?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
The results of a survey on the helminth parasites of the catfishes Bagrus spp. Forsskål, 1775 and Chrysichthys auratus Geoffroy,1809 inhabiting Damietta branch of the River Nile at Mansoura city, Dakahlia Governorate, Egypt, have been demonstrated. During the period of March 2020 to February 2021, five species were detected from Bagrus spp. and represented by one monogenean Quadriacanthus bagrae from the gills, three digeneans Acanthostomum absconditum, Acanthostomum spiniceps and Haplorchoides cahirinus and a single nematode species Capillostrongyloides fritschi from the intestine. Only one monogenean species Protoancylodiscoides mansourensis was detected from the gills of Chrysichthys auratus. Except for a few months, most monogeneans, digeneans and nematodes of Bagrus spp. and C. auratus were recorded in all months of the year. The digeneans A. absconditum, A. spinicips, and H. cahirinus are eudominant and A. absconditum was the most dominant while the nematode C. fritschi is subrecedent. In Bagrus spp., the digenean parasites attained the highest prevalence over the monogeneans and nematode parasites throughout the year (77.7%). There was a definite seasonal change in the studied helminths, but no general common trend or pattern was observed. Most helminth parasites showed a higher prevalence in male fishes than females while they attained a higher abundance in females than males. There was a significant difference in the mean prevalence as well as in the mean intensity of most examined parasite species in male fishes and females but a significant difference in the mean abundance of males and females was only recorded for the digenean parasites. Most parasites showed a higher prevalence as the condition factor of the host increased.
Introduction
The catfishes Bagrus bajad Forsskål, 1775 and Bagrus docmak Forsskål, 1775 (Siluriforms: Bagridae) are well distributed in the River Nile, Lake Manzala, and freshwater parts of other lakes in Egypt. Their ‘economic value’ is attributed to readily marketable size, delicious flesh, superb flavor, acceptance by Egyptians, and availability in fish farming [Citation1]. Additionally, the freshwater catfish Chrysichthys auratus Geoffroy, 1809 (Claroteidae) is a highly valued omnivorous catfish [Citation2]. There are a few ecological and biological studies on helminth parasites of C. auratus in Egypt [Citation3]. This species has recently become important to River Nile communities because of its affordability, and relative abundance compared with other bagrid fishes in River Nile.
Fish parasites cause problems for both commercial and artisanal fishers [Citation4] and are still thought to be the main cause of fish sickness and mortality worldwide, which results in great economic loss [Citation5]. The prevalence of these parasites rises sharply in locations with high levels of aquatic pollution, which has a severe negative impact on fish health, nutritional value and reproductive ability. Investigations of parasite communities are greatly important for enriching our knowledge of parasite community structure and dynamics [Citation6] and estimating environmental influences on their infection levels [Citation7]. Parasite community structure may experience changes related to variations in biotic and abiotic factors and these variations can be reflected in species composition and abundances [Citation8]. Changes in diversity and structure of parasite communities of different fish hosts have therefore received increasing attention due to the possible application of parasites as indicators of ecosystem integrity and health [Citation9]. Temperature is one of the most important ecological parameters in relation to the seasonal population dynamics of helminth parasites [Citation10,Citation11]. It was intriguing to determine whether the parasites exhibit any equivalent monthly and seasonal changes in their prevalences, mean intensities, and abundance in Egypt, where monthly and seasonal fluctuations in day duration and temperature are moderate.
The condition factor of a fish is an indication of a ‘healthy’ or compromised organism [Citation12]. It reflects the physiological state of the fish in relation to its welfare [Citation13]. Measurement of the condition factor requires only measuring the whole length and weight of each individual fish. A good condition factor is rather a reflection of the availability of suitable food sources for fish and may only indicate a compromised environment if the exposure to harmful substances is prolonged and result in physiological symptoms and altered fish-feeding behavior [Citation14]. Furthermore, the low condition factor of fish may represent immunologically compromised individuals due to factors other than environmental factors such as endoparasites helminth infection and parasitemia. Therefore, the present study was extended to determine the host condition factor (weight and length) as well as the host sex of the catfishes Bagrus spp. and C. auratus and their relationship with the infection levels of their helminth parasites. Additionally, the present study aimed to investigate the community structure, dominance, monthly occurrence, and seasonal infection levels (prevalence, mean intensity and abundance) of some helminth parasites infecting the catfishes Bagrus spp. and C. auratus in Nile Delta, Dakahlia Governorate, Egypt.
Materials and methods
Study area and host collection
A total of 141 individuals of the catfish Bagrus spp. (B. bajad and B. docmak) and 113 individuals of C. auratus were collected by trammel nets from Damietta branch of the River Nile at Mansoura City in Dakahlia Governorate, Egypt (coordinates: 31°02ʹ47.2”N, 31°21ʹ12.2”E), at the period of March, 2020 to February, 2021. Freshly caught individuals of the catfishes were transported in an aerated container with River Nile water to the parasitological Laboratory at Mansoura University.
