ABSTRACT
Diabetes mellitus is characterized by structural abnormalities, oxidative stress and neuroinflammation. This study aimed to determine the antioxidative therapeutic effects of peanut supplementation in improving brain damage resulted from streptozotocin (STZ)-induced diabetes. Forty male Wistar albino rats (Rattus novergicus) were categorized into four groups: control, peanut-supplemented, diabetic, diabetic and peanut-supplemented. The brain was dissected and subjected to biochemical analyses which indicated the role of diabetes in downregulating the expression of superoxide dismutase (SOD), dopamine (DA), serotonin (5-HT), adenosine triphosphate (ATP) and upregulating the levels of malondialdehyde (MDA), tumor necrosis factor-α (TNF-α), 5-lipoxygenase (5-lOX), 8-hydroxy-deoxy guanosine (8-OHdG), Amyloid-β, α-amylase and tau protein expression levels. peanut treatment enhanced the diabetic-dependent brain histopathological features in the cerebrum and hippocampus, the immunohistochemical localization indicated significant downregulation at the expression of synaptophysin and caspase 3. Peanut supplementation significantly downregulated the gene expression of BAX, SOD, Myloperoxidase (MPO), Tumor necrosis factor- α (TNF-α), peroxisome proliferator-activated receptors (PPAR-α and PPAR-γ) and upregulated glial fibrillary acidic protein (GFAP) expression. In conclusion, peanut supplementation showed therapeutic potential against brain damage induced by diabetes. Peanut treatment significantly protected the brain from diabetic-related oxidative stress, and increased dopamine and serotonin levels by restoring the redox balance.
Graphical Abstract
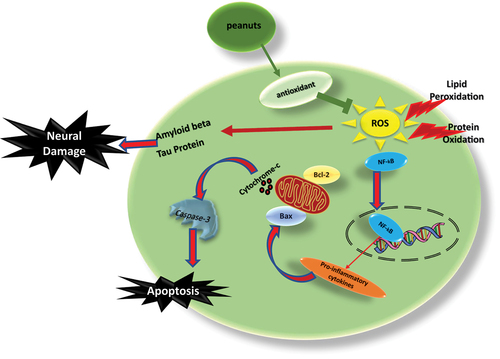
Introduction
Diabetes mellitus is a chronic metabolic disorder of hyperglycemia which results in abnormalities in carbohydrate, protein and lipid metabolism[Citation1]. It can be classified into two types according to either lack of insulin secretion (Type 1) or increased cellular tolerance to insulin (Type 2), which is considered as one of the world’s most common diseases and a leading cause of mortality [Citation2]. Moreover, diabetes has been recently proven to increase the incidence of metastasis in mouse tumor models of Lewis lung carcinoma and melanoma (B16F10) cells by improving the adhesion between cancer cells and endothelial cells which is a critical step in cancer cell evasion [Citation3].
Diabetes is also associated with microvascular complications including diabetic encephalopathy with progressive tissue damage in the central nervous system [Citation4]. This complication is characterized by impairment of cognitive functions and electrophysiological changes [Citation5]. These functional changes are accompanied by structural and neurochemical abnormalities as well as several pathological changes in the brain that have been attributed to chronic elevation in intracellular glucose concentration [Citation6].
Oxidative stress upraising is usually implicated in the development of such neurodegenerative disorders [Citation7]. Mitochondria are essential cell organelles that provide energy and regulate several cellular processes including signaling to cell apoptosis. These cellular structures are preserved by a highly effective antioxidant system against the continuous production of reactive oxygen species (ROS), such as superoxide anion or hydrogen peroxide, by the mitochondria electron transport chain [Citation8]. Increased production of ROS, such as superoxide anion or hydrogen peroxide, may disrupt cellular processes in the mitochondria. A major disruptive effect of ROS is lipid peroxidation, a process that occurs when ROS attack membrane phospholipids [Citation9].
The prevalence of unsaturated fatty acids and abundance of oxygen in the brain makes it more susceptible to lipid peroxidation. The human brain intakes 20% of the overall oxygen (O2) supply to maintain a normal adenosine triphosphate (ATP) neuronal activity [Citation10]. Insufficient O2 supply to the brain tissues causes ATP deficiency and impaired neural activity that leads to transient ischemia and neurodegeneration [Citation11]. Therefore, lipid peroxidation levels, along with protein nitration, carbonylation, and RNA and DNA oxidative damages, are among the biomarkers of oxidative stress-induced neurodegenerative disorders, cancer and ischemia [Citation11,Citation12].
A paucity of literature has been devoted to understanding deleterious mechanisms involved in diabetes and its complications. Streptozotocin (STZ) is a glucosamine-nitrosourea compound that has been widely used in many studies to induce diabetes in a variety of animals by affecting degeneration and necrosis of pancreatic β-cells [Citation13]. Rodents with STZ-induced diabetes are the most common model that has been developed to decipher the mechanisms involved in diabetes and to study potential prophylactic/therapeutic strategies for ameliorating diabetic complications.
The role of phytochemicals such as polyphenols in regulating the level of oxidative stress and inflammation has been fully illustrated [Citation14]. In the past decade, research has proven that diets rich in nuts and peanuts (Arachis hypogaea) are beneficial to human health. These benefits are brought by their unique nutritional profile, nutrient density, fatty acid profile and bioactive compounds. More than three-quarters of the fat in peanuts is unsaturated, with nearly half of them being monounsaturated, oleic acid [Citation15]. In addition to beneficial fats, peanuts are a rich source of B-vitamins, vitamin E, magnesium, copper and phosphorus [Citation16]. Furthermore, they are a source of plant protein, arginine, dietary fibers and bioactive substances (e.g. flavonoids, resveratrol and plant sterols) [Citation15,Citation16].
Peanut active antioxidant compounds can prevent oxidative damage by chelating with ionized copper and iron, thus blocking the production of highly reactive hydroxyl species [Citation17]. Therefore, peanut intake can be an effective approach to protect against inflammation, oxidation damage, and heavy metal toxicity. Peanuts can also slow down age-related neuronal degeneration by reducing oxidative stress in the neurons [Citation17]. The goal of this study was to demonstrate the therapeutic potential of peanut supplementation against brain damage resulted from STZ-induced diabetes.
Materials and Methods
Animals modeling and experimental designation
Forty male Wistar albino rats (Rattus novergicus) weighing 110–120 gm were included in the study. The trials were approved by the Faculty of Science’s Experimental Animal Ethical Committee in Mansoura University, Egypt (No. MZ 170010). The National Institute of Health’s guidelines for the use of laboratory animals were followed in this work (NIH Publication No, 8523, updated 1996). Animals were housed in an aerated environment with a light/dark cycle of roughly 12 hours and light intensity exposure at 180–200 lx. A typical diet and unlimited access to water were provided. Afterwards, the rats were divided into four groups: control, peanut-supplemented group (20% of standard diet) for one-month, diabetic group (60 mg/kg body wt. STZ and 100 mg nicotinamide/kg body weight), diabetic and peanut-supplemented group (started after 15 days of development of diabetes and continue for one-month treatment). At the end of treatment, the studied groups were fastened overnight, euthanized using chloral hydrate (300 mg/kg body weight), and sacrificed. The brain was dissected and processed for investigations.
