ABSTRACT
Aluminum (Al) is a ubiquitous element on planet earth and its exposure to human systems is increasing steadily due to industrialization. Its accumulation in the biological systems have deleterious effects such as neurodegeneration and cognitive impairment. Centella asiatica L. (CA) is a herb used in the Ayurveda system of medicine as a neurotonic and for enhancement of memory. This study aimed at assessing the protective role of CA in aluminum chloride (AlCl3) induced cognitive dysfunction in rats. Results have shown that AlCl3 toxicity caused significant (p < 0.05) cognitive dysfunction of both spatial and non-spatial memory in rats, histopathological aberration of cerebral cortex and significant (p < 0.05) increase in the level of acetylcholinesterase in their brain. However, administration of CA irrespective of the dose given prevented all the observed changes which are comparable to a standard drug donepezil. CA prevented cognitive impairment and neurodegeneration in rats by decreasing the level of acetylcholinesterase. Hence, CA could be developed as a memory enhancing drug.
Background
Aluminum (Al) is an abundant metal on earth’s crust and its exposure into the biological system is constantly increasing due to growing global industrialization [Citation1,Citation2]. Daily lifestyle of humans makes it easy for Al to gain entrance into their bodies, as they are constantly exposed through diet, medicines (adjuvant, antacids, buffered aspirin and allergen), cosmetics and utensils. Hence, it is of utmost importance to understand the effects of Al exposure on humans [Citation3]. Studies have shown, chronic exposure to Al leads to progressive neurodegeneration of different parts of the brain including hippocampus and cerebral cortex. It also resulted into undesirable biochemical changes [Citation4,Citation5]. Although, the role of Al or aluminum chloride (AlCl3) in aggravating neurodegenerative diseases such as Parkinsonism and Alzheimer’s disease (AD) is debatable, chronic exposure to Al resulted into learning and memory impairments and cognitive dysfunction as observed in both clinical and animal experiments [Citation6,Citation7]. Further, rodents exposed to Al have shown increased activities of acetylcholinesterase and reduced levels of acetylcholine which invariably resulted to memory dysfunction [Citation8–10].
Large-scale research is ongoing in search of medicinal plants with antioxidant properties as well as memory enhancing capabilities [Citation11]. Centella asiatica (L.) (CA) urban, belonging to Apiaceae family, locally known as pegaga in Malay is a perennial herbaceous creeper [Citation12,Citation13]. CA is considered to be an invigorating herb and believed to enhance memory and intelligence in Ayurvedic system of medicine. CA’s medicinal values are attributed to its numerous active constituents which includes madecassic acid, asiaticoside, asiatic acid, braminoside, bramoside, madecassoside and flavonoids [Citation14]. Asiatic acid exerted neuroprotection against AlCl3-induced apoptosis, oxidative stress and amyloid pathology in rats [Citation15]. Further, it also exhibited a powerful neuroprotective ability against cerebral ischemia in a mouse model [Citation16]. Existing literature has shown that CA extract increased dendritic arborization in neuronal cells [Citation17], has antioxidant ability [Citation18] and has improved mental state of psychologically impaired children [Citation19]. Against this backdrop, this study evaluated the protective potentials of CA against AlCl3-induced cognitive dysfunction in rat. Motor activity of the rats was evaluated followed by cognitive abilities, afterward the rats were euthanized and the level of acetylcholenesterase in their brains was evaluated besides the histology of their cerebral cortices.
Methods
Ethics statement
Before the commencement of the study, the protocol was revised and approved by the Animals Ethics Committee, Universiti Putra Malaysia (UPM/IACUC/AUP-R096/2016). Thirty female albino rats weighing between 200–250 g, aged 2 months, purchased from a local vendor (Perniagaan Usaha Cahaya, Batu Caves, Selangor), were used in this study. The rats were kept in a Standard Animal House 2–3 per cage, at temperature (22°C ±3°C) and 12/h light /dark cycle. Additionally, the rats were given free access to food and water. They were kept 7 days for acclimation with the laboratory conditions before the commencement of the experiments proper.
