ABSTRACT
Alternate day fasting (ADF) is beneficial in delaying aging and associated morbidities by reducing oxidative stress and inflammation. Hesperidin is a flavonoid antioxidant known for its antioxidant and anti-inflammatory properties. In this study, we aimed to evaluate the synergistic role of hesperidin supplementation and ADF regimen in Wistar rats on biomarkers of aging and inflammation. Middle-aged male Wistar rats (12–15 months) were divided into four groups (n = 6): Group I. Control (given 1.0 mL of carboxymethylcellulose orally); Group II. ADF (chow diet given on alternate days); Group III. Hesperidin (100 mg/kg BW orally); Group IV. ADF+ Hes (ADF regimen and hesperidin dupplemented) for three weeks. We measured clinical parameters and some crucial biomarkers of oxidative stress like FRAP, GSH, PMRS, MDA, PCO, AOPP, NO, insulin, adiponectin and inflammatory cytokines (TNF-α. IL-6, CRP and COX). A significant increase (p < 0.05) in insulin, FRAP and GSH, and a significant decrease (p < 0.05) in MDA and NO were observed in the ADF+Hes group. IL-6, CRP and COX were also significantly reduced (p < 0.05) suggesting hesperidin-induced benefits. We conclude that hesperidin intake during ADF may serve as a crucial purpose in improving signature biomarkers of health and metabolism.
Introduction
Aging is a biological phenomenon which leads to loss of metabolic homeostasis, eventually damaging internal cellular integrity and increasing risk for major age-related pathologies [Citation1]. One of the most robust mechanisms to attenuate aging is caloric restriction (CR), an intervention which involves restricting the amount of caloric intake without reducing nutritional efficiency and this strategy has been proposed to help in attaining longer and healthier lifespan [Citation2]. CR has been applied successfully in many model organisms although studies in humans suggest several complications associated with nutritional intake and the observed efficacy of CR is not found to be the same as observed in other organisms. Moreover, it also has some repercussions associated with bone health, loss of libido amongst others [Citation3]. A slightly different approach from CR is Intermittent Fasting (IF), another dietary restriction (DR) strategy based on limited feeding along with subsequent periods of total abstinence from food (fasting) and is an effective method known to reduce biomarkers of aging and promote healthspan [Citation4]. Alternate day fasting (ADF) is one of the subtypes of IF regimens and is defined as an alternate sequence of feeding and fasting days which may be classified as periodic caloric restriction.
Of the several common denominators of aging, the most widely reviewed area is that of reactive oxygen species (ROS) because ROS is an important causative factor for aging-induced oxidative damage. Although ROS generation in negligible amounts corresponds to the concept of mitohormesis [Citation5], but an acute generation of ROS leading to aging can be prevented by IF. ROS-induced mitohormesis is beneficial in activating the protective response of IF against oxidative stress and aging [Citation6]. IF initiates major cellular and biochemical pathways in defense against ROS which are known to extend lifespan [Citation7]. Since longer periods of fasting may not be feasible to practice, therefore certain intermittent bouts of fasting and feeding and additional antioxidant supplementation is an area which is still open to research scrutiny.
Antioxidants are dietary supplements taken for the protection against oxidative stress which also helps to delay the onset of age-related diseases [Citation8]. Several studies have administered antioxidant supplementation in rats to observe its potential benefits in delaying aging and protection from diseases [Citation9,Citation10]. Hesperidin (3,5,7 trihydroxyfavanone 7-rhamnoglucoside) is one such antioxidant polyphenol, a flavanone isolated from orange peels and abundantly found in citrus fruits [Citation11]. The anti-inflammatory and antioxidant properties of hesperidin and its protective response to cardiovascular disease and type II diabetes have been explored [Citation12].
Hesperidin plays a pivotal role in maintaining glucose and lipid metabolism, neurological disorders, oxidation levels and inflammation [Citation13]. Its molecular mechanism of action targets certain metabolic pathways which share their mode of action with IF [Citation14]. Therefore, the combined effect of intermittent fasting and hesperidin intake may provide additional benefits or produce synergistic effects with IF. The present study aims to investigate the synergistic effects of an IF diet along with antioxidant supplementation (hesperidin) on the biomarkers of aging and inflammation by measurement of oxidative stress level, general biochemical parameters and major inflammatory markers in male Wistar rats.
