interleukin-6 (IL-6), and tumor necrosis factor-α (TNF-α) [Citation21]. Furthermore, trihoney expressed antioxidative stress function via the suppression of lipid peroxidation [malondialdehyde (MDA)], oxidized low-density lipoprotein (Ox-LDL), and enhancement of antioxidant enzyme activity both systemically and locally in the aortic wall [Citation22]. The present study attempts to investigate the protective function of trihoney against atherosclerotic plaques. In this work, a histopathological study involving implementation of special staining techniques was conducted in order to examine the vascular protective function of trihoney against atherosclerotic lesions.
Materials and methods
Chemicals and reagents
Pure cholesterol powder Nacalai-Tesque (Kyoto, Japan). Cholesterol-free extra virgin coconut oil (Philippines). Sudan IV 25 g (Sigma Aldrich, USA), Masson’s Trichrome (MT) stain and Verhoeffi’s staining kit (Clin-Tech, UK). Other solvents, chemicals, and hematoxylin and eosin (H&E) stain were supplied by Sigma Aldrich (USA) and Leica Biosystems (Germany).
Trihoney and atorvastatin
Trihoney is a product manufactured by the Department of Nutrition Sciences, Kulliyyah of Allied Health Sciences, International Islamic University Malaysia (IIUM). It is a combination of three types of natural honey: trigona, mellifera, and dorsata, at a ratio of 45%, 15%, and 10% respectively. Trihoney was administered orally in two doses (0.3 g/kg/day and 0.6 g/kg/day) to the respective animal groups. The doses were calculated based on human and rabbit Km factors [Citation23]. Atorvastatin 40 mg film-coated tablets (Prague-Czech) were crushed into a fine powder, reconstituted in 1 mL of distilled water and given by oral gavage using a clean syringe [Citation24–26], at a dose of 2 mg/kg body weight.
Preparation of a 1% cholesterol diet
Preparation of a 1% cholesterol diet was performed according to Alfarisi et al. [2020, Citation20] as follows: 40 g of pure cholesterol powder (Nacalai-Tesque, Kyoto, Japan) was emulsified in 80 mL (= 80 g) of cholesterol-free extra virgin coconut oil (a product from the Philippines). The cholesterol emulsion was evenly poured over 3,880 g of standard rabbit pellets (Perternakan Hong Lee Sdn. Bhd, Malaysia). The prepared food (1% cholesterol, 2% coconut oil rabbit pellet) was then packed in zipped bags and kept at a temperature of 20 to 22°C for use.
Animal
Thirty NZW rabbits of male gender were purchased from a certified experimental animal supplier (A Sapphire Enterprise, Seri Kembangan, Selangor, Malaysia), with animal weights that ranged from 2 to 2.5 kg, and animals aged 20 weeks. The animals were randomly housed in stainless-steel cages designed for rabbits as a single rabbit per cage with free access to water and standard rabbits’ pellet. In addition to the standard animal care, A housing condition of 12 hours dark/light cycle, the temperature of 15 to 21°C, and humidity of 45 to 65%. Animals were acclimatized for two weeks. All animals in the control and treatment groups had open access to a daily food amount of 160 g. The amount was estimated based on the normal reference data of NZW rabbits and our calculation for their daily food consumption during this experiment. The animal handling procedure was conducted following the guidelines of the Malaysian Code of Practice for the Care and Use of Animals for Scientific Purposes (AEPC) [Citation27]. Meanwhile, the protocol of this experiment was approved by the Institutional Animal Care and Use Committee of International Islamic University Malaysia (IACUC-IIUM) with ID approval (IIUM/IACUC- Approval /2017 [Citation19]).
At the end of 12 weeks, animals were sacrificed at the animal surgical laboratory of the Central Research and Animal Facility, International Islamic University Malaysia (CREAM)-IIUM. For humane euthanasia; deep general anesthesia was given by a combination of ketamine and xylazine through the intramuscular route [Citation28] at doses of 50 mg/kg and 10 mg/kg respectively [Citation29]. Laparotomy and sternotomy were performed for full exposure of the aorta [Citation30]. Systemic perfusion secured through left ventricular approach and infusion of ice-cold normal saline and euthanasia were achieved by exsanguination of blood through opened right atrium [Citation31,Citation32].