Experimental induction of type 2 diabetes
Diabetes was induced in rats by a single intraperitoneal injection of STZ 60 mg/kg body wt. (Sigma, USA) dissolved in freshly prepared citrate buffer (0.1 M, pH 4.5) and 100 mg nicotinamide/kg body wt. Negative control rats received intraperitoneal doses of the same volume of isotonic saline solution.
Preparation of a peanut diet
Fresh peanut grains were purchased from the market, weighed, crushed and mixed into a standard diet at a 20% concentration. The diet was freshly prepared once a week and then fed to rats in the study groups.
Phytochemical Analysis
Total phenolic compounds content
The total phenolics content of different peanut extracts (water, methanol, and petroleum ether) was measured using the modified Folin Ciocalteu colorimetric assay [Citation18]. A known volume (1 ml) of peanut extract or standard solutions of gallic acid (10, 20, 40, 60, 80, 100 and 150 μg gallic acid /ml) was added to a 10 ml volumetric flask. The absorbance was measured at 765 nm. The quantity of the phenolics present in the extracts was calculated from the calibration curve and expressed as gm gallic acid equivalent/100 gm dried peanut material.
Total flavonoids content
Flavonoids content of different peanut extracts was measured using aluminum chloride colorimetric assay. A known volume (1 ml) of each extract or standard solution of catechin (20, 40, 60, 80, 100 and 200 μg catechin/ml) was added to a 10 ml volumetric flask containing 4 ml distilled water. Afterwards, 0.3 ml of 5% (w/v) NaNO2, 0.3 ml of 10% (w/v) AlCl3 and 2 ml of 1 M NaOH was added to the mixture [Citation19]. The solution was then mixed well, and the absorbance was measured at 510 nm. Total flavonoids content was expressed as g catechin equivalent/100 g peanut.
Total Alkaloids content
The alkaloids content was calculated as a mg alkaloid/100 g peanut. A volume of 50 ml of 10% acetic acid in ethanol was added to 1 g of the sample. Then, the sample was covered, allowed to stand for 4 h and filtered. Then, concentrated ammonium hydroxide was added on top of the extract till the precipitation was complete. The precipitate was then filtered and dried to a constant weight [Citation20].
Total tannins content
Tannin content was estimated using Vanillin hydrochloride assay. One gram of peanut was dissolved in 50 ml of methanol and mixed occasionally by swirling. After 20–28 h, the mixture was centrifuged, and then the supernatant was collected. Then, 5 ml of Vanillin hydrochloride reagent was added to 1 ml of the supernatant. The absorbance of the resultant color was measured at 500 nm after 20 minutes [Citation21]. The standard curve was prepared using 0–100 µg of tannic acid, and tannins content was expressed as g tannic acid equivalent/100 g of peanut.
Evaluation of the antioxidant activity of the peanut extracts
Free radical scavenging method (DPPH)
The effect of the extracts on scavenging DPPH radicals was estimated according to 20-Kitts et al. [Citation22] with slight modifications [Citation23]. Five dilutions of each sample were prepared in methanol. An aliquot of 1 ml of the prepared concentrations of the tested extracts was added to 1 ml of DPPH˙ (0.135 mM). The absorbance of samples and blank was measured at 517 nm after 30 minutes of incubation in darkness. The percentage of remaining DPPH˙ of each tested concentration at the steady state was calculated as follows:
% DPPH˙ remaining = [DPPH˙]T/[DPPH˙]T=0 X 100
These values were plotted against mg of peanut extract to show the concentration of antioxidants required to decrease the initial DPPH concentration by 50% (IC50) using the exponential curve.
Histopathological studies
Brain specimens were fixed in 10% phosphate-buffered formalin (pH 7.4), dehydrated in ascending grades of ethyl alcohol, cleared in toluene, and mounted in molten paraplast 58–62°C. Serial 5 µm thick histological sections were cut, stained with hematoxylin and eosin (H&E) and examined under bright field light Olympus CX31 microscopy to visualize the changes in the cerebrum and hippocampus.
Ultrastructure studies
Cerebral biopsies were preserved in phosphate-buffered 2.5% glutaraldehyde (pH 7.4) followed by post-fixation in 1% phosphate-buffered osmium tetroxide. The specimens were then dehydrated in higher grades of ethyl alcohol, followed by propylene oxide, and embedded in epoxy resin. The LKB-Ultratome IV was used to cut ultrathin sections that were put on grids. The thin sections were stained with lead citrate and uranyl acetate and investigated on a Joel 100CXl transmission electron microscope (Mansoura University, Egypt).
Biochemical assays
Brain specimens of the experimental groups were weighed, homogenized in 10% ice-cold 2.5 mM-tris buffer (pH 7.5) and centrifuged at 14,000 × g for 15 minutes at 4°C. The supernatants were kept in −20 for further biochemical analyses.
Dopamine and serotonin determination
Dopamine and serotonin contents were measured using ELISA rat Kits from CUSABIO Technology LLC, Houston, USA as directed by the manufacturer: ELISA Kit catalogue Nu. CSB-E08660r was used to measure dopamine, while the Kit Cat Nu. E-El-0033 was used to measure serotonin (5-HT) [Citation24].
Assessments of neurodegenerative markers
The brain α-amylase and tau protein expressions were assessed using rat ELISA kit (CUSABIO Technology LLC, USA) according to the manufacturer’s protocol. ATP was measured by ELISA kit (My Biosource, USA) according to the manufacturer’s protocol. The absorbance was measured at 540 nm within 30 minutes to avoid color fading. The protein concentrations were calculated using a standard curve of the assayed parameter [Citation25].
8-hydroxy-2-deoxy guanosine (8-OHdG)
The expression of 8-hydroxy-2-deoxy guanosine (8-OHdG) was assayed using ELISA kit (CUSABIO Technology LLC, USA) according to the manufacturer’s protocol. Tissue lysates were incubated in wells containing horse reddish peroxidase tagged antibodies of 8-OH-dG at 37°C for 1 h, followed by the addition of tetramethylbenzidine substrate for color production. The difference in expression was measured at 450 nm (Bio TekELx 808, USA) [Citation26].
Assessments of tumor necrosis factor-α (TNF-α), 5-lipoxygenase (5-LOX)
The expression of tumor necrosis factor-α (TNF-α) and 5-lipoxygenase in brain lysates were measured using an ELISA kit from (CUSABIO Technology LLC, USA) according to the manufacturer’s instruction. The absorbance was measured at 540 nm within 30 minutes (Bio TekELx 808, USA). The concentration of each sample was assessed using the generated standard curve [Citation27].
Determination of superoxide dismutase (SOD) and malondialdehyde (MDA)
The expression of superoxide dismutase (SOD) activity and malondialdehyde (MDA) were assessed in the brain tissue lysates. For the determination of SOD, 100 μL of brain tissue lysates, 100 μl xanthine oxidase, 100 μl nitroblue tetrazolium and 3100 μL phosphate buffer solution (PBS) were used. The absorbance was measured at 500–600 nm (Bio TekELx 808, USA) [Citation28]. MDA levels were measured using thiobarbituric acid. The reaction of MDA with thiobarbituric acid produces a thiobarbituric acid reactive substance, which has a pink color. The change in absorbance was measured calorimetrically at 532 nm (Bio TekELx 808, USA) [Citation29].