Determination of the fish biological parameters
Host sex was determined through examining the external genitalia and dissection of the internal reproductive organs. The weight and length of each host individual were recorded to determine the condition factor. The condition factor (k) of fish samples was determined according to Omar [Citation15] from the equation:
K = 100 W/L3
Where K = condition factor, W = weight of fish, L = total length of fish.
The condition factor of investigated fishes, Bagrus spp. and Chrysichthys auratus varied between 0.003 and 2.04. The collected fish samples were grouped into three condition factor classes as follows: class I, from 0.003 to 0.682 (poor condition), class II, from 0.683 to 1.361 (good condition), and class III, from 1.362 to 2.040 (excellent condition).
Community structure and species diversity of helminth parasites
Parasitological indices evaluated in this study include dominance, prevalence, mean intensity, and abundance. The dominance of intestinal parasite species was calculated according to Roohi et al. [Citation16]. as follows:
Where N = abundance of a specific parasite species and N sum = sum of the abundance of all parasite species found.
The helminth parasites were classified based on their dominance values according to Kasprzak and Niedbala [Citation17] as follows: eudominant (< 10%), dominant (5.1% – 10%), subdominant (2.1%-5%), recedent (1.1–2%) and subrecedent (> 1.0%) of a given species.
Identification of helminth parasites
Identification of collected Quadriacanthus bagrae and P. mansourensis was done according to Paperna [Citation18] and El-Naggar [Citation3], respectively. Identification of collected digenean parasites, Acanthostomum spp. and Haplorchoides was done according to Moravec [Citation19] and Chen [Citation20], respectively, while that of Capillostrongyloides fritschi followed the method of Moravec [Citation21].
Ecological parameters
All individuals of each parasite species were counted in each fish. The prevalence (%), mean intensity and abundance of each parasite species were calculated seasonally and collectively throughout the year to illustrate the relationship between seasons and infection levels of helminth parasites [Citation22].
Statistical data analyses
The relationship between seasons and infection levels of the helminth parasites infecting catfish Bagrus spp. and Chrysichthys auratus was illustrated using One-way ANOVA that was chosen on SPSS package (version 25 IBM for windows). Significance in differences in the infection levels of helminth parasites between male and female fishes was determined using the student’s t-Test. The probability levels were selected as follows: non-significant (p < 0.05), significant (p ≤ 0.05), highly significant (p ≤ 0.01) and very highly significant (p ≤ 0.001). In case of significant differences, the Multiple Range Comparisons (Least Significant Difference) were selected from the Post Hoc tests (Tukey HSD) on the same statistical package to detect the variances between infection levels of different helminth parasites and different seasons.
Results
Community structure, monthly occurrence, dominance pattern and infection levels of identified helminth parasites
General features of monthly occurrence
During the present study, five helminth parasitic species were detected from Bagrus spp. These are: one monogenean, Quadriacanthus bagrae Paperna, 1979, from the gills, three digeneans Acanthostomum absconditum, Acanthostomum spiniceps Looss, 1901 and Haplorchoides cahirinus Chen, 1949 from the intestine, and a single nematode species, Capillostrongyloides fritschi Travassos, 1914 from the intestine. Only one monogenean species, Protoancylodiscoides mansourensis El-Naggar, 1987 was detected from the gills of C. auratus.
All monogenean species (Q. bagrae and P. mansourensis) and the digenean parasite, A. absconditum were detected in all months of the year, whereas A. spiniceps was recorded in all months of the year except October and November (). The digenean H. cahirinus was found during all months of the year except April, June and August. Finally, the nematode parasite, C. fritschi was completely absent during June, October and February ().
Table 1. Monthly occurrence of helminth parasites infecting Bagrus spp. and Chrysichthys auratus inhabiting Damietta branch of the River Nile. (+), Present; (-), absent.
Dominance pattern in gastrointestinal parasites of Bagrus spp.
All digenean species, A. absconditum, H. cahirinus, and A. spiniceps are eudominant while the nematode, C. fritschi is subrecedent (). The digeneans are more dominant than the nematode and A. absconditum is the most dominant species.
Table 2. Dominance (%) of the intestinal parasites of Bagrus spp.