Experimental study
Thirty male NZW rabbits were grouped into the following 6 groups, 5 rabbits/group, n = 5:
Group 1: Normal diet (C), n = 5.
Group 2: Normal diet with trihoney dose of 0.6 g/kg/day (C + H), n = 5.
Group 3: 1% cholesterol diet (HCD), n = 5.
Group 4: 1% cholesterol diet with 0.3 g/kg/day of trihoney (HCD+H1), n = 5.
Group 5: 1% cholesterol diet with 0.6 g/kg/day of trihoney (HCD+H2), n = 5.
Group 6: 1% cholesterol diet with 2 mg/kg/day of atorvastatin (HCD+At.), n = 5.
Aorta harvesting
The aortic arch was dissected 1 mm above the aortic root, and 3 mm distal to the commencement of the left subclavian artery to achieve consistency in the sampling site [Citation33], then freed from surrounding fat and connective tissues ensuring the endothelium was not damaged. One ring measuring 4 mm of the proximal part of the aortic arch was cut from each rabbit [Citation34] and fixed in 10% neutral buffer formalin (NBF) for histopathology processing [Citation24,Citation35]. The remaining thoracic and abdominal aorta until 1 cm distal to bifurcation into two common iliac arteries, was dissected carefully for the quantification of fatty streaks and the Sudan IV study.
Macroscopic histopathological study with Sudan IV stain
Sudan IV staining was employed according to the protocol described by Fan et al. (2001) with some modifications as follows: the pinned aorta was fixed overnight in 10% NBF at room temperature, rinsed thrice for 10 minutes each with phosphate buffer saline (PBS), and fixed for five minutes with 70% ethanol at room temperature. The aorta was then stained with Sudan IV containing solution (5 g Sudan IV, 500 mL 70% ethanol, 500 mL acetone) for 15 minutes at room temperature. Next, the aorta was rinsed twice for 3 minutes each with 70% ethanol at room temperature, followed by rinsing for five minutes with PBS for the removal of ethanol. The dish was then filled with PBS until the stained aorta and pins were covered. Images of the whole stained aorta were taken with a digital camera [Citation30,Citation32]. The quantification of the atherosclerotic lesions was performed using a computerized image analysis system (Image J software 1.52a National Institute of Health USA). The system measured the sudanophilic lesions areas relative to the total photographed surface area of the aorta and expressed as a percentage of the whole aorta [Citation35] according to the following formula: [Percentage (%) lesion area = (Area of the lesion /Total aortic area) ×100%].
Microscopic histopathological study
Hematoxylin and Eosin (H&E), Verhoeff-van Gieson (VGS), and Masson’s trichrome staining were performed according to the manufacturer’s protocols. Verhoeff-van Gieson staining was employed to demonstrate the junction between the tunica intima and tunica media of the aorta to visualize thinning, disruption or loss of elastic fibers during atherosclerosis [Citation36]. Meanwhile, Masson’s trichrome staining was employed in examining the collagen deposition within the aortic wall [Citation37] and assessing the fibrous cap cellular components on top of atherosclerotic plaques [Citation38].
Measurement of tunica intima, tunica media thickness, and histopathological description of atherosclerotic plaque
After the digital scanning of aortic cross-sections using Leica Aperio CS2 digital scanner, histological changes were documented and the thickness of tunica intima, tunica media, and atherosclerotic plaques was measured using Aperio Image Scope V12.2.2.5015 software. The measurements were taken from the thickest and most well-visualized areas [Citation39].
Statistical analysis
The Statistical Package for Social Sciences (SPSS version 21 Chicago, Illinois, USA) software was used for the data processing and analysis. Data were expressed as mean (M) and standard deviation (SD) and analyzed using a one-way analysis of variance (ANOVA), followed by a post hoc test in determining if any significant differences existed between two or more independent groups. Statistical significance was considered at p < 0.05. Correlations were investigated using Pearson’s correlation coefficients (r).
