ABSTRACT
Diabetes-related retinopathy (DR) is one of the sight-threatening diseases. The present case–control study screened 134 patients with type 2 diabetes and 36 healthy ones without diabetes. The genotyping of SNPs rs2010963 and rs699947 of the VEGF gene were done by tetra primers ARMS-PCR. The mutant GG genotype of rs2010963 was significantly associated with DR (OR = 10.29; 95% CI = 2.20–59.06; p = 0.004). No significant associations existed between patients with DR and those with NDR or controls in the genotype or allele frequencies of rs699947 polymorphism. The haplotype analysis for rs2010963 and rs699947 shows the CG haplotype was significantly different between DR and NDR OR 2.0 (95% CI:1.02–3.93), p = 0.043. and between DR and control OR 2.42 (95% CI: 1.2–4.65), p = 0.0108. The two SNPs showed moderate Linkage disequilibrium (LD) between DR and control, D’ = 0.64 also, moderate LD between DR cases and NDR, D’ = 0.75. No significant importance for rs699947 SNP with diabetic retinopathy. The 2010963 SNP in the VEGF gene is associated with the risk of NPDR and PDR. The findings also suggest a moderate LD in the two SNPs and their CG haplotype associated with DR progression.
Introduction
Diabetes mellitus is a widespread disease associated with high morbidity rates [Citation1,Citation2]. Diabetes results in various consequences, classified as macrovascular (in a large blood vessel) problems, such as heart disease and stroke, and microvascular (in a small blood vessel) diseases, such as kidney disease. Diabetic retinopathy (DR), a microvascular complication of diabetes, is the leading cause of vision loss among the general population in different countries, notably among the working-age population and the elderly [Citation3,Citation4]. The disease of DR occurs due to damage to the retina as a consequence of diabetic mellitus complications that lead to permanent damage to the eyes and sometimes even vision loss [Citation5]. About 415 million individuals across the globe were diagnosed with diabetes in 2015, with estimates indicating that the number would increase to 642 million by the year 2040 [Citation6]. Due to the rise in the number of individuals with diabetes and the increased duration of the disease, diabetic retinopathy, and eye disease are growing globally [Citation7,Citation8].
Diabetic Retinopathy (DR) is a leading cause of blindness. DR mutilates a diabetic patient’s retinal blood vessels. The DR is classified into two main subtypes: non-proliferative diabetic retinopathy disease (NPDR) and proliferative diabetic retinopathy disease (PDR) [Citation9]. During the early stages, the disease is known as NPDR, which is classified into three subcategories: mild, moderate, and severe. One microaneurysm (MA), a tiny red dot on the terminal of blood vessels, is seen in the mild stage. The MAs ascend to a deeper layer of the retina in the Moderate stage and create a flame-shaped hemorrhage. The severe stage is characterized by more than 20 intraretinal hemorrhages in each of the four quadrants, with obvious venous bleeding and significant intraretinal microvascular changes in the affected retina. When DR progresses to the advanced stage of the disease, it results in neovascularization, the natural development of new blood vessels in the form of functioning microvascular networks that grow on the surface of the retina, and the disease known as PDR [Citation10].
The VEGF (VEGF) gene is located on chromosome 6 p 21.1. About 4 kb, the size of its coding region. The VEGF gene has eight exons that may be combined in different ways to produce a wide variety of mRNAs via alternative splicing. This gene’s polymorphism exceeds the 150 SNPs in exons or promoters [Citation11]. These SNPs are primarily clustered in the VEGF gene’s promoter and 5’-UTR [Citation12]. The 5’-UTR has many different binding sites for transcription factors, and the polymorphisms in this region cause gene expression changes [Citation13]. This study aimed to investigate whether DR patients had an association with genotypes of two SNPs in the VEGF gene and whether a specific set of variants of these SNPs inherited together.
Methods
Subject. Recruit 134 patients with diabetes and 36 healthy controls in a case–control study. The ophthalmologist evaluated the patient’s eyes, sought the presence of any signs of DR, and grouped them into 64 subjects with NPDR, 39 subjects with PDR, and 31 diabetic patients without retinopathy (NDR). The sociodemographic characteristics of patients were presented in our previous study [Citation14].