Real-time PCR expression of genes expressed in brain damage
Total RNA concentration was separated using RNeasy kit (Qiagen, US) and the RNA content was determined using the NanoDrop ND-2000 spectrophotometry method. The incidence of DNA contamination was excluded using RNA purification kit (Qiagen, US). Complementary DNA (cDNA) was synthesized using RevertAid First Strand cDNA Synthesis Kit (Thermo Co, BIO-6505, USA). The fold change of each gene was performed in triplicate and compared to the fold change of GAPDH as the internal standard. The relative expression of genes was calculated using the 2−ΔΔC method Citation[30]. The reverse and forward primer sequences for all of the studied genes are presented in .
Table 1. List of the real time PCR primers.
Assessments of cysteine-aspartic protease-3 (caspase-3) and synaptophysin in immunohistochemistry
Sections of rat brain (5 μm thickness) were cut and mounted on positively charged glass slides (Fisher Thermo Scientific, Nepean, Ontario, Canada). The sections were digested in 0.05% trypsin (pH 7.8) for 15 min at 37°C for antigen retrieval and treated overnight at 4°C with cysteine-aspartic acid protease 3 (1:50) or mouse anti-synaptophysin (1:75) (Thermo Fisher Scientific, Fremont, CA, USA). The sections were then treated with horseradish peroxidase streptavidin to develop the immunoactivity and stained with Mayer hematoxylin as a counter stain. The negative control sections were incubated in PBS containing 1% non-immune serum. The tissue sections were investigated and photographed using a Leica BM5000 microscope (Leica Microsystems, Wetzlar, Germany) [Citation31].
Statistical analyses
Statistical analyses, including two-way ANOVA, post hoc analysis, between the control and studied groups were carried out using SPSS (version 13). Data are presented as means ± standard error (SE). The data were considered statistically significant at p < 0.05.
Results
Phytochemistry of peanuts
In comparison to methanolic extract, peanut water extract had medium levels of phenolic, flavonoids, tannins, alkaloids and antioxidant activity. However, petroleum ether extract showed the minimum values of these nutrients except higher inclusion of alkaloids. When compared to water and methanolic extract, petroleum ether extract showed higher antioxidant activity ().
Table 2. Phytochemicals content and antioxidant activity of different peanuts extracts.
Histopathology and ultrastructure changes
Cerebrum
Histologically, the cerebral cortex of male albino rats of the control group and peanut-supplemented group exhibited the morphology of normal six layers. The thick molecular layer had normal histological features with the presence of few cells and dense plexus of nerve fibers. Meanwhile, The external pyramidal and granular layers were characterized by the presence of numerous pyramidal and granular cells with few pyramidal and granular cells at the internal pyramidal and internal granular layers. The pyramidal cell had a multipolar shape with basophilic cytoplasm and a large, rounded vesicular nucleus. The granular cells were characterized by large open-face nuclei with little cytoplasm and prominent nucleolus ().
Figure 1. Histopathological changes of cerebral cortex and hippocampus of diabetic and peanut treated rats (a). Photomicrographs of sagittal histological section of cerebral cortex. A. Control. A1. Dietary peanut supplementation. B-B3. Diabetes showing blood vessel infiltration, glial cell condensation, angiogenesis of blood vessels, glial cell infiltration and gliosis. C-C1. Diabetes and Peanut supplementation. Note the improvement of the cerebral cortex. Abbreviations; BVI, blood vessel infiltration; Gl, gliosis. Star illustrated apoptotic neuronal cells. I. Molecular layer. II. External granular layer. III. Pyramidal layer. IV. Inner granular layer. (b) Photomicrographs of sagittal histological section of hippocampus of male rat. A. Control. B. Dietary peanut supplementation. C. Diabetes showing chromatolysis or grouping nuclear chromatin (apoptosis) of pyramidal cells. D. Diabetic and dietary supplemented Peanut showing an improvement.Abbreviations; ML, molecular layer, PL, polymorphic layer; PyL, pyramidal layer.
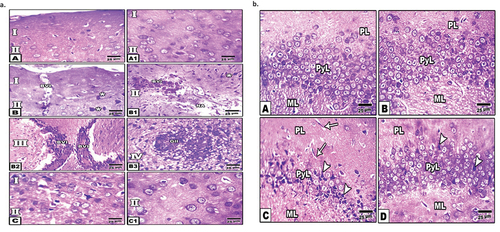
In the diabetic group, there were evidences for cell degeneration including loss of the cell processes, presence of eosinophilic cytoplasm, and darkly stained small nuclei. Furthermore, many congested blood vessels and glial cell condensation were observed. Several signs for neo-angiogenesis as well as infiltration of glial cells and gliosis were recorded (). The diabetic and peanut-supplemented (treated) group showed apparent enhancement in pathological features of cerebral tissue with a normal architecture to some extent. However, the number of cells was comparatively less compared to the control group (1a)
In transmission electron microscopy, control pyramidal cells showed centrally located nuclei with a peripheral thin coat of heterochromatin. The cytoplasm appeared rich in the rough endoplasmic reticulum, mitochondria with well-developed cristae and Golgi apparatus with a characteristic pattern of Nissl bodies, and axons with non-myelinated sheath were detected ().
Figure 2. Transmission Electron micrographs of cerebral cortex. (a) TEM of control. A -C. Showed normal pyramidal cell with nuclei having peripheral heterochromatin, and euchromatin containing vesicular nucleoli and thin cytoplasm containing rich RER, and mitochondria. D. Showing non-myelinated axons. Abbreviations; M, mitochondria, NMA, non-myelinated axon; N, nucleus, NE, nuclear envelope; Nu, nucleolus; RER, rough endoplasmic reticulum. (b) TEM of cerebral neurons of diabetic rat. A - B. Showed Pyramidal cell with nuclei having densely compacted heterochromatin and cytoplasm with vesiculated rough endoplasmic reticulum and atrophied mitochondria. C. Showed congested blood capillaries. D. showed widespread of demyelinated axons. Abbreviations; DM; damaged mitochondria; DMA, demyelinated axons; KN, karyolitic nuclei; PN, pyknotic nuclei. (c) Transmission electron micrographs of cerebral cortex of diabetic rat received dietary peanut. A-C. Showed an improvement at pyramidal cells with peripheral heterochromatin and cytoplasm containing rich RER, and mitochondria. Arrow head in figure A. showed satellite cells present adjacent to some neurons. D. Showed the myelinated axons.
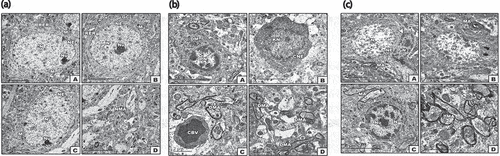
In the diabetic group, there was an alteration of the cerebral cortex cells. The pyramidal cells showed abnormal condensation of nuclear heterochromatin and appeared either pyknotic or karryolyzed. The pyramidal cells possessed disorganization of the cytoplasmic organelles. The rough endoplasmic reticulum became either fragmented or vesiculated and was irregularly distributed throughout the cytoplasm. The mitochondria were atrophied and lacked differentiation of their internal compartments. Nissl bodies exhibited a disorganized pattern. Several blood vessel congestions were detected. The neuronal axon exhibited the presence of a non-myelinated sheath. ().