Chemicals and equipment
Sigma Aldrich (St. Louis, MO, USA) supplied AlCl3 and cresyl violet, while ELISA kits for acetylcholinesterase were bought from Wuhan Fine Biotech Co., Ltd. Open field box and T-maze apparatus were locally fabricated from the home laboratory, all other chemicals used were of analytic grade. A standardized 60% hydro-ethanol extract of Centella asiatica (ref. no. AuRins-MIA-1-0) was used [Citation20,Citation21] for the study. Briefly, 60% aqueous ethanol extraction was carried-out with plant material to solvent ratio of 1:10 (weight to volume). Dried powder of CA was soaked in to the solution for 8 h at temperature of 60°C. Afterward it was filtered, concentrated and freeze dried. The yield of the crude extract in relation to the dried weight of the plant material was 16%. HPLC analysis was conducted using Agilent 1290 Infinity II LC [Citation20].
Study design and treatment protocol
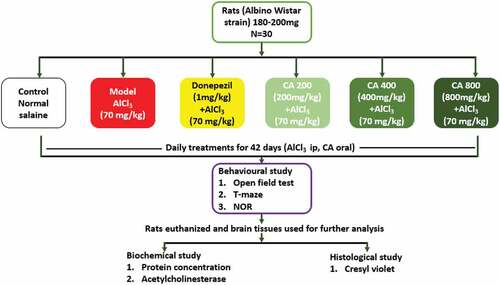
The rats were grouped randomly into six (n = 5). Group 1 (control) received distilled water orally and normal saline intraperitoneally, Group 2 received AlCl3 (70 mg/kg b.w) intraperitoneally for 42 days. Group 3 rats were co-administered with Donepezil (dissolved in distilled water, 1 mg/kg b. w) and AlCl3 for 42 days. While groups 4, 5 and 6 rats were co-administered with CA extract 200, 400 and 800 mg/kg b. w respectively and AlCl3 for 42 days. At completion of the treatment regimen, rats were assessed for motor and cognitive behaviors followed by euthanisia and their brains were excised for histological and biochemical analysis ().
Behavioral assessment
Open field test (OFT)
The OFT is centered on overall activities of rodents when placed in a new environment, the apparatus used [Citation22] and the protocol followed were earlier described [Citation23].
T-maze spontaneous alternation test
The T-maze task was design to test the spatial learning and memory in rodents, the apparatus used and the protocol followed was adopted from Deacon [Citation24].
Novel object recognition (NOR) test
The novel object recognition test was carried out to evaluate the cognitive abilities of rats as earlier documented [Citation25]. Hence, the apparatus used and the protocol followed were adopted from previous studies [Citation21,Citation25].
Histological studies
The integrity of the cells on the cerebral cortices of the rats was checked through cresyl violet stain [Citation26], while the scoring of histopathological changes was carried-out by an independent pathologist blinded to the groupings [Citation27]. The slides were viewed using an Olympus BX51 and DP72 under 40x, 100x and 400x magnification with different spots per specimens. The Olympus Cellˆ F Imaging software used to capture images of the slide.
Biochemical studies
Protein determination
The protein concentration of the brain homogenate was ascertained through bovine serum albumin as a standard and according to Bradford method [Citation28].
Determination of AChE activity
In the brain tissue homogenate, AChE activity was assessed using ELISA Kits (Wuhan Fine Biotech Co., Ltd.) according to their manufacturer’s instructions.
Statistical analysis
For biochemical analysis, OFT, T-MAZE and scoring of histology, statistical analyses were conducted using one way ANOVA followed by Tukey’s post hoc test. While for NOR test, two-way ANOVA followed by Tukey’s post hoc test was employed. All data were expressed as the mean ± standard error of mean (SEM). P value of < 0.05 was considered to be statistically significant.