Materials and methods
All chemicals and reagents
2, 4-Dinitrophenylhydrazine (DNPH), 5, 5-Dithiobis nitro benzoic acid (DTNB) and 4, 7-Diphenyl-1,10-phenanthroline disulfonic acid sodium salt (DPI) were obtained from Sigma (St. Louis, MO, USA) and TCI Chemicals Pvt. Ltd (India). Total cholesterol (TC), triglyceride (TG), high-density lipoprotein (HDL), serum alanine transaminase (ALT) and aspartate aminotransferase (AST) kits were obtained from Span diagnostics and ERBA diagnostics (Transasia, India). All other chemicals were of analytical grade and available from Merck (Germany) and SRL (India).
Experimental study design and treatments
Middle-aged male Wistar rats, 12–15 months age, were used in the study. They were acclimatized and housed in standard living conditions of a 12/12-hour light/dark cycle at a controlled temperature of 23 ± 2°C, and relative humidity levels of 50% ±20%, with ad libitum access to drinking water and standard supplement rich pellets. The initial body weight of the rats was 220 ± 10 g for control group, 225 ± 8 g for ADF group, 232 ± 10 g for hesperidin-treated groups and 215 ± 6 g for the ADF and hesperidin supplemented group. All animal care and laboratory methods were conducted according to the guidelines of the Control and Supervision of Experiments on Animals (CPCSEA) and Institutional Animal Ethics Committee (IAEC), University of Allahabad, India (IAEC/AU/2019(1)/09).
The experimental groups were organized as follows
Group I: Normal control. This group was administered 1.0 mL of carboxymethylcellulose (CMC) (0.5% dissolved in water) orally per day as vehicle control.
Group II. Experimental Alternate day fasted rats. This group was given their normal chow diet on alternate days i.e. fed on one day and fasted the next day (chow diet was placed and removed on alternate days) described earlier in detail [Citation15].
Group III. Hesperidin (control). This group received hesperidin orally (100 mg/kg body weight) dissolved in CMC once regularly, as described earlier [Citation16].
Group IV. Hesperidin + Alternate day fasted rats. This group is similar to group II but was additionally supplemented with hesperidin. This group received oral administration of hesperidin (100 mg/kg body weight) (same as given to group III).
Chow diet was placed and removed at 10 a.m. for alternate day fed rats. The sample size of rats assigned to each group was six rats per group and the total duration of dosing was three weeks.
Collection of blood, separation of plasma, serum and RBCs
After completing the duration of treatment, the rats were sacrificed under light anesthesia (pentobarbital 50 mg/kg body weight) and blood was collected in heparinized syringes via a cardiac cut. Plasma was isolated by centrifugation of blood at 800 g for 10 minutes at 4°C, after which packed red blood cells (PRBCs) were obtained. The PRBCs obtained after removal of the buffy coat and upper 15% of the packed RBCs were subsequently washed thrice with cold phosphate-buffered saline (PBS) which were later stored in glucose phosphate buffer saline (GPBS) (Glucose+ PBS) at 4°C. The PRBCs were used on the same day for biochemical assays while plasma was stored at −80°C for further evaluation. Serum was isolated by collecting blood in non-heparinized syringes and allowed to clot. This was then centrifuged at 3000 g for 5 min and sera was isolated and stored at −80°C for further analysis.
Determination of fasting blood glucose, serum triglyceride, total cholesterol, HDL and serum ALT and AST levels
Fasting glucose level was measured by Accu-check active blood glucose monitoring system. Determination of serum total cholesterol (TC), triglyceride (TG), high-density lipoprotein (HDL), serum alanine transaminase (ALT) and aspartate aminotransferase (AST) measurements were made on an Erba Mannheim Chem-7 analyzer.
Estimation of total antioxidant activity by FRAP
The total antioxidant capacity of the plasma was measured by the FRAP assay as per the established protocol [Citation17]. This assay is based upon the ability of ferric ion to get reduced to ferrous ion at low pH and form colored complex product ferrous tripyridyltriazine. Absorbance values are obtained by change in absorbance at 593 nm. Readings were taken at 30 s intervals for 5 min and values were calculated and expresssed as (μmol Fe(II)/L plasma).
Determination of erythrocyte reduced glutathione (GSH)
Erythrocyte GSH was determined by following the established protocol [Citation18]. This method exploits the reducing ability of the thiol 5, 5’-dithiobis [2-nitrobenzoicacid] (DTNB) to reduce the sulfhydryl (–SH) group to form a yellow anionic product measured at 412 nm. GSH concentration is expressed in terms of mg/mL PRBCs.