In the diabetic and peanut-supplemented group, there was a marked improvement in the cytoarchitecture structure compared with the control group. The pyramidal cells became intact with almost normal nuclei, nucleoli and cytoplasmic organelles. There were obvious nuclei with peripheral margination of heterochromatin. The cytoplasm appeared with rough endoplasmic reticulum and mitochondria. The presence of satellite cells was confirmed. The neuronal axon showed the presence of myelinated sheath ().
Hippocampus
The hippocampus of the control group and the peanut-supplemented group exhibited normal structure. It was composed of three main cell layers: polymorphic layer, pyramidal layer and molecular layer. The polymorphic layer consisted of neuronal processes (axons and dendrites), blood capillaries, glial cells, and scattered nerve cells. The pyramidal layer included pyramidal cells with triangular, pyramid-shaped cell bodies, nodded axons, multiple branched dendrites with spines and large vesicular nuclei. The molecular layer consisted of neuronal axons and dendrites ().
In the diabetic group, there was an alteration of the hippocampus cells manifested by the loss of normal structure. Distorted pyramidal layers reveal marked shrinkage and degenerative changes in pyramidal cells with pyknotic nuclei and empty areas of pyramidal cell loss. Severe dilation of blood capillaries and enlargement of neurons were noticed in molecular and polymorphic layers. In the treated group, the hippocampus of diabetic rats supplemented with peanut showed an improvement in the normal histological structure of the polymorphic layer, pyramidal layer and molecular layer with a mild reduction in pyramidal cells of the pyramidal layer and moderately dilated blood capillaries ().
Immunohistochemical localization of caspase 3 and synaptophysin
Image analyses of cerebral neurons and pyramidal hippocampus cells displayed a significant overexpression of caspase-3 in diabetic rats indicating a marked increase in cell apoptosis. In the peanut-treated group, peanuts significantly over-countered the pro-apoptotic effect of diabetes which was indicated by the significant downregulation of caspase-3 expression compared to the diabetic group. Neither the peanut-supplemented group nor the control group has shown caspase-3 expression ().
Figure 3. (a) Photomicrographs of immunohistochemical localization of caspase 3 at cerebrum (A1-D1) and hippocampus (A2-D2). Note the negative immune reaction in control and peanut supplementation (N). Diabetic cerebrum (C1) and Diabetic hippocampus (C2) showed an increased expression of caspase-3 in neuronal cells. Diabetic and peanut treated cerebrum (D1) and hippocampus (D2) showed a downregulation at caspase-3 expression. Abbreviations; D+N, diabetic and peanuts; N, peanut. Star pointed out the increased immune reaction. (b) Histogram illustrating Image analysis of caspase 3 immunolabeling in cerebrum and hippocampus. Showed the significant overexpression of caspase 3 in diabetic group compared to control group. This elevation at caspase-3 was significantly downregulated by peanut treatment. Each result represent the mean ± SD (n = 5); * indicated the significance at p < 0.05 compared to the control. $ indicated the significance at p < 0.05 compared to the diabetic group. Abbreviations; C, Control; D, diabetes; N, Peanut.
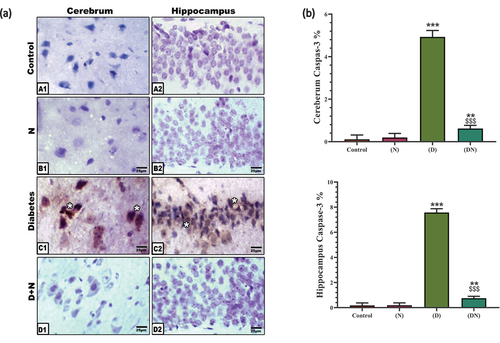
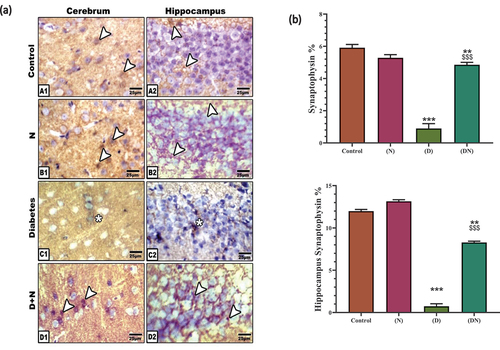
Image analyses of the expression showed a significant reduction of synaptophysin in the synaptic axons of the cerebrum and hippocampus in the diabetic rats compared to the control and peanut-supplemented groups. The experimental diabetic group supplemented with peanut exhibited a significant improvement in synaptophysin immune reaction ().
Figure 4. (a) Photomicrographs of immunolocalization of synaptophysin at cerebrum (A1-D1) and hippocampus (A2-D2). Note moderate immune reaction in control and peanut supplementation (N) in cerebrum and hippocampus. (C1-C2). Diabetic cerebrum (C1) and hippocampus (C2) showed a decreased expression of synaptophysin in neuronal cells. (D1-D2). Diabetic and peanut treated cerebrum (D1) and hippocampus (D2) showed an improvement at immune reaction. Arrow head pointed out the synaptophysin immune reaction. Star pointed out the decreased immune reaction. (b) Chart illustrating Image analysis of synaptophysin immunostaining of cerebrum and hippocampus indicated a significant decrease at the expression of synaptophysin in diabetic group compared to control group which was significantly over-countered by peanut supplementation. Each result represent the mean ± SD (n = 5); * indicated the significance at p < 0.05 compared to control. $ indicated the significance at p < 0.05 compared to diabetic group. Abbreviations; C, Control; D, diabetes; D+N, diabetes and peanut; N, Peanut.
Biochemical observations
As shown in (a-k), the brain tissues of the diabetic group showed a significant reduction in SOD, dopamine, serotonin, and ATP compared to the control group. The diabetic group also had a significant increase in the levels of MDA, TNF-α, 5-lipoxygenase, 8-OHdG, amyloid-β, α-amylase and tau protein compared to the control group.
Figure 5. Histogram illustrating the effect of Diabetes and/or peanut supplementation on brain biochemical activity. Charts showed the diabetes dependent significant decrease at the brain SOD activity (a) increase of brain MDA content (b) decrease of brain dopamine content (c) decrease of brain serotonin content (d) increase of brain TNFα content (e) increase of brain 5-lipoxyginase content (f) decrease of brain ATP content (g) increase of brain 8-hydroxy-deoxy guanosine content (h) increase of brain amyloid-β content (i) increase of brain α- amylase content (j) increase at brain tau protein content (k) compared to control group. These levels showed a significant therapeutic improvement post-dietary supplementation of peanut (DN). Each result represented as the mean ± SD (n =7); * indicated the significance at p < 0.05 compared to control. $ indicated the significance at p < 0.05 compared to diabetic group. Abbreviations; C, Control; D, diabetes; N, Peanut; DN, diabetic and peanut supplemented group.