Selection criteria. Participants included those 18 years or older with a medical history consistent with type 2 diabetes under the criteria of the World Health Organization in its 2019 categorization standards [Citation15]. One or two eyes with any signs of the DR were included. Individuals affected by inflammatory retinal disorders (uveitis, retinal vasculitis) and all type 1 diabetes were excluded. Eight samples were excluded from statistical analysis because they did not give any variation; it is suspected they showed genotypes differently for rs2010963 (C > T instead of C > G).
Genotyping. Genomic DNA was extracted from whole blood in EDTA tubes using G-spin™ Total DNA Extraction Mini Kit (iNtRON Biotechnology, Korea) and stored at −20°C in the biotechnology laboratory of Babylon University until the genotyping. The genotyping of the SNPs in the study were tetra primers ARMS-PCR technique. In brief, the outer primers of rs2010963 and rs699947 SNPs generate the confirmative amplicon for the 5’ UTR and promoter region where the SNPs resides. The inner reverse primers were designed for the mutant allele by mismatching the 3’ end of the primer. Also, the third nucleotide of the 3’ end was mismatched to increase the specificity. The PCR steps were as follows: 40 cycles of denaturation at 95°C for 35 s and annealing at 65°C decreased gradually every cycle to reach the lowest annealing at 58°C for 40 s and 72°C for extension and final extension for 40 s and 7 min respectively. The PCR cycles are preceded by initial denaturation at 95°C for 2 min for activation. Followed by agarose gel electrophoresis (2% and 75 V) for 1 hour. The SNPgen® tool was used to design rs2010963 primers, whereas rs699947 primers were obtained from an Elfaki et al. study [Citation16], as shown in .
Table 1. Primers utilized in the detection of SNPs by the tetra primers ARMS–PCR.
Statistical analysis. The SPSS version 26.0 for Windows was used (Chicago, IL, USA). Genotype and allele frequency of VEGF polymorphisms were compared between the control group and the case groups using the Chi-square test [Citation17]. The binary and multinominal logistics were used to estimate the risk allele and genotype associated with DR, NPDR, and PDR. The haploview 4.2 (Broad Institute) program was used to calculate the haplotype frequencies of the VEGF rs2010963 and rs699947 SNPs and to build linkage disequilibrium (LD).
Results
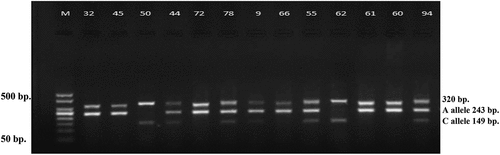
The genotyping of rs2010963 and rs699947 using the tetra primer-ARMS PCR. The bands were presented as follows the band of the C allele is 140 bp., and the G allele band is 177 bp. The heterozygous (CG) is shown in the presence of the two bands (140 bp. and 177 bp.) for rs2010963. The bands of rs699947 were presented as follows the band of the A allele is 243 bp. The C allele band is 149 bp. The heterozygous (AC) is shown in the presence of the two bands (149 bp. and 243 bp.) .
Figure 1. Agarose gel electrophoresis for the tetra primer-ARMS PCR product for genotyping rs2010963 SNP. (75 V for 60 min, M: DNA marker 50–500 bp, 2% Agarose).

Figure 2. Agarose gel electrophoresis of the tetra primer-ARMS PCR for genotyping rs699947 SNP (75 V for 60 min, M: DNA marker 50–500 bp, 2% Agarose).
The genotypic and allelic frequencies of rs2010963 in the healthy control (HC), NDR, and DR groups are detailed in . The genotype distribution of rs2010963 was in Hardy–Weinberg equilibrium (HWE) in the control group and cases groups using Yate’s correction (p-value ≥ 0.054). The Chi-square test results demonstrated that the frequencies of genotypes and alleles among study groups were statistically significant, with p-value = 0.027 and 0.024, respectively. The results revealed that the heterozygote genotype (CG) has a higher frequency among all of the controls, NDR, NPDR, and PDR; the lowest frequency is for the CC genotype. The G Allele was a higher frequency in case groups; in contrast, the C allele was a higher frequency than the G allele in the control group (P = 0.024). The genotypic and allelic frequencies of rs699947 in the HC, NDR, and DR groups are detailed in . Neither the NPDR nor the PDR groups showed any significant differences in genotype or allele frequency for the rs699947 polymorphism compared to the control or NDR groups. The C allele frequency among case groups was 64.8% and 70.5%, whereas in the controls and NDR groups was 63.6% and 56.9%, respectively; thus, no significant differences were found between controls and cases regarding the C allele.