Results
Effect of trihoney on the number of atherosclerotic lesions and correlation with the serum lipid profile and oxidized low-density lipoprotein
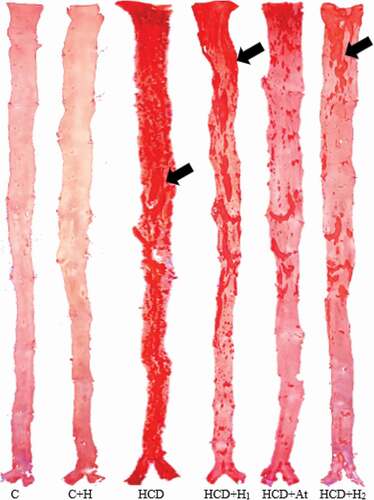
depicts the percentage of atherosclerotic lesions in the aorta of various experimental animal groups. The control group demonstrated extremely few atherosclerotic lesions and the control group that received trihoney revealed no atherosclerotic lesions. However, the high cholesterol diet group exhibited an extensive and significant (p < 0.05) increase in atherosclerotic lesions compared to the control groups. Atherogenic groups that received trihoney exhibited a significant (p < 0.05) reduction in the atherosclerotic lesions in comparison to the high cholesterol diet group. The effect of trihoney was comparable to that of atorvastatin. illustrates the differences in sudanophilic percentage areas between various experimental groups. Furthermore, a significant positive correlation was recorded between the percentage of atherosclerotic lesions in the aorta and serum total cholesterol (TC), low-density lipoprotein cholesterol (LDL-c), oxidized low-density lipoprotein (Ox-LDL), and cardiovascular disease risk ratio (TC/HDL) as demonstrated in .
Table 1. Comparison of percentage of atherosclerotic aortic lesions.
Table 2. Correlation (r) between aortic lesion area percentage, serum lipid profile and oxidized low-density lipoprotein.
Figure 1. Effect of trihoney on quantity of atherosclerotic plaques.
Effects of trihoney on the thickness of tunica intima and tunica intima/tunica media thickness ratio and correlation with the lipid profile
Experimental groups that received trihoney revealed a significant (p < 0.05) less tunica intima thickness and a significant (p < 0.05) reduction in tunica intima/tunica media thickness ratio compared to the high cholesterol diet group. The effects of treatment with trihoney were highly comparable to that with atorvastatin (). A significant positive correlation was found between serum lipid profile, atherosclerotic plaque thickness, tunica intima/tunica media thickness ratio, and cardiovascular risk ratio as displayed in . A photomicrograph of a representative section of the aorta from each group stained with H&E stain is presented in .
Table 3. Comparison of atherosclerotic plaque thickness, and tunica intima/Tunica media thickness ratio.
Table 4. Correlation (r) between atherosclerotic plaque thickness, tunica intima/Tunica media thickness ratio and serum lipid profile.
Effects of trihoney and 1% cholesterol diet on the histology of aortic wall
Hematoxylin and eosin study
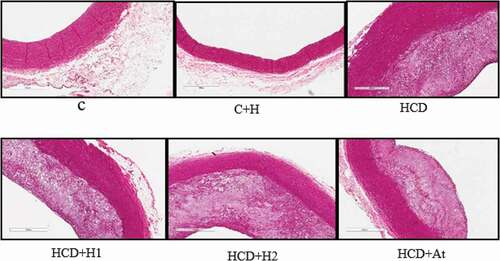
Microscopic examination of the intimal lesions in the high cholesterol diet group () revealed massive intimal lesions caused by circumferential thickening of intima with 30% occlusion, sheets of foamy macrophages, layers of smooth muscle cells (SMCs), foamy smooth muscle cells (FSMCs), massive extracellular fat deposition but no lipid core was seen. According to the American Heart Association (AHA) classification of atherosclerotic plaques, this atherosclerotic lesion is classified as type III. The experimental group that received trihoney at a higher dose exhibited more recruitment of SMCs in the subendothelial layer compared to the atherogenic groups (HCD+H1 and HCD+At). This finding is a feature of type II atherosclerotic lesions according to AHA classification. In the high cholesterol diet group, SMCs recruited more in the deeper part of the intimal atherosclerotic lesion, which is specific for type III lesions. Additionally, the group that received trihoney at a higher dose had a clear demarcation between intima and media maintained by the internal elastic lamina. This demarcation is more obvious in the treatment groups than in the high cholesterol diet group.