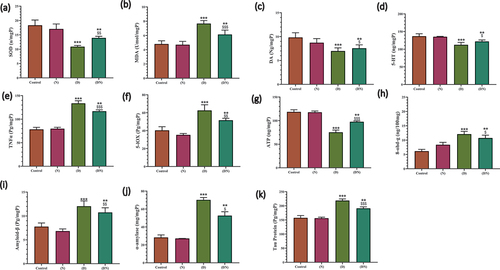
On the other hand, the diabetic and peanut-supplemented group showed significantly upraised the levels of SOD, dopamine, serotonin, and ATP compared with the diabetic group. Meanwhile, the levels of MDA, TNF-α, 5-lipoxygenase, 8-OHdG, amyloid-β, α-amylase and tau protein were significantly reduced, compared with the diabetic group.
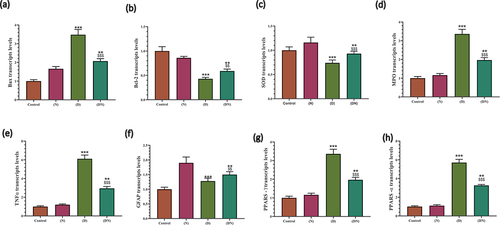
Gene expression of BAX, BCL2, SOD, MPO, TNF-α, GFAP, PPAR-α, PPAR-γ
As demonstrated in (a-h), the diabetic group had a significant reduction in the gene expression of B-cell lymphoma 2 (BCL2) and SOD as well as a significant elevation at the transcription levels of BAX, Myeloperoxidase (MPO), TNF-α, glial fibrillary acidic protein (GFAP), peroxisome proliferator-activated receptors (PPAR-α and PPAR-γ) compared to the control group. These effects were significantly over-countered with peanut supplementation compared to the diabetic group.
Figure 6. RT-PCR validation of peanut cytoprotective effect against diabetic related oxidative stress, inflammation, apoptosis and differentiation of brain neurons. Charts illustrated the significant effect of diabetes at RNA expression of BAX (a) Bcl-2 (b) SOD (c) MPO (d) TNFα (e) GFAP (f) PARP-γ (g) PARP-α (h) in brain of male rats, which was significantly opposed in rats that received dietary peanut (DN) compared to diabetic group. Each result represent the mean ± SD (n = 3); * indicated the significance at p < 0.05 compared to control group. $ indicated the significance at p < 0.05 compared to diabetic group. Abbreviations; C, Control; D, diabetes; N, Peanut; DN, diabetic and peanut supplemented group.
Discussion
The relation between diabetes and oxidative stress is a double-edged sword, where several studies illustrated the critical and pivotal role of oxidative stress in the occurrence and development of diabetes mellitus by activating different metabolic pathways including hexosamine, glycolytic, polyol, protein kinase C, and advanced glycation end-product pathways [Citation32]. Meanwhile, Hyperglycemia itself plays a fundamental role in upregulating the level of oxidative stress which occurred via induction of mitochondrial dysfunction and endoplasmic reticulum stress. These effects provoke the ROS built-up that, in turn, activates the inflammatory process and pro-inflammatory cytokines generation [Citation33].
STZ-induced diabetes provides a reliable model of endogenous chronic oxidative stress and hyperglycemia. Thus, the pathological and molecular alterations related to brain oxidative stress induced by STZ were employed to evaluate the potential protective role of peanuts against diabetes-related neurodegeneration. Diabetic rats showed substantial depletion at the expression of antioxidant enzymes SOD as indicated by gene and enzyme expression assays. This imbalance in antioxidant enzymes led to a significant increase in MDA as an indication of oxidative stress which was revoked by peanut supplementation. The role of antioxidant enzymes in sustaining the redox balance was illustrated by previous research [Citation34]. A significant increase in SOD activity was recorded in the plasma of patients with type 2 diabetes [Citation35].
MPO is the most abundant protein in human neutrophils, and it plays a major role in inflammation, oxidative stress, lipoprotein oxidation, and atherosclerosis [Citation36]. In vivo studies have shown a higher MPO activity in the vessels of diabetic Zucker rats than in non-diabetic rats [Citation37].
In accordance with oxidative stress upregulation, a significant increase in pro-inflammatory cytokines TNF-α and 5-lipoxygenase enzyme was recorded in the diabetic group compared with the controls in this study.
Literature survey indicated the role of 5-lipoxygenase upregulation in promoting lipid peroxidation in an in vitro model as well as in brain tissue [Citation38,Citation39]. On the other hand, it is responsible for the transformation of essential fatty acids into leukotrienes, which play a pivotal role in stimulating immune cell chemotaxis and induction of inflammatory responses in patients with asthma and allergy [Citation40].
The increased level of oxidative stress and inflammation at neuronal cells led to a progression of neuronal cell damage in both hippocampus and cerebrum indicated by histopathological and electron microscopic studies. Histopathological results revealed a marked increase in angiogenesis accompanied by breakdown of cerebral cortex neuronal cells. In the hippocampus, a considerable atrophy at the pyramidal layer with a marked missing of the pyramidal neurons, which attained pleomorphic appearance. In agreement with these results, electron microscopic studies showed neuronal cell death in the hippocampus of STZ-induced diabetes, after 21 days of STZ injection [Citation41]. This study supported the fact that diabetes induced by STZ is capable of inducing apoptosis in the hippocampus of diabetic rats [Citation42].
These results are in consent with several mechanisms recorded myelin alterations in the brain of STZ–diabetic rats, such as decreased myelin-associated glycoprotein, autoantibodies to myelin basic protein, and myelin damage induced by nitric oxide [Citation6,Citation43]. Previous research illustrated that the increased levels of MDA, as well as impairment of antioxidant enzymes [Citation44], led to the generation of active forms of oxygen [Citation45,Citation46] which was directly implicated in the induction of cell death. Cell death can result in cognitive defects and increment at the risk of brain disorders [Citation47].
Alzheimer’s disease (AD) and other neurodegenerative disorders were found to be accompanied by a significant increase at oxidative stress and lipid peroxidation [Citation48], involving the production of free radicals that directly damages cell membranes and reproduce secondary byproducts leading to neurodegenerative disorders [Citation49].
The histologically recorded upraising of angiogenesis, tightly grouped glia cells in the cerebrum, marked damage to pyramidal cells and extensive presence of pleomorphic cells in hippocampus necrotic sites of diabetic rats mostly reflect the diabetic-dependent elevation of the levels of TNF-α and 5- lipoxygenase of the diabetic group. Moreover, the role of diabetes in neural degeneration was indicated by the significant reduction in GFAP expression which is essential for the formation of stable astrocytic cells indicating diabetic-dependent neuronal damage. The increased synthesis of GFAP is considered as a key indicator of glial reactivity and its downregulation caused reactive gliosis [Citation50]. GFAP levels have been reported to be a consequence of hyperglycemia [Citation51]. In this study, peanut supplementation markedly enhanced the diabetic-dependent downregulation of GFAP expression. Diabetes-related inflammation and pro-inflammatory cytokines play a relevant role in the development of microvascular diabetic complications, including nephropathy [Citation52]. The current findings verified the former studies that illustrated the role of increased inflammation in activating signal transduction pathways of cell apoptosis [Citation53,Citation54].