Infection levels of helminth parasites of the catfishes Bagrus spp. and Chrysichthys auratus
The overall infection rate of Bagrus spp. with helminth parasites was greater than that of C. auratus (). In Bagrus spp., the digenean group attained the highest prevalent value. Amongst all helminth parasites of Bagrus spp., the highest and lowest prevalences were recorded for Q. bagrae and C. fritschi, respectively, while the highest and lowest mean intensity were registered for H. cahirinus and C. fritschi, respectively (). Concerning the overall abundance, A. absconditum was the most abundant, while C. fritschi was the least abundant species. In C. auratus, the overall prevalence, mean intensity and abundance were illustrated for the monogenean P. mansourensis ().
Table 3. Infestation levels of identified helminth parasites of the catfishes Bagrus spp. and Chrysichthys auratus (pooled data).
Seasonal fluctuations of infection levels of helminth parasites infecting Bagrus spp. and Chrysichthys auratus
Seasonal mean prevalence
The highest mean prevalence of Q. bagrae, A. spiniceps, and C. fritschi was attained during spring while their lowest mean prevalence was recorded during Summer for Q. bagrae and Winter for A. spiniceps and C. fritschi (). Also, the highest mean prevalence of A. absconditum, H. cahirinus, and P. mansourensis was registered during autumn and their lowest mean prevalence during spring, summer, and winter, respectively ().
Table 4. Seasonal fluctuations in the mean prevalence of helminth parasites infecting Bagrus spp. and Chrysichthys auratus. Highest value = (+), Lowest value = (-). Groups with the same letter are not significantly different and groups that are significantly different get different letters.
Seasonal differences in the mean prevalence were significant for A. absconditum (F = 6.89, p = 0.01) and A. spiniceps (F = 5.51, p = 0.02) and non-significant for the other helminth species among various seasons. Multiple Range Comparisons revealed that a significant difference in the mean prevalence of A. absconditum between Spring and Autumn (p = 0.02) was higher than its mean prevalence between Spring and Winter (p = 0.04). Additionally, a significant difference in the mean prevalence of A. spiniceps between Spring and Winter (p = 0.03) was higher than its mean prevalence between Spring and Autumn (p = 0.05).
Seasonal mean intensity
The highest mean intensity of Q. bagrae, P. mansourensis, and A. spiniceps was recorded during summer while their lowest mean intensity was registered in autumn for Q. bagrae and A. spiniceps and in spring for P. mansourensis (). The parasites A. absconditum and C. fritschi reached their highest mean intensities during spring while their lowest intensities were recorded during winter for A. absconditum and summer for C. fritschi (). In contrast, H. cahirinus recorded its highest mean intensity during Winter and its lowest one in Summer. Statistical analyses showed a non-significant difference between mean intensities of all studied helminth parasites.
Table 5. Seasonal fluctuations in mean intensity of helminth parasites infecting Bagrus bajad and b. docmac spp. and Chrysichthys auratus. Highest value = (+), Lowest value = (-). Groups with the same letter are not significantly different and groups that are significantly different get different letters.
Seasonal mean abundances
The highest mean abundance of A. absconditum, H. cahirinus, and C. fritschi was attained during autumn and their lowest mean abundance during spring for A. absconditum and summer for both H. cahirinus and C. fritschi (). Q. bagrae and P. mansourensis reached their highest mean abundance during winter and lowest mean abundance during autumn for Q. bagrae and spring for P. mansourensis (). The highest mean abundance of A. spiniceps was recorded during Spring and lowest mean abundance during Autumn (). Statistical analyses showed a non-significant difference between mean abundances of all studied helminth parasites.
Table 6. Seasonal mean abundance of helminth parasites infecting Bagrus spp. and Chrysichthys auratus. Highest value = (+), Lowest value = (-). Groups with the same letter are not significantly different and groups that are significantly different get different letters.
The relationship between mean prevalence, mean intensity, and mean abundance with host sex of Bagrus spp. and C. auratus
The helminth parasites Q. bagrae, A. spincips, C. fritschi, and P. mansourensis are more prevalent in males than in females while the remaining parasites A. absconditum and H. cahirinus attained higher mean prevalence in females than in males (). Concerning the mean intensity, H. cahirinus, C. fritschi, and P. mansourensis attained higher intensities in males than in females while the remaining parasites Q, bagrae, A. absconditum and A. spincips had higher intensities in females than in males (). Q. bagrae and A. spincips are more abundant in males than in females while the remaining parasites A. absconditum, H. cahirinus, C. fritschi, and P. mansourensis are more abundant in females than in males ().
Table 7. Mean prevalence (%), mean intensity, and mean abundance of parasites infecting males and females of Bagrus spp. and Chrysichthys auratus. Groups with the same letter are not significantly different and groups that are significantly different get different letters.