Results
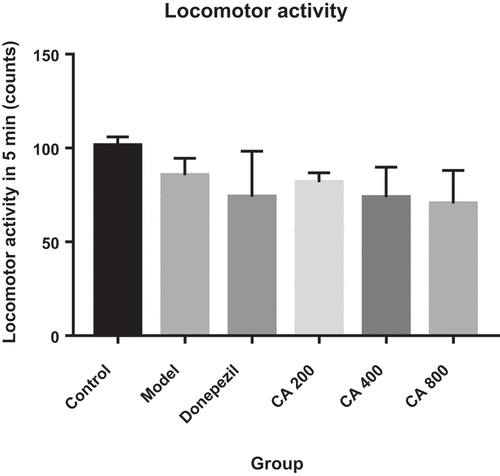
Effects of CA on the locomotor activities of AlCl3-induced rats in open field test
One way ANOVA did not show any statistically significant differences [F (5, 21) = 2.034, p = ns] in the distances moved among the groups of rats ().
Figure 2. Effect of CA on locomotor activities. Control (distilled water + normal salin), Model (AlCl3 70 mg/kg. bwt), Donepezil (Donepezil + AlCl3 70 mg/kg.bwt), CA 200 (CA 200 mg/kg.bwt + AlCl3), CA 400 (CA 400 mg/kg.bwt + AlCl3) and CA 800 (CA 800 mg/kg.bwt + AlCl3). Data represent as mean ± SEM (n = 5).
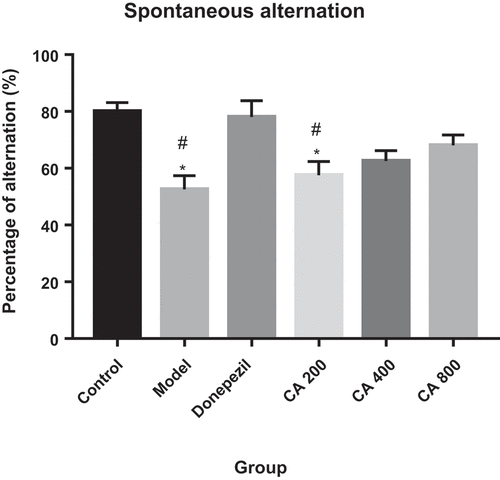
Effects of CA on spontaneous alternation in AlCl3-induced rats during T-maze test
As shown in , rats from control group scored 80%, model 52.5%, Donepezil 78%, CA 200 57.5%, CA 400 62.5% and CA 800 68%. One-way ANOVA showed significant differences in the number of alterations [F (5, 24) = 6.135, p = 0.0009]. Tukey’s test further showed a marked of correct alternations in model group (52.5 ± 6.168, p = 0.0026) and CA 200 group (57.5 ± 5.72, p = 0.0172) in comparison to control group (80 ± 7.07). Additionally, there is statistically significant decrease of correct alternations in model group (78 ± 5.72, p = 0.0056) and CA 200 group (57.5 ± 4.598, p = 0.0353), when compared to Donepezil group (78 ± 12.58) of rats.
Figure 3. Effect of Centella asiatica on spontaneous alternation in rats using T-maze. Control (distilled water + normal saline), Model (AlCl3 70 mg/kg. bwt), Donepezil (Donepezil 10 mg/kg + AlCl3 70 mg/kg.bwt), CA 200 (200 mg/kg.bwt + AlCl3), CA 400 (400 mg/kg.bwt + AlCl3) and CA 800 (800 mg/kg.bwt + AlCl3). Data represent mean ± SEM (n = 5), one way ANOVA. * = p < 0.05, Model and CA 200 vs. Control group. # = p < 0.05, Model and CA 200 vs. Donepezil group.