The implication of diabetes in induction of neuronal apoptosis was molecularly validated by the upregulation of proapoptotic BAX and downregulation of BCL2 mRNA transcripts which lead to mitochondrial dysfunction confirmed by a significant increase in 8-OHdG and decrease in ATP of diabetic rats compared to control. Several studies elucidated that diabetic-related oxidative stress leads to an oxidative damage to the mitochondrial DNA [Citation55], which is indicated by the upregulation of 8-OHdG as the most abundant indicator of mitochondrial DNA oxidative damage [Citation56]. Other studies also demonstrated that ATP synthesis rate was decreased by 27% and mitochondrial function was compromised by ~45% as well in type 2 diabetic patients, suggesting that impaired mitochondrial function may be an important bioindicator of diabetes [Citation57,Citation58].
The increased BAX and the decrease in antiapoptotic BCL-2 mRNA transcription levels in diabetic rats suggested a mitochondrial-dependent apoptotic pathway which was further confirmed by immunohistochemical localization of the upregulation at Caspase-3 expression in diabetic rats compared to controls. The role of BAX in the induction of cell apoptosis was found to be carried out via increasing the permeability of the mitochondrial membrane and release of cytochrome C stimulating caspases activity and cell apoptosis [Citation59]. The present findings are consistent with the studies which indicated that STZ-related hyperglycemia led to apoptotic histopathological changes in rat hippocampus [Citation60]. The peanut oral supplementation significantly over-countered the diabetic proapoptotic effects by significantly downregulating the expression of BAX and caspase-3 and upregulating the expression of BCL2.
The observed findings revealed that the diabetic-related inflammation led to injured brain structure and reduced brain function which were correlated with the significant upregulation of brain tau protein, amyloid-β and α-amylase. In diabetes, amyloid-β and tau proteins have been considered as the major components of senile plaques and neurofibrillary tangles, respectively. The hyperphosphorylation of tau protein would stimulate its aggregation to form neurofibrillary tangles [Citation61]. The hippocampus of STZ-induced diabetic rats showed a significant increase in the levels of amyloid precursor protein (APP), amyloid-β expressions and phosphorylated tau [Citation62,Citation63]. The upregulation of brain α-amylase enzyme level in diabetic rats indicated their high susceptibility to neuronal damage which was reversed by peanut oral supplementation. These results were supported by other studies which revealed the role of altered levels of α-amylase in brains leading to variations of glucose readiness and neuropathological changes observed in patients with Alzheimer’s disease [Citation64].
The diabetic-dependent increase in inflammation and neuronal cell apoptosis led to an apparent reduction of dopamine and serotonin secretion compared to the control group. Reduction of the assayed neurotransmitters reflects the increased pattern of neuronal cell damage shown by increased brain α-amylase, amyloid-β peptide, and tau protein. The decrease in neurotransmitters serotonin and dopamine has been linked to alterations in adaptive behavior, including decision making, reinforcement learning and emotional and functional activation. The role of diabetes in the alteration of dopamine signaling was markedly enhanced with Peanut supplementation which led to an upregulation of dopamine expression. The present findings were in line with many studies which revealed that brain insulin resistance alters dopamine revenue which persuades anxiety and depressive behaviors [Citation65–67].
Furthermore, the data illustrated the significant effect of peanuts in downregulating PPAR-α and PPAR-γ mRNA expressions that have been upregulated in the diabetic group as an indication of its therapeutic effect on insulin resistance. PPARs are nuclear hormone receptors that regulate the expression of genes involved in lipid metabolism and insulin resistance [Citation68,Citation69]. PPAR-α mRNA levels are increased in STZ-induced diabetic rat liver [Citation70]. It was also reported that the expression of PPAR-γ mRNA in skeletal muscle is highly regulated by insulin levels and increased in both obese non-diabetic and type II diabetic rats which proposed the role that PPAR-γ may play in skeletal muscle insulin resistance [Citation71].
Finally, the bioactivity of peanut was attributed to its potent antioxidant activity due to its high contents of phenolics, flavonoids and tannins which resulted in a significant improvement in SOD activity and in decreasing lipid peroxidation that led to downregulation at tau protein, amyloid-β, α- amylase, 8-OHdG, TNF-α and 5-LOX levels of the brain of treated rats. These changes helped in the improvements of brain function, indicated by the enhancement of neurotransmitters levels, and improved histological changes in the hippocampus and cerebral cortex.
These findings were in line with other studies, which mentioned that peanut consumption has beneficial effects on improving insulin resistance, oxidative stress, inflammation, and vascular reactivity [Citation72]. Furthermore, a diet high in oleic acid, which can be easily achieved through the consumption of peanuts and olive oil, have a beneficial effect on type II diabetes and ultimately reverse the negative effects of inflammatory cytokines observed in obesity and non-insulin-dependent Diabetes mellitus [Citation73].
A variety of animal and human studies have shown that resveratrol, which is found in peanuts, administration for diabetic subjects could enhance the antioxidant defense, reduce lipid and protein oxidation and decrease apoptosis rate [Citation74]. It can improve energy expenditure through the promotion of mitochondrial biogenesis and antioxidant activity [Citation75]. The anti-inflammatory activities of resveratrol are also characterized by decreased levels of interleukin IL-6, IL-8, and TNF-α [Citation76].
In conclusion, peanut supplementation played a pivotal role in opposing the STZ-related oxidative damage which modulated the inflammatory and apoptotic-related pathways. Dietary Peanut exerted these effects by increasing the antioxidant activity and suppressing both of the inflammatory and pro-apoptotic pathways in the rat’s brain. These findings suggested that peanut supplementation may have therapeutic potentials against brain damage resulted from STZ-induced diabetes [Citation77].
Acknowledgments
Authors dedicate this work to the soul of Prof. Hassan I Elssayad who passed away of Covid-19 last September
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- Maraschin JDF. Classification of diabetes. Diabetes. 2013;2013:12–19.
- Dandona P, Aljada A, Chaudhuri A, et al. Metabolic syndrome: a comprehensive perspective based on interactions between obesity, diabetes, and inflammation. Circulation. 2005;111(11):1448–1454.
- Jeong H-S, Lee D-H, Kim S-H, et al. Hyperglycemia-induced oxidative stress promotes tumor metastasis by upregulating vWF expression in endothelial cells through the transcription factor GATA1. Oncogene. 2022;41(11):1634–1646.
- Brands AM, Kessels RP, de Haan EH, et al. Cerebral dysfunction in type 1 diabetes: effects of insulin, vascular risk factors and blood-glucose levels. Eur J Pharmacol. 2004;490(1–3):159–168.
- Allen KV, Frier BM, Strachan MW. The relationship between type 2 diabetes and cognitive dysfunction: longitudinal studies and their methodological limitations. Eur J Pharmacol. 2004;490(1–3):169–175.