There was a significant difference in the mean prevalence between males and females of all identified parasite species of Bagrus spp. and C. auratus (p ≤ 0.05). However, there was a significant difference in mean intensity between males and females of all identified parasite species of Bagrus spp. and C. auratus (p ≤ 0.05) except C. fritschi which showed no significant difference between males and females of Bagrus spp. (p ≥ 0.05). Finally, there was a significant difference in the mean abundance of A. absconditum, A. spincips and H. cahirinus between males and females of Bagrus spp. (p ≤ 0.05). In contrast, Q. bagrae, P. mansourensis, and C. fritschi showed no significant difference between male and female Bagrus spp. and C. auratus (p ≥ 0.05).
The relationship between prevalence, mean intensity and abundance with condition factors
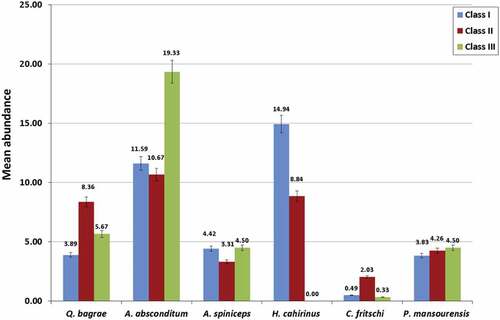
Except for Q. bagrae and H. cahirinus, a high mean prevalence was recorded for the other parasites, A. absconditum, A. spinicips, C. fritschi and P. mansourensis as the condition factor of the host increases. There was a non-significant difference of prevalence between different condition factor classes for all studied helminth parasites (p ≥ 0.05) ().
Figure 1. Relationship between mean prevalence of helminth parasites and condition factor classes of the host.
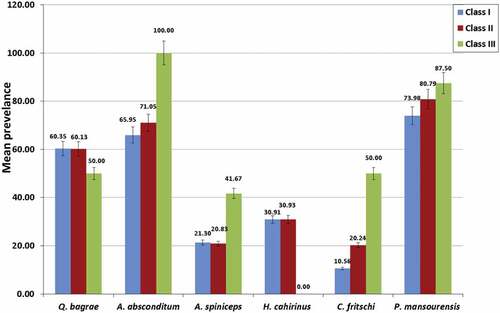
The mean intensity of A. absconditum and P. mansourensis increases as the host condition factor rises while the mean intensity of H. cahirinus declines as the condition factor increases. The mean intensity of both Q. bagrae and C. fritschi had no regular pattern with the host condition factor. There was a non-significant difference in mean intensity between different condition factor classes for all studied helminth parasites (p ≥ 0.05) ().
Figure 2. Relationship between mean intensity of helminth parasites and condition factor classes of the host.
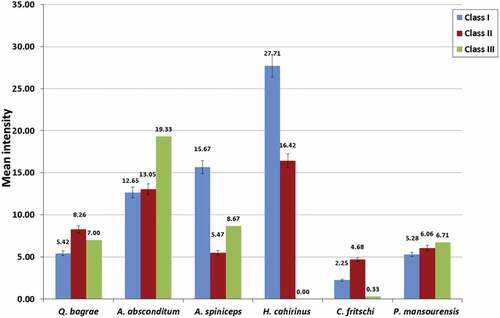
The mean abundance of Q. bagrae, A. absconditum, A. spiniceps, and C. fritschi has no regular pattern with the host condition factor. However, the mean abundance of P. mansourensis increases with the increase of the host condition factor and the opposite occurs for H. cahirinus. There was a non-significant difference in the mean abundance between different condition factor classes for all studied helminth parasites (p ≥ 0.05) ().
Discussion
The results of a survey on the helminth parasites of the catfishes Bagrus spp. Forsskål, 1775 and Chrysichthys auratus Geoffroy, 1809 inhabiting Damietta branch of the River Nile at Mansoura City, Dakahlia Governorate, Egypt have been demonstrated. The dominance pattern and seasonal changes in their prevalence, mean intensity, and abundance as well as the effects of some host biological factors on their infection level were studied. The helminth community structure of Bagrus spp. and C. auratus of Damietta branch of the River Nile in the Nile Delta indicates a rich assemblage, with high species richness, diversity, and variability in prevalence, mean intensity, and abundance values of most constituent species.