Table 2. Distribution of Alleles and genotypes of rs2010963 and rs699947 between Controls, NDR, NPDR and PDR.
The VEGF polymorphisms were selected for logistic regression analysis for the genetic association study. First; we made a binary logistic regression analysis of the comparison between control and DR subjects and NDR and DR subjects; for rs2010963, the results revealed that the homozygous GG genotype of minor alleles was significantly associated with DR (OR = 10.29; 95% CI = 2.20–59.06; p = 0.004). No statistically significant association with DR for the heterozygous CG genotype (). The second is a multinomial logistic regression analysis between groups of DR (NPDR and PDR), which once compared with the controls and then for the NDR group. The analysis showed that minor alleles (GG) were also strongly associated with NPDR and PDR (OR = 6.33; 95% CI = 1.20–33.39; p = 0.030) and (OR = 34; 95% CI = 2.9–392.94; p = 0.005) respectively (). However, the heterozygous CG genotype was unrelated to NPDR or PDR state (). In contrast, for rs699947, the heterozygous AC and homozygous (AA & CC) genotypes in binary logistic regression and multinomial logistic regression were statistically insignificant in DR status, and subtypes of DR (NPDR & PDR) , respectively.
Table 3. Binary Logistic Regression for rs2010963 & rs699947 SNP between Controls, NDR and DR.
Table 4. Multinomial Logistic Regression for rs2010963 & rs699947 SNP between Controls, NDR, NPDR and PDR.
Moreover, in the case of rs2010963, the GG genotype and recessive model (CC+CG vs. GG) frequencies were significantly higher in the DR compared with controls (OR = 0.10; 95% CI = 0.02–0.46; p = 0.002) and (OR = 5.63; 95% CI = 1.75–18.38; p = 0.003), respectively. Interestingly, when analyzing the association between groups of DR (NPDR and PDR) with controls and NDR, the dominant model (CC vs. CG+GG) was statistically significant only with PDR (OR = 8.44; 95% CI = 1.20–99.07; p = 0.026). In contrast, the recessive model (CC+CG vs. GG) was associated with NPDR and PDR subjects (p = 0.016 and 0.001, respectively) (). In contrast, for rs699947, the comparisons between DR status and subtypes of DR (NPDR & PDR) with controls regarding dominant, recessive, codominant, and heterozygous models revealed similar results (p-value > 0.05) for all models . Thus, our samples have no significant importance for this SNP with diabetic retinopathy.
Table 5. Association of Different Genetic Models for rs2010963 & rs699947 SNP between Controls, NDR, and DR.
Table 6. Association of Different Genetic Models for rs2010963 & rs699947 SNP between Controls, NDR, NPDR, and PDR.