Determination of erythrocyte plasma membrane redox system (PMRS)
The estimation of erythrocyte PMRS activity was done by the method described previously [Citation19], based on the property of reduction of ferricyanide. Briefly, 0.2 ml of PRBCs was suspended in PBS containing 5 mM glucose and 1 mM potassium ferricyanide and was made to a final volume of 2.0 ml. The resulting suspension was incubated at 37°C for 30 min and centrifuged at 800 g for 15 min. The absorption of the supernatant was assayed for ferrocyanide content at 535 nm using 4, 7‐diphenyl‐1, 10‐phenanthrolinedisulfonic acid disodium salt. Data are expressed in µmol ferrocyanide/mL PRBC/30 min.
Estimation of lipid peroxidation in terms of malondialdehyde (MDA) content
MDA content was determined following the established protocol with slight modification [Citation20]. The lipid peroxidation is based on the reaction between thiobarbituric acid (TBA) and malondialdehyde (MDA) and their adduct formation MDA- TBA2. The MDA concentration was calculated using the extinction coefficient (ε = 153,000), which is expressed in units nmol/mL of packed RBC. The absorbance readings were taken at 532 nm.
Determination of plasma protein carbonyl (PCO)
Plasma protein carbonyl level was measured according to the procedure of Levine [Citation21]. Protein carbonyls react with 2,4-dinitrophenylhydrazine and produce hydrazones as supernatant. The total protein carbonyl generated in plasma was estimated by taking spectra measurements of the obtained supernatant at 370 nm against control. Plasma protein content was estimated by the method of Lowry [Citation22] using bovine serum albumin as standard. Carbonyl content was calculated using an absorption coefficient of 22,000 M−1 cm−1 and data were expressed in units of nmol/mg protein.
Determination of plasma advanced oxidation protein products (AOPP)
Plasma AOPP was measured following the method of Witko-Sarsat [Citation23] with slight modifications [Citation24]. Chloramine-T solution (0–100μ mol/L) was used as a calibrator, which calculated the value of oxidized protein products at 340 nm and AOPP concentration was expressed as μmol of chloramine-T equivalent/liter.
Estimation of plasma Nitric oxide (NO) level
Nitric oxide level was measured in the plasma by employing Griess reaction [Citation25]. The samples were incubated with Griess reagent (0.1% naphthalene diamine HCl; 1% sulfanilamide in 5% phosphoric acid mixed as 1:1) and the pink-colored product thus formed was measured at 540 nm. Production of NO was calculated by comparing standard sodium nitrite concentration and the results are expressed as μmol/L.
Estimation of cytokines levels in serum of rats
Serum insulin and adiponectin level and cytokine levels (TNF-α, IL-6, CRP and COX) estimation was done following the instructions of the manufacturing company (Krishgen Biosystem, India). The detailed steps have been elucidated in our earlier lab report [Citation16].
Statistical analysis
Data are expressed as mean ± SD for six independent experiments. Statistical analysis was performed using Graph Pad Prism version 5.01 software. Differences between the groups were assessed by one-way ANOVA followed by Bonferroni’s post-hoc test comparing all pairs of columns. A probability (P) value p < 0.05 was considered as statistically significant.
Results
Effect of ADF and hesperidin supplementation on general characteristic and biochemical parameters
shows the changes observed in body weight, blood glucose and other experimental parameters (serum TC, TG, HDL, ALT and AST) in all the groups. A significant decline (p < 0.05) of (10.54%) in the body weight of rats on ADF group was observed in comparison with control. Other groups had no significant change. The fasting blood glucose of group II (ADF) and group III (hesperidin) was significantly reduced (p < 0.05) in comparison with control rats whereas the ADF and hesperidin combined group also showed significant reduction (p < 0.05) in blood glucose when compared to ADF alone. Serum TG was significantly reduced (p < 0.05) in ADF group when compared to control and in ADF and hesperidin combined group in comparison with ADF. The TC decreased significantly (p < 0.05) in group II and III both with respect to control rats and in group IV with respect to ADF group. Serum ALT showed significant reduction (p < 0.05) only in group IV (ADF+ Hesperidin) in comparison with ADF group and serum AST was found to reduce (p < 0.05) in group III when compared to control and in group IV when compared to group III. The level of HDL reduced significantly (p < 0.05) during ADF and hesperidin treatment, respectively, when compared to control and also in the ADF+ hesperidin-treated group with respect to ADF group.
Table 1. General characteristic and serum experimental parameters.