The present investigation has revealed that the catfishes B. bajad and B. docmak harbored one monogenean species, Q. bagrae, three digenean species, A. absconditum, A. spiniceps and H. cahirinus, and one nematode called C. fritschi while the catfish C. auratus harbored only one monogenean species, P. mansourensis at Damietta branch of the River Nile. Mansour et al. [Citation23] recorded the same digeneans A. absconditum, A. spiniceps, and H. cahirinus from the same host (Bagrus spp.) inhabiting Damietta branch of the River Nile and its tributaries. Moreover, Mansour et al. [Citation23] detected two nematode species, Spinitectus allaeri Campana-Rouget, 1961 and C. fritschi. However, S. allaeri was not observed in the present study. Also, no cestode parasites were recorded either in the present study or by Mansour et al. [Citation23]. However, Imam et al. [Citation24] described cestodes from the intestine of B. bajad. Saoud and Wannas [Citation25] made a helminthological survey on C. auratus, B. bajad, and B. docmak collected along different seasons from Aswan High Dam Lake and found no parasites on all examined fish of C. auratus. Meanwhile, Saoud and Wannas [Citation25] detected three digeneans (A. absconditum, A. spiniceps and H. cahirinus) and one Acanthocephalan (Paragorgorhynchus sp.) from the gastrointestinal canal of both B. bajad and B. docmak. Osman et al. [Citation26] reported the digeneans A. spiniceps, A. absconditum and H. cahirinus and the nematodes, S. allaeri, Procamallanus laevichonchus wedl,1862 and C. fritschi from B. docmak collected from three different regions of Menoufiya Governorate: Bahr Shebeen, Albagoreya, and Sabal Drainage Canals. Neither S. allaeri nor P. laevichonchus has been recorded in the present study. Taha [Citation27] recorded only the nematode parasite Capillaria sp. and the acanthocephalan Polymorphus sp. from B. bajad in Ismailia Canal.
The present study showed that, most monogeneans, digeneans, and nematodes of Bagrus spp. and C. auratus are recorded in most months of the year. This may indicate clearly that the catfishes Bagrus spp. and C. auratus in the Nile delta are more suitable for infection with helminth parasites.
In the intestinal tract of the catfishes B. bajad and B. docmak, the digenean parasites are more dominant than the nematode parasites. According to Kasprzak and Niedbala [Citation17], the digeneans A. absconditum, A. spinicips, and H. cahirinus are eudominant and A. absconditum is the most dominant while the nematode C. fritschi is subrecedent. These findings coincide with those of Groenewold et al. [Citation28] who found that the digenean, Cryptocotyle lingua Creplin,1825, was dominant among helminth intestinal parasites of four fish species of the Wadden Sea. Clark [Citation29] proposed two concepts for controlling the community. In the first, a few species may determine the occurrence of other species in the area and in the second, the physical features of the biotope play a role in controlling the occurrence of most species directly and independently.
In Bagrus spp, the digeneans attained the highest prevalence, if compared with the monogenean and nematode parasite species. The present data agree with that reported by Saoud and Wannas [Citation25]. However, Abdel-Gaber et al. [Citation30] reported that the nematode species have the highest prevalence followed by cestodes in C. gariepinus from Lake Manzala. Mgbemena et al. [Citation31] reported the highest prevalence of nematodes, especially Camallanus sp., followed by Capillaria sp. with a corresponding low prevalence of the cestode, Tetracampose ciliotheca from C. gariepinus in Nigeria.
There was a definite seasonal impact on the parasitic infection level of the studied helminths, but no general common trend or pattern was observed, and each species showed its response to the seasonal changes throughout the year. Some parasite species like Q. bagrae, A. spiniceps, and C. fritschi attained their highest prevalence in Spring, while others (A. absconditum, H. cahirinus and P. mansourensis) in Autumn and the seasonal differences were only significant for A. absconditum and A. spiniceps. Except for H. cahirinus, all other helminths acquired their highest mean intensity during Spring or Summer and most helminth parasites attained their highest abundance in cold seasons (Autumn and Winter). There was no significant difference either in the mean intensity or in the mean abundance between different seasons for all studied parasites. These findings suggest that the change in temperature may affect the infection level of each parasite species but also there are other factors that may have the same effect like environment physicochemical parameters and host biotic factors (feeding habits, sex, and condition factor). Despite our findings, previous studies have recorded variations in the infection level of helminth parasites in the different seasons.
Osman et al. [Citation26] studied the seasonal population dynamics of the helminth parasites infecting B. docmak and found that the prevalence of infection with different parasites was higher in Spring and Summer followed by Winter and Autumn in Bahr Shebeen, Albagoreya, and Sabal Drainage canals in Menoufiya Governorate, Egypt. The authors attributed this to the temperature which was suitable for parasite transmission, extensive feeding of the host fish, and the availability of intermediate hosts of the helminth parasites at this time of the year. Similar results were reported by Imam [Citation32], Moravéc [Citation19], Wannas [Citation33], Noor El-Din [Citation34], Negm El-Din [Citation35], Sharaf El-Din [Citation36] and Taha [Citation27]. Aydogdu et al [Citation37] found that the infection with the digenean Saccocoelium obesum Looss,
1902 and acanthocephalan Neoechinorhynchus agilis Rudolphi, 1819 was high in summer and spring, respectively. Accordingly, the choice and composition of food and the presence or absence of intermediate hosts are very important for the presence of the intestinal helminth parasitic fauna of the thick-lip gray mullet (Chelon labrosus) [Citation37]. The relationship between abundance and intensity of parasites and seasonality was influenced by climatic factors, as well as aquatic fauna and flora and hence fish immune response [Citation38].