Masson’s trichrome and Verhoeff-van Gieson staining
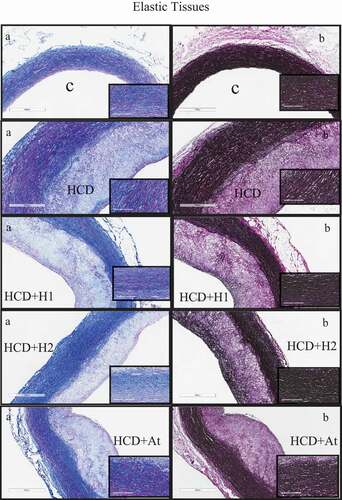
Masson’s trichrome staining of aortic sections visualized the collagen contents of all stained sections as blue color, while SMCs were stained red color. Atherosclerotic plaques of the trihoney treated group (HCD+H2) had less collagen and more elastin condensed in the upper part of the atherosclerotic plaque. Sections of the control group that were stained by VGS () showed clear concentric compacted elastic tissue layers (black in color) in tunica media and continuous internal and external elastic laminae. In this normal group, bundles of collagen and SMCs were sandwiched between the elastin layers in tunica media. No elastic tissue fragmentation was found. In contrast, VGS sections from the high cholesterol diet group () presented a fragmentation of internal and external elastic laminae as well as the disrupted orientation of elastic layers in tunica media, especially at the thickest points of the plaques. Trihoney treated animals () had concentric compact layers of elastic tissues in tunica media, in addition to well retained continuous internal and external elastic laminae. Trihoney treated group that received a higher dose showed condensed elastic tissues in the subendothelial layer, which was not visualized in other atherogenic groups. Atorvastatin received group had compact layers of medial elastic tissues, in addition to evidence of interrupted internal elastic lamina at the thickest points of the atherosclerotic plaque.
Figure 3. Study of atherosclerotic plaque using special stains demonstrating collagen and elastic tissues.
Discussion
In this experiment, supplementation with a 1% cholesterol diet resulted in the development of extensive gross atherosclerotic lesions (Atherosclerotic plaques) and caused highly significant histopathological changes, such as an increased thickness of tunica intima due to the development of fatty streaks and changes in tunica intima/tunica media thickness ratio. These changes are consistent with previous studies [Citation35,Citation40]. The development of atherosclerotic plaques within the vascular bed is considered the macroscopic pathological hallmark of atherosclerosis [Citation41]. Moreover, it has been recognized to be proportionate to the severity of atherosclerosis and assigned the main contributor to the subsequent medical complications [Citation5].
This experiment revealed that trihoney was very effective in reducing the number of atherosclerotic plaques. Trihoney also expressed a considerable reduction effect on the thickness of the atherosclerotic plaque and the tunica intima/tunica media thickness ratio. Microscopic examination using MT and VGS staining demonstrated that trihoney treated groups were protected against fragmentation of internal and external elastic laminae, as well as evidence of more condensation of elastic tissue and recruitment of the SMCs in the upper intima compared to other atherogenic groups. Although the thickness of tunica intima due to an atherosclerotic plaque is an indicator of the severity of atherosclerosis, the prevalence of cardiovascular events is not solely associated with the degree of the stenosis or plaque volume but also with plaque instability [Citation5]. As far as plaque stability is concerned, SMCs recruitment in the atherosclerotic intima has been described as a plaque stability factor [Citation5]. This particular event was visualized following the treatment with trihoney. Elastic layers of tunica media in the trihoney treated groups were compact and comparable to that of the control group, thereby maintaining the elasticity and preserving the physiological functions of the aortic wall. Contrarily, the medial elastic layers were disrupted in the high cholesterol diet group. This finding might be due to the deposition of more collagen by SMCs (invested between medial elastic lamellae), which might induce aortic wall stiffness and impair the aortic wall physiology. These events align with the report by Tham et al. [Citation42] that an increased aortic wall stiffness occurred due to collagen deposition following angiotensin-II induced aortic wall injury.