- Mastrocola R, Restivo F, Vercellinatto I, et al. Oxidative and nitrosative stress in brain mitochondria of diabetic rats. J Endocrinol. 2005;187(1):37–44.
- Angelova PR, Abramov AY. Role of mitochondrial ROS in the brain: from physiology to neurodegeneration. FEBS Lett. 2018;592(5):692–702.
- Abate M et al. (2020). Mitochondria as playmakers of apoptosis, autophagy and senescence. Seminars in Cell & Developmental Biology, 98 139–153. 10.1016/j.semcdb.2019.05.022
- Ighodaro OM. Molecular pathways associated with oxidative stress in diabetes mellitus. Biomed Pharmacother. 2018;108:656–662.
- Dienel GA. Brain glucose metabolism: integration of energetics with function. Physiol Rev. 2019;99(1):949–1045.
- Cobley JN, Fiorello ML, Bailey DM. 13 reasons why the brain is susceptible to oxidative stress. Redox Biol. 2018;15:490–503.
- Butterfield DA, Reed T, Newman SF, et al. Roles of amyloid β-peptide-associated oxidative stress and brain protein modifications in the pathogenesis of Alzheimer’s disease and mild cognitive impairment. Free Radic Biol Med. 2007;43(5):658–677.
- Agrawal R, Sethiya NK, Mishra SH. Antidiabetic activity of alkaloids of Aerva lanata roots on streptozotocin-nicotinamide induced type-II diabetes in rats. Pharm Biol. 2013;51(5):635–642.
- Hussain T, Tan B, Yin Y, et al. Oxidative stress and inflammation: what polyphenols can do for us? Oxid Med Cell Longev. 2016;2016:1–9.
- Higgs J. The beneficial role of peanuts in the diet–an update and rethink! Peanuts and their role in CHD. Nutrition & Food Science. normal physiological functions and human disease. Int J Biochem Cell Biol. 2002;39(1):44–84.
- Griel AE, Eissenstat B, Juturu V, et al. Improved diet quality with peanut consumption. J Am Coll Nutr. 2004;23(6):660–668.
- Yang J, Halim L, Liu RH (2005). Antioxidant and antiproliferative activities of common nuts. In Institute Of Food Technologists annual meeting and food expo, New Orleans, LA (pp. 16–20).
- Wolfe K, Wu X, Liu R. Antioxidant activity of apple peels. J Agric Food Chem. 2003;51:609–614.
- Zhishen J, Mengcheng T, Jianming W. Research on antioxidant activity of flavonoids from natural materials. Food Chem. 1999;64:555–559.
- J B H. Phytochemical Dictionary: handbook of Bioactive Compounds from Plants. 2nd. London:Taylor and Francis;1999. 221–234.
- Sadasivam S, Manickam A. Biochemical Methods. Third ed. New Delhi IndiA: New Age International Publishers; 2008. p. 203–204.
- Kitts DD, Wijewickreme AN, Hu C. Antioxidant properties of a North American ginseng extract. Mol Cell Biochem. 2000;203(1):1–10.
- Liyana-Pathirana M, Shahidi F. Antioxidant activity of commercial soft and hard wheat (Triticum aestivum L.) as affected by gastric pH conditions. J Agric Food Chemi. 2005;53:2433–2440.
- Namkung SM, Choi JS, Park JH, et al. Detection of dopamine and serotonin by competitive enzyme-linked immunosorbent assay. Korean J Clin Lab Sci. 2017;49(3):220–226.
- Takechi R, Lam V, Brook E, et al. Blood-brain barrier dysfunction precedes cognitive decline and neurodegeneration in diabetic insulin resistant mouse model: an implication for causal link. Front Aging Neurosci. 2017;9:399.
- Attia S. Modulation of Irinotecan-Induced Genomic DNA Damage by Theanine. Food ChemToxicol. 2012;50:1749–1754.
- Singh DP, Chopra K. Flavocoxid, dual inhibitor of cyclooxygenase-2 and 5-lipoxygenase, exhibits neuroprotection in rat model of ischaemic stroke. Pharmacol Biochem Behav. 2014;120:33–42.
- Ohkawa H, Ohishi N, Yagi K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Analomi Biochemi. 1979;95(2):351–358.
- Bajpai VK, Sharma A, Kang SC, et al., Antioxidant, lipid peroxidation inhibition and free radical scavenging efficacy of a diterpenoid compound sugiol isolated from Metasequoia glyptostroboides. Asian Pacific J Tropical Med. 2014;7:9–15.
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2 (-Delta Delta C (T)) method. Methods. 2001;25:402–408.
- Rashed LA, Ibrahim Mohamady MD. Effect of diabetes mellitus on rat cognitive functions and related hippocampal synaptic plasticity markers. Med J Cairo Univ. 2011;79(2):213–227.
- Adiele RC, Adiele CA. Metabolic defects in multiple sclerosis. Mitochondrion. 2019;44:7–14.
- Golembewski EK, Wales SQ, Aurelian L, et al. The HSV-2 protein ICP10PK prevents neuronal apoptosis and loss of function in an in vivo model of neurodegeneration associated with glutamate excitotoxicity. Exp Neurol. 2007;203(2):381–393.
- Jafari Anarkooli I, Sankian M, Ahmadpour S, et al. Evaluation of Bcl-2 family gene expression and Caspase-3 activity in hippocampus STZ-induced diabetic rats. Exp Diabetes Res. 2008;2008. DOI:10.1155/2008/638467
- Ozkaya YG, Agar A, Yargicoglu P, et al. The effect of exercise on brain antioxidant status of diabetic rats. Diabetes Metab. 2002;28(5):377–384.
- Gholamian-Dehkordi N, Luther T, Asadi-Samani M, et al. An overview on natural antioxidants for oxidative stress reduction in cancers; a systematic review. Immunopathol Persa. 2017;12(2):e12.
- Stefanatos R, Sanz A. The role of mitochondrial ROS in the aging brain. FEBS Lett. 2018;592(5):743–758.
- Young IS, Woodside JV. Antioxidants in health and disease. J Clin Pathol. 2001;54(3):176–186.
- Bradley-Whitman MA, Lovell MA. Biomarkers of lipid peroxidation in Alzheimer disease (AD): an update. Arch.Toxicol. 2015;89(7):1035–1044.
- Skoumalová A, Ivica J, Santorová P, et al. The lipid peroxidation products as possible markers of Alzheimer’s disease in blood. Exp Gerontol. 2011;46(1):38–42.
- Takei Y. Age-dependent decline in neurogenesis of the hippocampus and extracellular nucleotides. Hum Cell. 2018;32(2):88–94.
- Domingueti CP, Dusse LMSA, Das Graças Carvalho M, et al. Diabetes mellitus: the linkage between oxidative stress, inflammation, hypercoagulability and vascular complications. J Diabetes Complications. 2016;30(4):738–745.
- Yu XC, Li Z, Liu XR, et al. The antioxidant effects of whey protein peptide on learning and memory improvement in aging mice models. Nutrients. 2021;13(6):2100.
- Navarro JF, Mora C. Diabetes, inflammation, proinflammatory cytokines, and diabetic nephropathy. ScientificWorldJournal. 2006;6:908–917.