Plasma antioxidant status during ADF and hesperidin supplementation
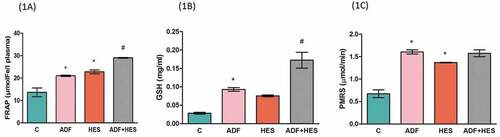
Plasma antioxidant status was determined using FRAP assay. FRAP is shown in and denotes significant increase (p < 0.05) in ADF rats and hesperidin group, respectively, in comparison with control and ADF and hesperidin combined group compared to ADF alone (group II). The RBC GSH content displayed in was significantly increased (p < 0.05) in group II when compared to control and group IV with respect to group II. represents the erythrocyte redox balance measured in terms of PMRS activity. It was observed that PMRS increased significantly (p < 0.05) in ADF rats and in hesperidin-treated group in comparison with control.
Figure 1. Effect of hesperidin treatment on antioxidant levels in rats maintained on alternate day fasting. (1a) Antioxidant levels measured by FRAP levels reported in terms of µmol Fe/l plasma. * denotes significant increase (p < 0.05) in comparison with control group and # denotes significant increase (p < 0.05) when compared to ADF group. (1b) Erythrocyte glutathione (GSH) content in units mg/ml. * significantly higher (p < 0.05) when compared to control and # significantly higher (p < 0.05) with respect to ADF group. (1c) PMRS activity (μmole ferrocyanide/ml PRBC/30 min). * significantly higher (p < 0.05) compared to control group. Values are expressed as mean ±SD (n = 6) performed in duplicates. C- control; ADF- alternate day fasting; Hes- hesperidin; ADF+Hes- alternate day fasting+ hesperidin.
ADF and hesperidin treatment induces protective effects against oxidative damage
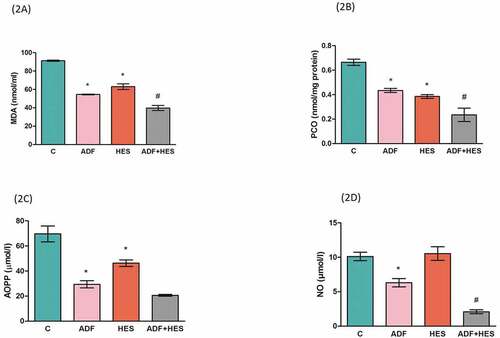
Lipid oxidized product shown in terms of MDA content is represented in . It was observed that MDA decreased significantly (p < 0.05) in both ADF and hesperidin-treated groups when compared to control and same in group IV compared to ADF alone. The protein carbonyl content is shown in . PCO level decreased significantly (p < 0.05) during ADF and in hesperidin supplementation both with respect to control group. The PCO levels were also significantly decreased (p < 0.05) in group IV when compared to group II. Plasma AOPP represented in showed significant decrease (p < 0.05) in ADF rats and hesperidin-treated rats with respect to control group. Plasma nitric oxide level () was significantly decreased (p < 0.05) during ADF with respect to control and in ADF and hesperidin-treated group when compared to ADF group.
Figure 2. Effect of hesperidin supplementation on biomarkers of oxidative stress in rats. (2a) Malonyldehyde (MDA) content in erythrocytes in unit nmol/ml. * significantly lower (p < 0.05) in comparison to control # significantly lower (p < 0.05) when compared to ADF group (2b) Protein carbonyl (PCO) content reported as nmol/mg protein. * significant decrease (p < 0.05) when compared to control, # significant decrease (p < 0.05) in comparison with ADF group. (2c) Advanced oxidation protein products (AOPP) measured as µmol/l. * significant decrease (p < 0.05) in comparison to control group. (2D) Nitric oxide (NO) level expressed as µmol/l. * significant decrease (p < 0.05) when compared to control; # significant decrease (p < 0.05) with respect to ADF group. C- control; ADF- alternate day fasting; Hes- hesperidin; ADF+Hes- alternate day fasting+ hesperidin.