Most helminth parasites of the present study showed a higher prevalence in males than in females while they attained a higher abundance in females than in males. There was a significant difference in the mean prevalence as well as in the mean intensity of most examined parasite species in males and females but a significant difference in the mean abundance in males and females was only recorded for the digenean parasites. The present findings coincide with Osman et al. [Citation26] who found that the prevalence of infection with all helminths of B. docmak was higher in males than in females. The influence of sex on animal susceptibility to infections was attributed to genetic predisposition and differential susceptibility owing to hormonal control [Citation39]. In contrast to the present finding, Taha [Citation27] recorded that females of B. bajad had a higher prevalence of helminth parasites than males. A similar result was reported by Okpasuo et al. [Citation40] who found that the females of bagrid species had higher prevalence and higher intensity than males. Gbankoto et al. [Citation41], Saha et al. [Citation42] and Edeh and Solomon [Citation43] attributed these results to the comparatively strong immunity system of males and biochemical changes in the quality and quantity of steroid hormones of females and males.
Saoud and Wannas [Citation25] found no significant differences in the incidence of different helminth species in both sexes of the catfishes C. auratus, B. bajad, and Bagrus docmak. These differences between previous studies indicate that there are factors other than sex that can affect and change the infection levels of the parasite species in different sexes of the host. These factors may include abiotic factors like water physicochemical parameters, concentrations of heavy metals, temperature, pH, and oxygen, and biotic factors like feeding habits and the size of the host.
The present investigation has revealed no significant difference between the mean prevalence, mean intensity and mean abundance of all studied helminth parasites and the host condition factor. However, most parasites showed a higher prevalence as the condition factor of the host increased. Most previous studies have concentrated on the impact of host length and weight on the infection level of helminth parasites [Citation23,Citation27]. Okpasuo et al. [Citation40] observed a significant and strong correlation between the condition factor of Auchenoglanis biscutatus Geoffory,1809 from Anambra River Basin, Nigeria and the parasite infection but reported a poor and insignificant correlation between the condition factor of B. bajad and B. docmak and their parasite infection. Length and weight growth of fish host are regulated by different endocrine systems and may not necessarily happen at the same time. Okpasuo et al. [Citation40] reported that A. occidentalis had the highest mean condition factor indicating that the environment of the river is more suitable for this species, while B. bajad had the least condition factor. Okpasuo et al. [Citation40] attributed the decrease in the condition factor of B. bajad to the rapid growth in length relative to weight.
Mansour et al. [Citation23] reported an increase in the prevalence of helminth parasites with the increase in the length of fish. Osman et al. [Citation26] observed an increase in the prevalence and mean intensity of infection in B. docmak in medium-weight fishes (200 gm). The increase in the prevalence with the increase of fish length may be due to the increase and growth of the internal organs of the hosts leading to the increase in the surface areas of infection as suggested by El-Naggar and Khidr [Citation44]; Khidr [Citation45] and Hagras et al. [Citation46] or could be due to the exposure time of infection [Citation47].
Ethical approval
No experiments were performed on live fishes. All applicable institutional, national, and international guidelines for the care and use of animals were followed. Mansoura University ethics approval no Sci-Z-Ph-2020-9.
Acknowledgments
This paper is a part of a Ph.D. Thesis to be submitted to Zoology Department, Faculty of Science, Mansoura University, Egypt. The paper was carried out with joint financial support from The Ministry of Social Solidarity.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Mashaly MI, El-Naggar AM, Hagras AE, et al. Microhabitat selection of ectoparasitic Monogenean populations of the Nile Catfish Clarias gariepinus. Jordan J Biol Sci. 2019;12(5):573–580
- Atobatele OE, Ugwumba OA. Condition factor and diet of Chrysichthys nigrodigitatus and Chrysichthys auratus (Siluriformes: bagridae) from Aiba Reservoir, Iwo, Nigeria. Rev Biol Trop. 2011;59(3):1233–1244.
- El-Naggar MM. Protoancylodiscoides mansourensis n. sp., a monogenean gill parasite of the Egyptian freshwater fish Chrysichthys auratus Geoffroy 1908. Arab Gulf J Sci Res. 1987;5:441–454.
- Edema CU, Okaka CE. A preliminary study of parasitic infections of some fishes from Ohkubo River, Benin city, Nigeria. Int J Biomed Sci. 2008;4(2):120–135.
- Khanum H, Easmi F, Hasan MDS, et al. Helminth and parasitic arthropod prevalence in catfish Clarias batrachus (l.) from ponds in Savar. Bangladesh J Zool. 2015;43(2):269–277.