To the best of our knowledge, this is the first study reporting the effect of the combination of natural honey on atherosclerotic plaque lesions in a hypercholesterolemic rabbit model. Atorvastatin was used in this study for comparison purposes based on its documented histological effects. Resultantly, atorvastatin depicted a reduction of plaque progression and some preservative effects on the orientation of elastic tissues despite some interruptions being noticed in the internal elastic lamina. These findings are supported by Luo et al. [Citation40] who found that atorvastatin at 2 mg/kg/day administered to hypercholesterolemic rabbits was able to reduce the atherosclerotic plaque size. In this experiment, although an atorvastatin dose of 2 mg/kg/day exerted a significant effect on the number of atherosclerotic lesions, trihoney at a dose of 0.6 g/kg/day exhibited a stronger impact. The impact of atorvastatin on the number of atherosclerotic lesions was attributed to its lipid-lowering effect and the stimulation of cholesterol efflux in macrophages [Citation40]. Recent understanding of the pathogenesis of atherosclerosis describes it as an imbalanced lipid metabolism integrated with misconducted immune response, thus leading to a chronic inflammatory process in the wall of the blood vessel [Citation1]. The development of atherosclerotic plaques is closely related to the level of serum cholesterol [Citation34]. This might explain the correlation between serum cholesterol level and thickening of tunica intima and increased intima/media thickness ratio in the pathogenesis of hypercholesterolemia. The current study reflected significant positive correlations between the percentage of aortic lesions, thickness of intimal lesions, cardiovascular risk ratio, and lipid profile. In a previous study, trihoney demonstrated hypocholesterolemic effects and suppression of adhesion molecules (ICAM-1 and VCAM-1) [Citation19]. Experimental studies revealed practical evidence of the direct relationship between IL-1β, and IL-6, as well as between TNF-α and the atherosclerotic plaque progression [Citation43]. Interestingly, trihoney had significant anti-inflammatory effects against pro-atherogenic cytokines such as IL-1β, IL-6, and TNF-α [Citation21]. Trihoney through the suppression of the pro-atherogenic inflammatory cytokines may retard the pathogenic migration of SMCs from tunica media to tunica intima. It might also alter the effects of secreted cytokines on SMCs and consequently suppress the accumulation of collagen within the tunica media while maintaining the normal medial orientation of elastic lamellae. Moreover, oxidized lipids derived from LDL are major contributors to the pathogenic process of atherosclerotic plaque development and progression [Citation10]. Trihoney had antioxidative effects against oxidative stress by suppressing lipid peroxidation and enhancement of antioxidant enzyme activity both systemically and locally in the aortic wall [Citation22]. Hence, we may propose that the underlying mechanism by which trihoney induced those beneficial effects was through lowering cholesterol and Ox-LDL and suppressing inflammatory and immunological pathogenic processes through anti-inflammatory and antioxidant functions.
Conclusion
The histological findings of this study suggest the vascular protective effects of trihoney against plaque formation and progression. Trihoney preserved the orientation of medial elastic tissues and exhibited plaque stability effect in the form of subendothelial SMCs recruitment and reduction of collagen deposition in tunica media. Trihoney might be suggested as an anti-atherosclerotic natural product that may retard the development of plaques if combined with other lipid-lowering pharmacotherapies.
Acknowledgments
Authors would like to acknowledge the histopathology laboratory at International Islamic University Malaysia Medical Centre (IIUMMC) for facilitating laboratory work.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Alfarisi HAH, Mohamed ZBH, Bin IM. Basic pathogenic mechanisms of atherosclerosis. Egypt J Basic Appl Sci. 2020;7(1):116–125.
- Hansson GK. Inflammation, atherosclerosis, and coronary artery disease. N Engl J Med. 2005;352:1685–1695.
- Tuttolomondo A, Di Raimondo D, Di Sciacca R, et al. Arterial stiffness and ischemic stroke in subjects with and without metabolic syndrome. Atherosclerosis. 2012;225(1):216–219.
- Moore KJ, Tabas I. The cellular biology of macrophages in atherosclerosis. Cell. 2011;145(3):341–355.