Effect of ADF and hesperidin treatment on insulin, adiponectin and inflammatory cytokine levels in rats
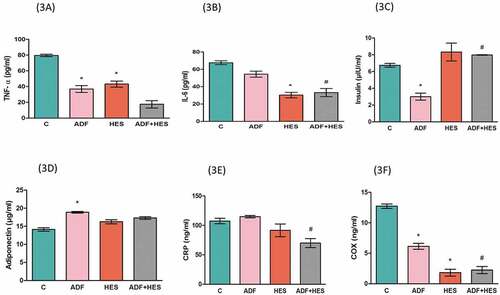
represents the serum level of insulin, adiponectin and inflammatory cytokines (TNF- α, IL-6, CRP and COX) in rats. Serum insulin () was significantly decreased (p < 0.05) in ADF rats in comparison with the control (group I) rats and in hesperidin and ADF combined group compared to ADF. Markers of inflammation TNF-α () showed significant reduction (p < 0.05) in group II and group III compared to control group, whereas IL-6 () was significantly reduced (p < 0.05) in hesperidin-treated rats when compared to control and hesperidin and ADF combined group in comparison with ADF group. Alternate day-fed rats (group II) showed a significant increase (p < 0.05) in serum adiponectin level () compared to control, whereas CRP () was found to decline in group IV only with respect to ADF group and COX () significantly decreased (p < 0.05) in group II and III both with respect to control and in group IV compared to group II.
Figure 3. Figure represents the serum levels of TNF-α, IL-6, Insulin, adiponectin, CRP and COX. The values are represented as TNF-α, IL-6 in terms of pg/ml; CRP and COX as ng/ml; insulin as µIU/ml and adiponectin in µg/ml units. The significant levels are indicated as (p < 0.05) * significant change with respect to control group and # (p < 0.05) significant change in comparison with ADF group. C- control; ADF- alternate day fasting; Hes- hesperidin; ADF+Hes- alternate day fasting+ hesperidin.
Discussion
Hesperidin is a citrus antioxidant having protective properties against oxidative stress-induced damage [Citation14]. The present study was undertaken to assess the effect of hesperidin administration on oxidative biomarkers in rats under ADF regimen. Hesperidin shares some common protective pathways with ADF. The role of hesperidin as an antioxidant is to provide protection against oxidative stress by upregulating the expression of Nrf2 gene, whereas ADF also mitigates oxidative damage and increases longevity by activating pathways induced by Nrf2 which corresponds to stress resistance in response to oxidative stress [Citation14]. Therefore, our results provide an overview of the synergistic benefits observed during antioxidant supplementation in rats which were maintained on a dietary restriction regimen.
An overall increase in FRAP was observed in the hesperidin supplemented fasting group which suggest a rise in antioxidant activity in their plasma. The plausible mechanism behind the rise in antioxidant levels is because of the ability of hesperidin to promote antioxidant activity by activating the ERK/Nrf signaling pathway [Citation14]. In addition, ADF reduces oxidative stress by activating antioxidant enzymes in plasma which further opens the way for the activation and functioning of defensive pathways of the cell.
ADF in rats has shown improvement in redox status because of rise in the reduced GSH levels. Our ADF and hesperidin supplemented group show additional improvement in GSH levels when compared to ADF alone. This change may be attributed to the synergistic effect of ADF which tends to increase the level of endogenous glutathione in blood by decreasing the oxidative stress level [Citation13,Citation26]. Hesperidin helps maintain the glucose and insulin concentration in blood which requires NADPH, that in turn, reinstates the GSH levels and thus maintains the redox state in blood and oxidative stress levels [Citation27].
Hesperidin has a protective effect on the glucose regulation and lipid peroxidation levels [Citation13]. Blood glucose is decreased in the ADF and hesperidin-treated groups, respectively. Moreover, the body weight is lowered significantly in the rats under ADF only. Therefore, ADF regimen has promising ability to promote weight loss and maintain glucose and insulin levels in blood. This result has been confirmed by several studies undergoing ADF or intermittent fasting in both rodents and humans [Citation28,Citation29]. The decrease in blood glucose during ADF and in ADF group supplemented with hesperidin leads to increased insulin sensitivity and the weight loss is because of fatty acid oxidation during fasting [Citation30]. Hesperidin attenuates blood glucose level and enhances insulin sensitivity due to the AMP-activated protein kinase- Peroxisome proliferator-activated receptor α (AMPK- PPARα) signaling pathway which also regulates the anti-oxidative property of flavanoids [Citation13]. The adiponectin level is elevated during ADF since it helps in maintaining the shift of fuel preference from glucose to fatty acids [Citation31]. On the other hand, although the PPAR- α gene is known to regulate the antiatherogenic effects during hesperidin supplementation, our results suggest no significant elevation in adiponectin levels in the hesperidin-administered groups due to lower ability of hesperidin to upregulate PPAR-α expression to the levels which causes rise in adiponectin in serum [Citation32].