- Esch GW, Bush AO, Aho JM. Parasite communities: patterns and processes. London (U.K): Chapman and Hall; 1990. p. 335.
- Khan RA, Thulin J. Influence of pollution on parasites of aquatic animals. Adv Parasitol. 1991;30:201–238.
- Violante-Gonzalez J, Mendoza-Franco EF, Rojas-Herrera A, et al. Factors determining parasite community richness and species composition in black snook Centropomus nigrescens (Centropomidae) from coastal lagoons in Guerrero, Mexico. Parasitol Res. 2010;107(1):59–66.
- Dusek L, Gelnar M, Sebelov S. Biodiversity of parasites in a freshwater environment with respect to pollution: metazoan parasites of chub (Leuciscus cephalus L.) as a model for statistical evaluation. Int J Parasitol. 1998;28(10):1555–1571.
- Khidr AA, Reda ES, Mashaly MI. Comparative parasitological and ecological studies on monogenean gill parasites infesting some cichlid fish in Manzala and Borollus lakes, north Nile delta, Egypt. Environ Sci. 2012;41(3):427–433.
- Mashaly MI, Allam HE, El-Naggar MM. Impacts of physicochemical and heavy metal parameters on infestation level of the monogeneans, Quadriacanthus spp. infesting Nile catfish, Clarias gariepinus of different water localities in Nile Delta. Egypt J Parasit Dis. 2020;44(3):579–589.
- Hoque MT, Yusoff FM, Law AT. Effect of hydrogen sulfade on liver-somatic index and fulton.s condition factor in Mystus nemurus. J Fish Biol. 1998;52:23–30.
- Lizama MAP, Ambrosio AM. Condition factor in nine species of fish of the characidea family in the upper Parana River floodplain, Brazil. Braz J Biol. 2002;62(1):113:124.
- Schmitt CJ, Dethloff GM. Condition factor and ogranosomatic indices. In: Schmitt CJ, Dethloff GM, editors. Biomonitoring of environmental status and trends (BEST) program: selected methods for monitoring chemical contaminants and their effects in aquatic ecosystems (USA: Geological Survey Information and Technology Report USGS/ BRD/ITR-2000-0005). 2000. p. 1–81.
- Omar RH, Hagras AA, El-Naggar AM, et al. Hematological and parasitological studies on Oreochromis niloticus Linnaeus 1757 in the Nile Delta Region. Egypt EJABF. 2021;25(1):795–819.
- Roohi JD, Sattari M, Asgharnia M, et al. Occurrence and intensity of parasites in European catfish, Silurus glanis L., 1758 from the Anzali wetland, southwest of the Caspian Sea, Iran. The Croat J Fish. 2014;72(1):25–31.
- Kasprzak K, Niedbala W. Biocenotic indices used in the ordering and analysis of data in quantitative studies. In: Górny M, Grüm L, Elsevier PWN, editors. Methods in Soil Zoology” (Warsaw, Poland: Wyd. Naukowe PWN). 1993. p. 379–396.
- Paperna I. Studies on monogenetic trematodes in Israel. 3. Monogenetic trematodes of the Cyprinidae and Claridae of the lake of Galilee. Isr J Aquac Bamidgeh. 1961;13:14–29.
- Moravec F. On two acanthostomatid trematodes, Acanthostomum spiniceps (Looss, 1896) and A. absconditum (Looss, 1901), from African bagrid fishes. Folia Parasitol. 1976;23:201–206.
- Chen HT. Systematic consideration of some heterophyid trematodes in the subfamilies Haplorchinae and Stellantchasminae. Ann Trop Med Parasitol. 1949;43(3–4):304–312.
- Moravec F. Redescription and systematic status of Capillaria philippinensis, an intestinal parasite of human beings. J Parasitol Res. 2001;87(1):161–164.
- Margolis L, Esch GW, Holmes J, et al. The use of ecological terms in parasitology. J Parasitol. 1982;68(1):131–133.
- Mansour MFA, Hassan HS, Khidr AA, et al. General survey on certain helminth parasites infecting some Nile fishes at El-Mansoura. Egypt Egypt Egypt J Aquat Biol Fish. 2003;7(4):423–446.
- Imam EAE, El-Aaskalany MA, Rashad SM. Studies on helminth parasites, of Syndontis shall and Bagrus bayad from Beni-Suef water resources. Assiut Vet Med J. 1991;24(48):137–152.
- Saoud MFA, Wannas MQA. A qualitative and quantitative survey on the helminth parasites of fishes from the Aswan high dam lake in Egypt. Qatar Univ Sci J. 1984;4:129–142.
- GYl O, Radwan NA, A.i K, et al. Helminth communities of Bagrus docmac and Malepterurus electricus in three water bodies at Menoufiya Governorate. Egypt Egypt J Exp Biol. 2008;4:177–192.