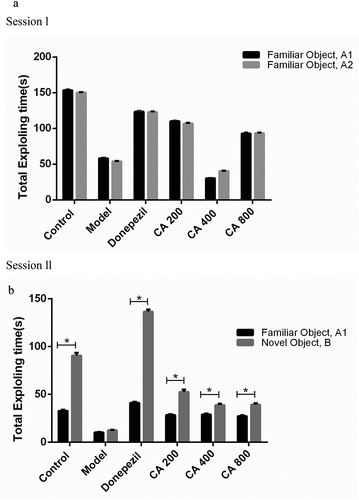
Effects of Centella asiatica against non-spatial memory deficit in AlCl3-induced rats in the NOR test.
In the acquisition trial (Session I), two-way ANOVA did not show any statistical significant differences in the time spent by the rats exploring the two familiar objects A1 and A2 (). In the retention trial (Session ll), two-way NOVA revealed statistical significant interactions between the treatment given and recognition time of the rats toward the novel object [F (5, 40) = 344.7 P < 0.0001] (). Bonferroni’s post hoc test confirmed a statistical significant difference (p < 0.05) in the preference for the novel object by all the groups of rats except for the model group.
Figure 4. A and b: Effect of different doses of Centella asiatica on the preference of both familiar objects (Session I) (a) and preference of familiar and novel object after 30 minutes (session II) (b) of introductory session. Data were analyzed using independent sample t-test. *P < 0.05. Control (distilled water + normal saline), Model (AlCl3 70 mg/kg. bwt), Donepezil (Donepezil 10 mg/kg + AlCl3 70 mg/kg.bwt), CA 200 (200 mg/kg.bwt + AlCl3), CA 400 (400 mg/kg.bwt + AlCl3) and CA 800 (800 mg/kg.bwt + AlCl3).
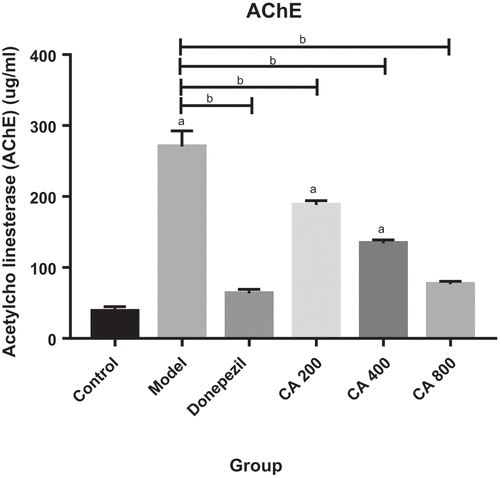
Effects of Centella asiatica on brain acetylcholinesterase (AChE) level in AlCl3 induced rats
. shows a statistically significant difference in the Model, CA 200 and CA 400 group as compared to control group. CA 800 group shows an effective treatment of CA on the rat’s brain as it reduces the AChE level until it does not show significant difference as compared to control. This figure also showed a statistically significant decreased of AChE level in all treatment groups when compared with model group.
Figure 5. Effect of Centella asiatica on the rat’s brain acetylcholinesterase (AChE) levels. Control (distilled water + normal saline), Model (AlCl3 70 mg/kg. bwt), Donepezil (Donepezil 10 mg/kg + AlCl3 70 mg/kg.bwt), CA 200 (200 mg/kg.bwt + AlCl3), CA 400 (400 mg/kg.bwt + AlCl3) and CA 800 (800 mg/kg.bwt + AlCl3). Data represent mean ± SEM (n = 5), one way ANOVA. a = p < 0.05, Model, CA 200 and CA 400 vs Control group. b = p < 0.05, Donepezil, CA 200, CA 400 and CA 800 vs Model group.