- Oikonomou E, Latsios G, Vogiatzi G, et al. Atherosclerotic Plaque. In: Dimitris Tousoulis, editor. Coronary artery disease. 1st ed. Athens, Greece: Elsevier Inc.; 2018. p. 31–41.
- Tomkin G. LDL as a Cause of Atherosclerosis. Open Atheroscler Thromb J. 2012;5(1)):13–21.
- Lorkowski S, Atherosclerosis: CP, Pathogenesis C. Features and Treatment. Encycl Life Sci. 2007;1–11. DOI:10.1002/9780470015902.a0004228.
- Bergheanu SC, Bodde MC, Jukema JW. Pathophysiology and treatment of atherosclerosis: current view and future perspective on lipoprotein modification treatment. Netherlands Heart J. 2017;25(4):231–242.
- Watanabe Y, Nagayama M, Suga T, et al. Characterization of atherosclerotic plaque of carotid arteries with histopathological correlation: vascular wall MR imaging vs. color Doppler ultrasonography (US). J Magn Reson Imaging. 2008;28(2):478–485.
- Sakakura K, Nakano M, Otsuka F, et al. Pathophysiology of atherosclerosis plaque progression. Heart Lung Circ. 2013;22(6):399–411.
- Libby P. The molecular mechanisms of the thrombotic complications of atherosclerosis. 2008;517–27. J Int Med. 2008;263(517–527). DOI:10.1111/j.1365-2796.2008.01965.x
- Adiguzel E, Ahmad PJ, Franco C, et al. Collagens in the progression and complications of atherosclerosis. Vasc Med. 2009;14:73–89.
- Majtanova N, Cernak M, Honey: MJ, et al. Remedy for eye diseases. Forschende Komplementarmed. 2016;23(6):1–6.
- Spilioti E, Jaakkola M, Tolonen T, et al. Phenolic acid composition, antiatherogenic and anticancer potential of honeys derived from various regions in Greece. PLoS One. 2014;9(4):1–10.
- Vallianou N, Gounari P, Skourtis A, et al. Honey and its anti-inflammatory, anti-bacterial and anti-oxidant properties. Gen Med. 2014;02(2). DOI:10.4172/2327-5146.1000132
- Abd Jalil MA, Kasmuri AR, Hadi H. Stingless bee honey, the natural wound healer: a review. Skin Pharmacol Physiol. 2017;30(2):66–75.
- Sahhugi Z, Hasenan SM, Jubri Z. Protective effects of gelam honey against oxidative damage in young and aged rats. Oxid Med Cell Longev. 2014;2014:1–8.
- Erejuwa OO, Sulaiman SA, Wahab MS, et al. Antioxidant protection of Malaysian tualang honey in pancreas of normal and streptozotocin-induced diabetic rats. Ann Endocrinol (Paris). 2010;71(4):291–296.
- Alfarisi HAS, Ibrahim M, Mohamed ZB, et al. Trihoney suppresses soluble adhesion molecules (ICAM-1 and VCAM-1 in hypercholesterolemic atherosclerotic rabbits: a comparative study with atorvastatin. Sains Malaysiana. 2020;49(6):1313–1322.
- Alfarisi HAH, Bin IM, Mohamed ZBH, et al. Hepatoprotective effects of a novel trihoney against nonalcoholic fatty liver disease: a comparative study with atorvastatin. Sci World J. 2020;2020:1–14.
- Alfarisi HAH, Bin IM, Azahari N, et al. Anti-inflammatory effects of trihoney in hypercholesterolemic atherosclerotic rabbits: a comparative study with atorvastatin. Malaysian J Med Heal Sci. 2020;16(2):230–236.
- Alfarisi HAH, Ibrahim M, Mohamed ZBH, et al. Trihoney reduces lipid peroxidation and enhances antioxidant enzyme activities in hypercholesterolaemic atherosclerotic rabbits. Int Food Res J. 2020;27(3):568–575.
- Reagan-Shaw S, Nihal M, Ahmad N. Dose translation from animal to human studies revisited. FASEB J. 2007;22(3):659–661.
- Du B, Xu G, Cao H, et al. Effects of atorvastatin on expression of ICAM-1 in atherosclerotic rabbits. J Cardiovasc Med. 2013;14(2):120–126.