The free radical scavenging ability of hesperidin helps it to reduce the oxidized content of protein and lipid, thereby reducing PCO, AOPP and MDA levels. Therefore, hesperidin has the ability to decrease the oxidized content and maintain the antioxidant status by reducing all biomarkers of oxidative stress [Citation14,Citation27]. During ADF, the PPARα gene functions to downregulate lipid and protein synthesis helping in lowering stress by reducing the content of lipid- and protein-oxidized molecules in rat erythrocytes [Citation33].
The PMRS is a finely functioned system present to maintain the intracellular redox conditions during times of oxidative stress [Citation19]. Hesperidin promotes Nrf2 activity as the key transcription factor that releases the stress and activates primary antioxidant enzymes during condition of oxidative stress. Hesperidin supplementation can maintain erythrocyte PMRS by increasing the activity of Nrf2 which in turn induces the production of antioxidant enzymes [Citation12].
Hesperidin has ability to lower the level of serum TG and TC associated with high fat diet because of its antioxidant property [Citation16]. In addition, ADF also lowers the cholesterol and TG in rats because fasting induces fatty acid oxidation and lipolysis [Citation34]. Our results indicate additional significant decrease in triglyceride and cholesterol level in the fasting rats supplemented with hesperidin because both ADF and hesperidin are equally effective in reducing these serum markers, therefore showing synergistic effects. ALT and AST are intracellular enzymes and are the principal markers of liver damage. The ADF and hesperidin supplemented group showed significantly lowered levels of ALT and AST in blood serum which is an indicator of protected hepatocytes because of intact cellular integrity and reduced necrosis [Citation26]. The ADF and hesperidin group, respectively, had a significantly low HDL when compared to control, in addition to synergistic reduction in the ADF and hesperidin combined group. Hesperidin lowers the HDL content in rats by converting the cholesterol in liver to bile acids, therefore reducing its content in blood circulation [Citation35].
The NF-kβ regulated pathway prominently regulates the generation of pro-inflammatory cytokines and attenuation of this pathway inhibits their expression [Citation36]. The major pro-inflammatory cytokine molecules which cause rise in oxidative-free radical generation are the IL-6, TNF-α, CRP and COX. Hesperidin supplementation in rats is known to inhibit the generation of these inflammatory factors since flavonoids possess the inherent ability to slow the NF-kβ pathway which helps in down- regulation of these inflammatory markers and prevent oxidative damage [Citation37].
The COX levels are significantly reduced in hesperidin and ADF- and hesperidin-treated group. Flavonoids have the ability to inhibit oxidative damage by restricting the expression of cyclooxygenase isoform which is consequently related to NO synthesis. This decline in COX in ADF and the ADF and hesperidin combined group also helps in lowering NO levels by scavenging NO oxidative radicals, thereby adhering to the property of hesperidin as a flavonoid and reducing inflammation and oxidative damage [Citation27].
Conclusion
Collectively, the findings from this study suggest that supplementation of hesperidin during ADF may provide some additional protection and serve beneficial functions to rescue from oxidative stress conditions due to the interplay of same metabolic pathways and mechanisms in both the conditions.
Availability of data and material
The information that helps the finding of this study is accessible from the corresponding author upon reasonable request.
Author contribution
SIR conceived and designed research. SB conducted experiments, analyzed data, and wrote the manuscript, RK and RK conducted experiments. All authors have read and approved the manuscript.
Ethics approvals
All the protocols and procedures were followed as mandated by the Ethical Committee of the University of Allahabad, Allahabad, India. The work described has been also carried out in accordance with The Code of Ethics of the World Medical Association (Declaration of Helsinki) for experiments involving animal.
Statements and Declaration
The authors report there are no competing interests to declare.
Acknowledgments
Sukanya Bhoumik is a recipient of Senior Research Fellowship from Council of Scientific and Industrial Research (CSIR), Government of India. The Department of Biotechnology, Government of India, has provided financial support under the ‘Research Resources, Service Facilities, and Platforms’ program. The Department of Biochemistry is funded by the DST, FIST Grant, New Delhi, and the SAP DRS I from UGC.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- López-Otín C, Blasco MA, Partridge L, et al. The hallmarks of aging. Cell. 2013;153(6):1194–1217. Available from: https://doi.org/10.1016/j.cell.2013.05.039
- Ingram DK, Roth GS. Calorie restriction mimetics: can you have your cake and eat it, too? Ageing Res Rev. 2015;20:46–62.