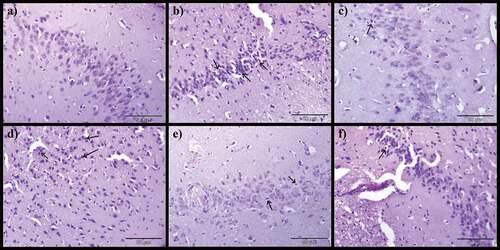
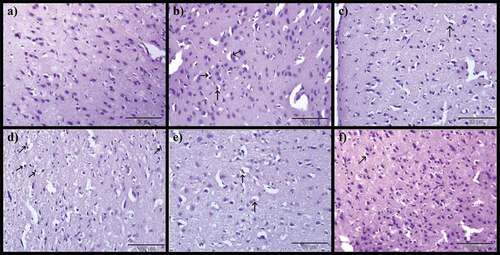
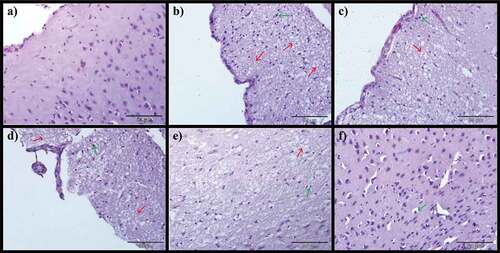
Histopathological alterations in the brain
demonstrates the potentials of CA on the brain of AlCl3-induced rat models. Normal histological appearance of the brain was noticed in control rats (), however, the AlCl3-induced rats showed severe encephalomalacia, diffuse gliosis (), focal gliosis () and severe precellular edema (). Notwithstanding, AlCl3-induced treated with Donepezil presented mild encephalomalacia with diffuse gliosis (), mild focal gliosis () and also mild precellular edema (). On the other hand, administration of CA to AlCl3-induced ameliorates most of the histological aberrations ( d-f, 7d-f and 8d-f) observed in the AlCl3-induced alone groups. The severity of pathological changes were scored and presented in .
Table 1. The severity of pathological changes.
Figure 6. Photomicrographs of cerebrum: a) Control group, b) Model group, c) Donepezil, d) CA 200, e) CA 400 and f) CA 800. H & E stain, magnification 40x, n = 3. Encephalomalacia of matrix brain (red arrow), diffuse gliosis (green arrow).
Discussion
The present study explored the protective potentials of CA on AlCl3-induced neurotoxicity in rat model. The AlCl3 toxicity resulted into diminishing of both non-spatial and spatial memories, cognitive dysfunction, neurodegeneration and increased level of AChE activities in rat model. However, co-administration of CA with AlCl3 irrespective of the dose given reversed the memory and cognitive dysfunctions, maintained the architecture of the brain cells besides attenuating the levels of AChE. These attributes of CA are comparable to that of donepezil, a commercially available anticholinesterase drug used for AD patients.
In this study, chronic exposure to AlCl3 alone or AlCl3 with CA or AlCl3 with donepezil does not affect the motor behavior of the rats in terms of locomotor activity. Therefore, impairments of motor activity of the rats might not have interfered with the cognitive tests carried-out in the present study, which is largely dependent on the rat’s movements. Similar results were documented in intracerebroventricular streptozotocin [Citation29] and AlCl3/d-gal [Chiroma Citation7] mediated cognitive impairments in rats. Hence, the present study continued with different behavioral paradigms to ascertain the cognition enhancing potentials of CA as claimed by folkloric usages [Citation2,Citation13].
T-maze test is used differently to assess the mental behaviors in rodents [Citation30], therefore alternation either rewarded or spontaneous in T-Maze is a superb method for detection of hippocampal impairment, possibly superior to Morris water maze [Citation24] since both procedures identify hippocampal abrasions. In this study, AlCl3-induced rats showed complete hippocampal lesion because they achieved less than 60% of correct alternation in T-maze spontaneous tasks. Notwithstanding, control, donepezil and CA exposed rat groups scored above 60% of correct alternations in the T-maze tasks. This study is in agreement with Rao [Citation31] and Chiroma [Citation10] who documented similar result on CA’s cognitive enhancing abilities.