- Jorge PAR, De AEA, Ozaki MR, et al. Effects of atorvastatin, fluvastatin, pravastatin, and simvastatin on endothelial function, lipid peroxidation, and aortic atherosclerosis in hypercholesterolemic rabbits. Arq Bras Cardiol. 2005;84(4):314–319.
- Song X, Liu H, Wang X, et al. Atorvastatin combined with poly-unsaturated fatty acid confers better improvement of dyslipidemia and endothelium function. Lipids Health Dis. 2014;13(1):1–5.
- Animal Ethics Policy Committee (AEPC). International Islamic University Malaysia Animal Ethics Policy. Kuantan; 2012. 14 p. Report No.:387 (21/12/2012).
- Rj de A T, de Noronha L, Torres RDRDA, et al. Increased intercellular adhesion molecule-1 immunoreactivity in the sclera-choroid complex in hypercholesterolemia experimental model. Rev Bras Oftalmol. 2014;73(4):210–215.
- Bolayirli IM, Aslan M, Balci H, et al. Effects of atorvastatin therapy on hypercholesterolemic rabbits with respect to oxidative stress, nitric oxide pathway and homocysteine. Life Sci. 2007;81(2):121–127.
- Fan J, Shimoyamada H, Sun H, et al. Transgenic rabbits expressing human apolipoprotein(a) develop more extensive atherosclerotic lesions in response to a cholesterol-rich diet. Arter Thromb Vasc Biol. 2001;21(1):88–94.
- Gourdon J. Large animal euthanasia. McGill University. Comparative Medicine & Animal Resources Centre. 2008. 2 p. Report No: 302.01.
- Mohanta S, Yin C, Weber C, et al. Aorta atherosclerosis lesion analysis in hyperlipidemic mice. Bio-Protocol. 2016;6(11):1–15.
- Li H, Cybulsky MI, Gimbrone MA, et al. An atherogenic diet rapidly induces VCAM-1, a cytokine-regulatable mononuclear leukocyte adhesion molecule, in rabbit aortic endothelium. Arterioscler Thromb. 1993;13(2):197–204.
- Yu Q, Li Y, Waqar AB, et al. Temporal and quantitative analysis of atherosclerotic lesions in diet-induced hypercholesterolemic rabbits. J Biomed Biotechnol. 2012;2012:1–7.
- Ibrahim M, Ahmed IA, Mikail MA, et al. Baccaurea angulata fruit juice reduces atherosclerotic lesions in diet-induced Hypercholesterolemic rabbits. Lipids Health Dis. 2017;16(1):1–8.
- Zhang C, Zheng H, Yu Q, et al. A practical method for quantifying atherosclerotic lesions in rabbits. J Comp Pathol. 2010;142(2–3):122–128.
- Thent ZC, Lin TS, Das S, et al. Histological changes in the heart and the proximal aorta in experimental diabetic rats fed with Piper sarmentsoum. African J Tradit Complement Altern Med. 2012;9(3):396–404.
- Phinikaridou A, Hallock KJ, Qiao Y, et al. A robust rabbit model of human atherosclerosis and atherothrombosis. J Lipid Res. 2009;50(5):787–797.
- Scholtes VPW, Johnson JL, Jenkins N, et al. Carotid atherosclerotic plaque matrix metalloproteinase-12-positive macrophage subpopulation predicts adverse outcome after endarterectomy. J Am Heart Assoc. 2012;1(6):1–12.
- Luo F, Guo Y, Ruan GY, et al. Combined use of metformin and atorvastatin attenuates atherosclerosis in rabbits fed a high-cholesterol diet. Sci Rep. 2017;7(1):1–10.
- Aziz M, Yadav K. Pathogenesis of Atherosclerosis A Review. Med Clin Rev. 2016;2(3):1–6.
- Tham DM, Martin-McNulty B, Wang Y-X, et al. Angiotensin II injures the arterial wall causing increased aortic stiffening in apolipoprotein E-deficient mice. Am J Physiol Integr Comp Physiol. 2002;283(6):R1442–9.
- Van TBW, Toldo S, Mezzaroma E, et al. Targeting interleukin-1 in heart disease. Circulation. 2014;128(22):1910–1923.