- Most J, Tosti V, Redman LM, et al. Calorie restriction in humans: an update. Ageing Res Rev. 2017;39:36–45. Available from: https://doi.org/10.1016/j.arr.2016.08.005
- Duregon E, Pomatto-Watson LCDD, Bernier M, et al. Intermittent fasting: from calories to time restriction. GeroScience. 2021;43(3):1083–1092. Available from: https://doi.org/10.1007/s11357-021-00335-z
- Bárcena C, Mayoral P, Quirós PM. Mitohormesis, an antiaging paradigm. In: International review of cell and molecular biology. Netherlands: Elsevier; 2018. p. 35–77. Available from: https://doi.org/10.1016/bs.ircmb.2018.05.002
- Stockman M-C, Thomas D, Burke J, et al. Intermittent fasting: is the wait worth the weight? Curr Obes Rep. 2018;7(2):172–185. Available from: https://doi.org/10.1007/s13679-018-0308-9
- Longo VD, Mattson MP. Fasting: molecular mechanisms and clinical applications.Cell Metab. 2014;19(2):181–192.
- Carocho M, Ferreira ICFR. A review on antioxidants, prooxidants and related controversy: natural and synthetic compounds, screening and analysis methodologies and future perspectives. Food Chem Toxicol. 2013;51: 15–25. Available from: https://doi.org/10.1016/j.fct.2012.09.021
- Hargreaves IP, Mantle D. Coenzyme Q10 supplementation in fibrosis and aging. In: Guest PC, editor. Reviews on biomarker studies in aging and anti-aging research. Cham: Springer International Publishing (Advances in Experimental Medicine and Biology; 2019. 103–112. Available from: https://doi.org/10.1007/978-3-030-25650-0_6
- Sadowska-Bartosz I, Bartosz G. Effect of antioxidants supplementation on aging and longevity. Biomed Res Int. 2014;2014:1–17. Available from: https://doi.org/10.1155/2014/404680.
- Li C, Schluesener H. Health-promoting effects of the citrus flavanone hesperidin.Crit Rev Food Sci Nutr. 2017;57(3):613–631.
- Man M-Q, Yang B, Elias PM. Benefits of hesperidin for cutaneous functions. Evid Based Complement Alternat Med. 2019;2019:1–19. Available from: https://doi.org/10.1155/2019/2676307.
- Xiong H, Wang J, Ran Q, et al. Hesperidin: a therapeutic agent for obesity. Drug Des Devel Ther. 2019;13:3855–3866. Available from: https://doi.org/10.2147/DDDT.S227499
- Parhiz H, Roohbakhsh A, Soltani F, et al. Antioxidant and anti-inflammatory properties of the citrus flavonoids hesperidin and hesperetin: an updated review of their molecular mechanisms and experimental models: HESPERIDIN AND HESPERETIN AS ANTIOXIDANT AND ANTI-INFLAMMATORY AGENTS. Phytother Res. 2015;29(3):323–331. Available from: https://doi.org/10.1002/ptr.5256
- Bhoumik S, Kumar R, Rizvi SI. Time restricted feeding provides a viable alternative to alternate day fasting when evaluated in terms of redox homeostasis in rats. Arch Gerontol Geriatr. 2020;91:104188.
- Kumar R, Akhtar F, Rizvi SI. Hesperidin attenuates altered redox homeostasis in an experimental hyperlipidaemic model of rat.Clin Exp Pharmacol Physiol. 2020;47(4):571–582.
- Benzie IFF, Strain JJ. The Ferric Reducing Ability of Plasma (FRAP) as a measure of “Antioxidant Power”: the FRAP assay. Anal Biochem. 1996;239(1):70–76. Available from: https://doi.org/10.1006/abio.1996.0292
- Beutler E, Gelbart T, Pegelow C. Erythrocyte glutathione synthetase deficiency leads not only to glutathione but also to glutathione-S-transferase deficiency. J Clin Investig. 1986;77(1):38–41. Available from: https://doi.org/10.1172/JCI112298
- Rizvi SI, Jha R, Maurya PK. Erythrocyte plasma membrane redox system in human aging.Rejuvenation Res. 2006;9(4):470–474.
- Esterbauer H, Cheeseman KH. 42] Determination of aldehydic lipid peroxidation products: malonaldehyde and 4-hydroxynonenal. In: Methods in enzymology. Netherlands: Elsevier; 1990. p. 407–421. Available from: https://doi.org/10.1016/0076-6879(90)86134-H
- Stadtman ER, Levine RL. 2006. Protein Oxidation. Annals of the New York Academy of Sciences. 899(1):191–208. Available from: https://doi.org/10.1111/j.1749-6632.2000.tb06187.x
- Lowry OH, Rosebrough N, Farr AL, et al. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951;193(1):265–275.