This study further assessed the role of CA in enhancing non-spatial memory and learning in rats using NOR test. NOR test is usually desirable since it requires no external reward, motivation or punishment, but rather just a little habituation and training besides it can be completed in a short period of time [Citation32]. The spatial learning and memory dysfunctions seen in AlCl3 alone exposed group of rats in this study was overcame by CA and donepezil treated rats. As the rats were able to distinguish between familiar and novel objects, because of the increased time they spent exploring the novel objects which signifies improved non-spatial memory. The current study supported the earlier report by Lin [Citation33] who documented asiaitcosite, an active phytochemical isolated from CA improved cognitive abilities in senescence-accelerated mice (SAMP8).
The present study shows increased level of AChE in the cerebrum of the rats, which decreases after supplementation with CA or donepezil. Previous studies reported increased activity of AChE in brain after Al exposure [Citation34,Citation35] and decreased AChE activity after administration of ethanol extract of CA [Citation5]. Acetylcholine (ACh) is an important neurotransmitter for memory and learning and is involved in several neuropsychiatric functions. According to Raina [Citation36], AChE is accountable for the breakdown of ACh in the synaptic cleft. Therefore, mitigating AChE activity can increase the ACh levels in the synaptic cleft and partly recover the cognitive deficit in patients with AD. The cognitive and memory enhancing abilities of CA as revealed by the behavioral studies could be as a result of its potential to decrease the level of AChE, which could be attributed to its phytochemical asiatic acid [Citation15].
The histology of the rat’s cerebrum showed that AlCl3 facilitates a steady pathological alterations which includes; focal and diffuse gliosis, precellular edema encephalomalacia with neuronal degeneration. Findings such as nuclear degradation, karyorrhexis, dense cytosolic staining and disorganization of pyramidal cells of the hippocampus were also reported by similar studies [Citation2,Citation37,Citation38]. The histopathological aberrations observed in the present study and the others reported could be as a result of increased oxidative stress due to Al exposure [Citation2]. Al is an abundant heavy metal on earth’s crust which is associated with etiology of AD, where it aggravates brain’s oxidative stress. Al exerts its effects on synaptic junction and axons leading to degeneration of cholinergic neurons which are chiefly associated with memory and learning impairments [Citation6]. Notwithstanding, CA administration prevented the neurodegeneration by preserving the cholinergic neurons of the brain which could further be attributed to its AChE inhibition capacity as seen earlier in this study.
Conclusion
Conclusively, CA protects against AlCl3-induced cognitive impairment and neurodegeneration. As its administrations improved cognition as well as non-spatial learning and memory, which could be attributed to CA’s ability to decrease brain’s level of AChE. Therefore, CA could be developed as cholinesterase inhibitor to enhance memory and as a neuro-protectant. However, further studies on active phytochemical constituents of CA and the molecular pathways involved in its mechanism of action are recommended.
Ethical issues
The protocol was revised and approved by the Institutional Animal Care and Use Committee, Universiti Putra Malaysia, with a reference number UPM/IACUC/AUP-R096/2016
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Exley C. Human exposure to aluminium. Environ Sci Processes Impacts. 2013;15(10):1807–1816.
- Ha F, Wu Y, Wang H, et al. The Reference Intervals of Whole Blood Copper, Zinc, Calcium, Magnesium, and Iron in Infants Under 1 Year Old. Biol Trace Elem Res. 2022;200(1):1–12.
- Grochowski C, Blicharska E, Bogucki J, et al. Increased aluminum content in certain brain structures is correlated with higher silicon concentration in alcoholic use disorder. Molecules. 2019;24(9):1721.
- Savory J, Herman MM, Ghribi O. Mechanisms of aluminum-induced neurodegeneration in animals: implications for Alzheimer’s disease. J Alzheimers Dis. 2006;10(2–3):135–144.