- Tiwari V, Kuhad A, Chopra K. Emblica officinalis corrects functional, biochemical and molecular deficits in experimental diabetic neuropathy by targeting the oxido‐nitrosative stress mediated inflammatory cascade. Phytother Res. 2011;25(10):1527–1536.
- Czubowicz K, Czapski GA, Cieślik M, et al. Lipoxygenase inhibitors protect brain cortex macromolecules against oxidation evoked by nitrosative stress. Folia Neuropathol. 2010;48(4):283–292.
- Czapski GA, Czubowicz K, Strosznajder RP. Evaluation of the antioxidative properties of lipoxygenase inhibitors. Pharmacol Rep. 2012;64(5):1179–1188.
- Kanaoka Y, Boyce JA. Cysteinyl leukotrienes and their receptors; emerging concepts. Allergy Asthma Immunol Res. 2014;6(4):288–295.
- Buée L, Troquier L, Burnouf S, et al. From tau phosphorylation to tau aggregation: what about neuronal death? Biochem Soc Trans. 2010;38(4):967–972.
- Currais A, Prior M, Lo D, et al. Diabetes exacerbates amyloid and neurovascular pathology in aging‐accelerated mice. Aging Cell. 2012;11(6):1017–1026.
- Kim B, Backus C, Oh S, et al. Increased tau phosphorylation and cleavage in mouse models of type 1 and type 2 diabetes. Endocrinology. 2009;150(12):5294–5301.
- Qu Z, Jiao Z, Sun X, et al. Effects of streptozotocin-induced diabetes on tau phosphorylation in the rat brain. Brain Res. 2011;1383:300–306.
- Ke YD, Delerue F, Gladbach A, et al. Experimental diabetes mellitus exacerbates tau pathology in a transgenic mouse model of Alzheimer’s disease. PloS one. 2009;4(11):e7917.
- Byman E, Schultz N, Wennström M. Brain alpha‐amylase: a novel energy regulator important in Alzheimer disease? Brain Pathol. 2018;28(6):920–932.
- Kurutas EB. The importance of antioxidants which play the role in cellular response against oxidative/nitrosative stress: current state. Nutr J. 2015;15(1):1–22.
- Ano Y, Ohya R, Takaichi Y, et al. Β-lactolin, a whey-derived lacto-tetrapeptide, prevents Alzheimer’s disease pathologies and cognitive decline. J Alzheimers Dis. 2020;73(4):1331–1342.
- Kleinridders A, Cai W, Cappellucci L, et al. Insulin resistance in brain alters dopamine turnover and causes behavioral disorders. Proc Nat Acad Sci. 2015;112(11):3463–3468.
- Widlansky ME, Hill RB. Mitochondrial regulation of diabetic vascular disease: an emerging opportunity. Transl Res. 2018;202:83–98.
- Shenouda SM, Widlansky ME, Chen K, et al. Altered mitochondrial dynamics contributes to endothelial dysfunction in diabetes mellitus. Circulation. 2011;124(4):444–453.
- Cividini F, Scott BT, Dai A, et al. O-GlcNAcylation of 8-oxoguanine DNA glycosylase (Ogg1) impairs oxidative mitochondrial DNA lesion repair in diabetic hearts. J Biol Chem. 2016;291(51):26515–26528.
- Schrauwen-Hinderling VB, Kooi ME, Hesselink MKC, et al. Impaired in vivo mitochondrial function but similar intramyocellular lipid content in patients with type 2 diabetes mellitus and BMI-matched control subjects. Diabetologia. 2007;50(1):113–120.
- Phielix E, Schrauwen-Hinderling VB, Mensink M, et al. Lower intrinsic ADP-stimulated mitochondrial respiration underlies in vivo mitochondrial dysfunction in muscle of male type 2 diabetic patients. Diabetes. 2008;57(11):2943–2949.
- Zhao H, Yenari MA, Cheng D, et al. Bcl‐2 overexpression protects against neuron loss within the ischemic margin following experimental stroke and inhibits cytochrome c translocation and caspase‐3 activity. J Neurochem. 2003;85(4):1026–1036.
- Soleymaninejad M, Joursaraei SG, Feizi F, et al. The effects of lycopene and insulin on histological changes and the expression level of Bcl-2 family genes in the hippocampus of streptozotocin-induced diabetic rats. J Diabetes Res. 2017;2017:1–9.
- Michalik L, Auwerx J, Berger JP, et al. International Union of Pharmacology. LXI. Peroxisome proliferator-activated receptors. Pharmacol Rev. 2006;58(4):726–741.
- Boden G. Role of fatty acids in the pathogenesis of insulin resistance and NIDDM. Diabetes. 1997;46(1):3–10.
- Vidal-Puig A, Jimenez-Liñan M, Lowell BB, et al. Regulation of PPAR gamma gene expression by nutrition and obesity in rodents. J Clin Invest. 1996;97(11):2553–2561.
- Liedtke W, Edelmann W, Bieri PL, et al. GFAP is necessary for the integrity of CNS white matter architecture and long-term maintenance of myelination. Neuron. 1996;17(4):607–615.
- Baydas G, Reiter RJ, Yasar A, et al. Melatonin reduces glial reactivity in the hippocampus, cortex, and cerebellum of strepto-zotocin-induced diabetic rats. Free Radic Biol Med. 2003;35(7):797–804.
- Fernández-Real JM, Ricart W. Insulin resistance and chronic cardiovascular inflammatory syndrome. Endocr Rev. 2003;24(3):278–301.
- Nishimura K, Sano M, Ohtaka M, et al. Development of defective and persistent Sendai virus vector: a unique gene delivery/expression system ideal for cell reprogramming. J Biol Chem. 2011;286(6):4760–4771.
- Moldoveanu E, Tanaseanu C, Tanaseanu S, et al. Plasma markers of endothelial dysfunction in type 2 diabetics. Eur J Intern Med. 2006;17(1):38–42.
- Zhang C, Yang J, Jennings LK. Leukocyte-derived myeloperoxidase amplifies high-glucose—induced endothelial dysfunction through interaction with high-glucose—stimulated, vascular none—leukocyte-derived reactive oxygen species. Diabetes. 2004;53(11):2950–2959.
- Hernández-Alonso P, Salas-Salvadó J, Baldrich-Mora M, et al. Beneficial effect of pistachio consumption on glucose metabolism, insulin resistance, inflammation, and related metabolic risk markers: a randomized clinical trial. Diabetes Care. 2014;37(11):3098–3105.
- Vassiliou EK, Gonzalez A, Garcia C, et al. Oleic acid and peanut oil high in oleic acid reverse the inhibitory effect of insulin production of the inflammatory cytokine TNF-α both in vitro and in vivo systems. Lipids Health Dis. 2009;8(1):1–10.
- Kim YH, Kim YS, Kang SS, et al. Resveratrol inhibits neuronal apoptosis and elevated Ca2+/calmodulin-dependent protein kinase II activity in diabetic mouse retina. Diabetes. 2010;59(7):1825–1835.
- Baum JI, Kim IY, Wolfe RR. Protein consumption and the elderly: what is the optimal level of intake? Nutrients. 2016;8(6):359 92016.