- Witko-Sarsat V, Friedlander M, Capeillère-Blandin C, et al. Advanced oxidation protein products as a novel marker of oxidative stress in uremia. Kidney Int. 1996;49(5):1304–1313. Available from: https://doi.org/10.1038/ki.1996.186
- Singh AK, Singh S, Garg G, et al. Rapamycin alleviates oxidative stress-induced damage in rat erythrocytes. Biochem Cell Biol. 2016;94(5):471–479. Available from: https://doi.org/10.1139/bcb-2016-0048
- Yamamoto K, MD Fazle Akbar SK, et al. Increased nitric oxide (NO) production by antigen-presenting dendritic cells is responsible for low allogeneic mixed leucocyte reaction (MLR) in primary biliary cirrhosis (PBC): dendritic cells in PBC. Clin Exp Immunol. 1998;114(1):94–101. Available from: https://doi.org/10.1046/j.1365-2249.1998.00696.x
- Pari L, Karthikeyan A, Karthika P, et al. Protective effects of hesperidin on oxidative stress, dyslipidaemia and histological changes in iron-induced hepatic and renal toxicity in rats. Toxicol Rep. 2015;2:46–55. Available from: https://doi.org/10.1016/j.toxrep.2014.11.003
- Mahmoud AM, Ashour MB, Abdel-Moneim A, et al. Hesperidin and naringin attenuate hyperglycemia-mediated oxidative stress and proinflammatory cytokine production in high fat fed/streptozotocin-induced type 2 diabetic rats. J Diabetes Complications. 2012;26(6):483–490. Available from: https://doi.org/10.1016/j.jdiacomp.2012.06.001
- Antoni R, Johnston KL, Collins AL, et al. Effects of intermittent fasting on glucose and lipid metabolism. Proc Nutr Soc. 2017;76(3):361–368.
- Seimon RV, Roekenes JA, Zibellini J, et al. Do intermittent diets provide physiological benefits over continuous diets for weight loss? A systematic review of clinical trials. Mol Cell Endocrinol. 2015;418:153–172. Available from: https://doi.org/10.1016/j.mce.2015.09.014
- Lushchak O, Strilbytska, O, Piskovatska, V, Storey, K.B, et al. 2018. Intermittent fasting. Reference module in biomedical sciences. Netherlands: Elsevier. B9780128012383621000. Available from: https://doi.org/10.1016/B978-0-12-801238-3.62133-5
- Golbidi S, Daiber A, Korac B, et al. Health benefits of fasting and caloric restriction. Curr Diab Rep. 2017;17(12):123. Available from: https://doi.org/10.1007/s11892-017-0951-7
- Liu L, Shan, S, Zhang, K, QiangNing, Z, Ping Lu, X, Yu Cheng, Yi, et al. Naringenin and hesperetin, two flavonoids derived from Citrus aurantium up-regulate transcription of adiponectin: FLAVONES DERIVED FROM CITRUS AURANTIUM UP-REGULATE TRANSCRIPTION OF ADIPONECTIN. Phytother Res. 2008;22(10):1400–1403. Available from: https://doi.org/10.1002/ptr.2504
- Bhoumik S, Rizvi SI. Anti‐aging effects of intermittent fasting: a potential alternative to calorie restriction? Biologia. 2021;76(8):2329–2336. Available from: https://doi.org/10.1007/s11756-021-00770-5
- Tinsley GM, La Bounty PM. Effects of intermittent fasting on body composition and clinical health markers in humans.Nutr Rev. 2015;73(10):661–674.
- Rekha SS, Pradeepkiran JA, Bhaskar M. Bioflavonoid hesperidin possesses the anti-hyperglycemic and hypolipidemic property in STZ induced diabetic myocardial infarction (DMI) in male Wister rats. J Nutrit Intermed Metabol. 2019;15:58–64.
- Scoditti E. Neuroinflammation and neurodegeneration: the promising protective role of the citrus flavanone hesperetin.Nutrients. 2020;12(8):2336.
- Spagnuolo C, Moccia S, Russo GL. Anti-inflammatory effects of flavonoids in neurodegenerative disorders. Eur J Med Chem. 2018;153:105–115.