- Amjad S, Umesalma S. Protective effect of Centella asiatica against aluminium-induced neurotoxicity in cerebral cortex, striatum, hypothalamus and hippocampus of rat brain-histopathological, and biochemical approach. J Mol Bio Diagnosis. 2015;6(1):1.
- Dumont M. Behavioral phenotyping of mouse models of neurodegeneration. In: Neurodegeneration. Totowa NJ: Humana Press; 2011. p. 229–237.
- Chiroma SM, Moklas MAM, Taib CNM, et al. D-galactose and aluminium chloride induced rat model with cognitive impairments. Biomed Pharmacother. 2018;103:1602–1608. 10.1016/j.biopha.2018.04.152.
- Auti ST, Kulkarni YA. Neuroprotective effect of cardamom oil against aluminum induced neurotoxicity in rats. Front Neurol. 2019;10:399.
- Batool Z, Sadir S, Liaquat L, et al. Repeated administration of almonds increases brain acetylcholine levels and enhances memory function in healthy rats while attenuates memory deficits in animal model of amnesia. Brain Res Bull. 2016;120:63–74.
- Chiroma SM, Baharuldin MTH, Mat Taib CN, et al. Protective effects of centella asiatica on cognitive deficits induced by D-gal/AlCl3 via inhibition of oxidative stress and attenuation of acetylcholinesterase level. Toxics. 2019;7(2):19. 10.3390/toxics7020019.
- Firdaus Z, Singh TD. An insight in pathophysiological mechanism of Alzheimer’s disease and its management using plant natural products. Mini Rev Med Chem. 2021;21(1):35–57.
- Gray NE, Alcazar Magana A, Lak P, et al. Centella asiatica: phytochemistry and mechanisms of neuroprotection and cognitive enhancement. Phytochem Rev. 2018;17(1):161–194.
- Chiroma SM, Moklas MAM, Norma CM, et al. Neuro-therapeutic benefits of Centella asiatica on some neurodegenerative diseases: a review. Res J Pharm Bio Chem Sc. 2017;8(6):549–556.
- Gohil KJ, Patel JA, Gajjar AK. Pharmacological review on Centella asiatica: a potential herbal cure-all. Indian J Pharm Sci. 2010;72(5):546.
- Rather MA, Thenmozhi AJ, Manivasagam T, et al. Neuroprotective role of Asiatic acid in aluminium chloride induced rat model of Alzheimer’s disease. Frontiers Biosc Scholar. 2018;10(2):262–275
- Krishnamurthy RG, Senut MC, Zemke D, et al. Asiatic acid, a pentacyclic triterpene from Centella asiatica, is neuroprotective in a mouse model of focal cerebral ischemia. J Neurosci Res. 2009;87(11):2541–2550.
- Mohandas Rao KG, Muddanna Rao S, Gurumadhva Rao S. Centella asiatica (L.) leaf extract treatment during the growth spurt period enhances hippocampal CA3 neuronal dendritic arborization in rats. Evid Based Complement Alternat Med. 2006;3(3):349–357.
- Gupta R, Flora SJS. Effect of Centella asiatica on arsenic induced oxidative stress and metal distribution in rats. J App Toxicol. 2006;26(3):213–222
- Appa Rao MVR, Srinivasan K, Rao KT, et al. The effect of Mandookaparni (Centella asiatica) on the general mental ability (Medhya) of mentally retarded children. J Res Indian Med. 1973;8(9):16.
- Wong JH, Muthuraju S, Reza F, et al. Differential expression of entorhinal cortex and hippocampal subfields α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) and N-methyl-D-aspartate (NMDA) receptors enhanced learning and memory of rats following administration of Centella asiatica. Biomed Pharmacother. 2019;110:168–180.
- Binti Mohd Yusuf Yeo NA, Muthuraju S, Wong JH, et al. Hippocampal amino‐3‐hydroxy‐5‐methyl‐4‐isoxazolepropionic acid GluA1 (AMPA GluA1) receptor subunit involves in learning and memory improvement following treatment with Centella asiatica extract in adolescent rats. Brain Behav. 2018;8(9):e01093.