- Taha RG. Seasonal variations, prevalence, and intensity of helminth parasites of some freshwater fish in Ismailia Canal, Egypt. J Egypt Soc Parasitol. 2018;48(2):301–308.
- Groenewold S, Berghahn R, Zander CD. Parasite communities of four fish species in the Wadden Sea and the role of fish discarded by the shrimp fisheries in parasite transmission. Helgol Mar Res. 1996;50(1):69–85.
- Clark KP. L’Homme et la Société. Paris: R. Laffont; 1966. p. 635.
- Abdel-Gaber R, Garhy M, Morsy K. Prevalence and Intensity of Helminth Parasites of African Catfish Clarias gariepinus in Lake Manzala, Egypt. Egypt Adv Biosci Biotechnol. 2015;6(7):464–469
- Mgbemena A, Arimoro F, Omalu I, et al. Prevalence of helminth parasites of Clarias gariepinus and Tilapia zillii in relation to age and sex in an afrotropical stream. Egypt J Aquat Biol Fish. 2020;24(5):1–11.
- Imam EA. Morphological and biological studies on the enteric helminths infecting some of the Egyptian Nile fishes particularly Polyonchobothrim clarias of Karmot Clarias lazera and Clarias anguillaris. [ Ph. D. Thesis], Parasitology. Cairo Univ. Egypt; 1971.
- Wannas MKA. Studies on certain helminth parasites of freshwater fishes from Lake Nasser. M. [ Sci., Thesis], Al-Azhar Univ. Egypt; 1977.
- El-Din SA N. Studies on some parasitic helminths in some freshwater fish. [ M. Sc. Thesis], Fac. Sci., Tanta Univ. (Egypt); 1981.
- Din KM N-E. Some morphological studies on the internal parasites of fish Delta Nile. [ M.V. Sc. Thesis], Benha branch (Moshtohor) (Egypt): Fac. Vet. Med., Zagzig Univ; 1987.
- El-Din SHM S. Taxonomical and ecological studies on parasites of some freshwater fish. [ M.Sc. Thesis], Tanta Univ. (Egypt): Fac. Sci; 1996.
- Aydogdu A, Emre N, Emre Y. Prevalence and intensity of parasitic helminths of thick lip grey mullet Chelon labrosus in hosts in Beymelek Lagoon Lake in Antalya, Turkey, according to season, host size, age, and sex of the host. Turk J Zool. 2015;39:643–651.
- Chubb JC. Seasonal occurrence of helminths in freshwater fish. Part I Monogenea Adv Parasitol. 1977;15:133–199.
- Wali A, Balkhi HM, Maqbool R, et al. Distribution of helminth parasites in intestines and their seasonal rate of infestation in three freshwater fishes of Kashmir. J Parasitol Res. 2016;16:8901518.
- Okpasuo OJ, Ezenwaji NE, Onah IE, et al. Parasites of freshwater and condition factor of bagrid fishes in Anambra River basin, Nigeria. J Pharm Biol Sci. 2016;6(4):13–26.
- Gbankoto A, Pampoulie C, Marques A, et al. Occurrence of myxosporean parasites in the gills of two Tilapia species from Lake Nokoué (Bénin, West Africa): effect of host size and sex, and seasonal patterns of infection. Dis Aquat Org. 2001; 44:217–222.
- Saha M, Bandyopadhyay KP, Roy A, et al. Impact of seasons, host age, size and sex on prevalence of protozoan parasites in ornamental fish. J Agric Vet Sci. 2015;8(10):54–59
- Edeh C, Solomon RJ. Endoparasites of Oreochromis niloticus and Clarias gariepinus found in Utako flowing gutter. Direct Research Agric Food Sci. 2017;4(12):361–373.
- El-Naggar MM, Khidr AA. Population dynamics of cichliogyrid monogeneans from the gills of three Tilapia spp. from Demietta branch of the River Nile in Egypt. Proc. Zool. Soc. U.A.R, 1986; 12:275–286.
- Khidr AA. Population dynamics of Enterogyrus cichlidarum (Monogenea: ancyrocephalinae) from the stomach of Tilapia spp. in Egypt. Int J Parasitol. 1990;20(6):741–745.
- Hagras AEM, El-Naggar MM, Mansour MF, et al. Influence of age, length, sex of the catfish Clarias lazera on infestation with six monogenean parasites. Bull Fac Sci Mansoura Univ. 1995;22(2):37–56
- Muzzall PM, Sweet RD, Mijewski CL. Occurrence of Diplostomum sp. (Trematoda: diplostomatidae) in pond-reared walleyes from Michigan. Prog Fish Cult. 1990;52(1):53–56.