- Chiroma SM, Baharuldin MTH, Taib CNM, et al. Protective effect of Centella asiatica against D-galactose and aluminium chloride induced rats: behavioral and ultrastructural approaches. Biomed Pharmacother. 2019;109:853–864.
- Dao D, Kovacsics C. Mood and anxiety related phenotypes in mice: characterization using behavioral tests. Vol. 2. Gould TD, Ed. New York: Humana Press; 2009.
- Deacon RM, Rawlins JNP. T-maze alternation in the rodent. Nat Protoc. 2006;1(1):7–12.
- Giorgetti M, Gibbons JA, Bernales S, et al. Cognition-enhancing properties of Dimebon in a rat novel object recognition task are unlikely to be associated with acetylcholinesterase inhibition or N-methyl-D-aspartate receptor antagonism. J Pharmacol Exp Ther. 2010;333(3):748–757.
- Mahdi O, Chiroma SM, Hidayat Baharuldin MT, et al. WIN55, 212-2 attenuates cognitive impairments in AlCl3+ d-Galactose-Induced Alzheimer’s disease rats by enhancing neurogenesis and reversing oxidative stress. Biomedicines. 2021;9(9):1270.
- Adeli S, Zahmatkesh M, Tavoosidana G, et al. Simvastatin enhances the hippocampal klotho in a rat model of streptozotocin-induced cognitive decline. Prog Neuro Psychopharmacol Biol Psychiatry. 2017;72:87–94.
- Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72(1–2):248–254.
- Kaundal M, Deshmukh R, Akhtar M. Protective effect of betulinic acid against intracerebroventricular streptozotocin induced cognitive impairment and neuronal damage in rats: possible neurotransmitters and neuroinflammatory mechanism. Pharmacol Rep. 2018;70(3):540–548.
- Deacon RM, Rawlins JNP. Hippocampal lesions, species-typical behaviours and anxiety in mice. Behav Brain Res. 2005;156(2):241–249.
- Rao MK, Rao MS, Rao GS. Treatment with Centalla asiatica (Linn) fresh leaf extract enhances learning ability and memory retention power in rats. Neurosci J. 2007;12(3):236–241.
- Antunes M, Biala G. The novel object recognition memory: neurobiology, test procedure, and its modifications. Cogn Process. 2012;13(2):93–110.
- Lin X, Huang R, Zhang S, et al. Beneficial effects of asiaticoside on cognitive deficits in senescence-accelerated mice. Fitoterapia. 2013;87:69–77.
- Fadl NN, Ahmed HH, Booles HF, et al. Serrapeptase and nattokinase intervention for relieving Alzheimer’s disease pathophysiology in rat model. Hum Exp Toxicol. 2013;32(7):721–735.
- Jayant S, Sharma BM, Sharma B. Protective effect of transient receptor potential vanilloid subtype 1 (TRPV1) modulator, against behavioral, biochemical and structural damage in experimental models of Alzheimer’s disease. Brain Res. 2016;1642:397–408.
- Raina P, Santaguida P, Ismaila A, et al. Effectiveness of cholinesterase inhibitors and memantine for treating dementia: evidence review for a clinical practice guideline. Ann Intern Med. 2008;148(5):379–397.
- Ahmad Rather M, Justin-Thenmozhi A, Manivasagam T, et al. Asiatic acid attenuated aluminum chloride-induced tau pathology, oxidative stress and apoptosis via AKT/GSK-3β signaling pathway in Wistar rats. Neurotox Res. 2019;35(4):955–968.
- Abdel Moneim AE. Evaluating the potential role of pomegranate peel in aluminum-induced oxidative stress and histopathological alterations in brain of female rats. Biol Trace Elem Res. 2012;150(1):328–336.