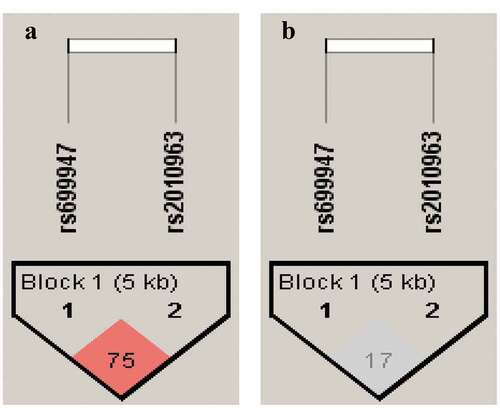
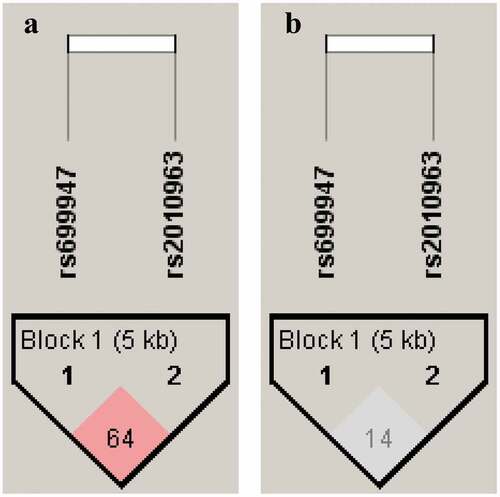
Haplotype analysis and linkage disequilibrium. The haplotype analysis results and the Linkage disequilibrium pattern (LD) between VEGF polymorphisms (rs2010963 and rs699947) are shown in . and , respectively. The predicted haplotypes with frequencies ≥0.03 were compared between the DR, NDR, and control groups. The frequency of CG haplotype was statistically significant between DR and NDR (OR = 2.0; 95% CI = 1.02–3.93; p = 0.043), and also there were significant differences between DR and controls (OR = 2.42; 95% CI = 1.2–4.65; p = 0.0108). The CC haplotype appears to have significant differences between DR and control groups only (OR = 0.56; 95% CI = 0.33–0.99; p = 0.0479) (). Pairwise LD parameter D’ was 0.75 with r2 = 0.17 for DR and NDR groups. In contrast, D’ was 0.64 with r2 = 0.14 for DR and control groups. The findings suggest a moderate LD in two SNPs (rs2010963 and rs699947).
Table 7. Haplotyping of rs2010963 and rs699947 of VEGF among DR and NDR.
Table 8. Haplotyping of rs2010963 and rs699947 of VEGF among DR and control.
Discussion
In this study, we looked at the possibility that VEGF polymorphisms are linked to the existence of DR in a well-defined group of Iraqi patients with type 2 diabetes. VEGF serves as the primary regulator in both normal and abnormal vascular development. It has the potential to enhance retinal vascular permeability, destroy the blood-retinal barrier, and generate new blood vessels in DR, all of which are directly linked to the emergence and progression of the disease [Citation18]. The VEGF polymorphism (rs2010963) was the one that received the most attention from researchers. Previous studies have demonstrated that rs2010963 is one of many SNPs associated with increased or decreased VEGF protein synthesis and may alter protein conformation [Citation19]. We’ve already shown solid evidence linking VEGF SNP rs2010963 to DR severity or existence. The rs2010963 locate on 5’ UTR of the VEGF gene; in the 5ʹUTR of the VEGF gene, the G + C content (up to 83%) upstream of the transcription start point is relatively high. The 5’-untranslated region of the VEGF gene (5ʹUTR) plays a key part in VEGF production. Change in the sequence of this region is thought to be linked to alterations in protein expression of the VEGF gene, which in turn may increase the risk of developing retinopathy [Citation20,Citation21] as well as NPDR in the Asian population [Citation22]. Yang et al. concluded that rs2010963 was a risk factor for PDR in most groups but not Caucasians, where no link was found between rs2010963 and PDR development [Citation23]. According to our findings, those with the GG genotype had a higher probability of developing PDR than those with the CC genotype. Compared with other studies, CG lowered the risk of PDR [Citation24]. On the other hand, a study by Fan showed that gene polymorphisms such
as rs2010963 have been strongly linked with Recent meta-analysis findings indicated that rs2010963 was associated with PDR in the overall population VEGF protein in the blood [Citation25]. The VEGF protein is a potent angiogenic agent in various diseases. It has been established that elevated serum and vitreous levels of VEGF in the presence of retinopathy are closely associated with proliferative diabetic retinopathy [Citation26]. A study from Egypt explored the association between rs699947 SNP and the susceptibility of DR, and it found no significant association between them. This result is consistent with our findings [Citation27]. Different studies also revealed no association between the presence of rs699947 SNP and the risk of DR [Citation28]. In a previous meta-analysis, the rs699947 in Asian individuals with type 2 diabetes are more likely to be associated with DR but not in white people [Citation29]. While a meta-analysis from China recognizes the reverse association that rs699947 polymorphism is strongly connected with DR after adjusting for outliers, rs2010963 polymorphism may not be linked to DR [Citation30]. Different meta-analyses on rs2010963; one revealed an association while the other did not [Citation31,Citation32].
In contrast, many others showed a positive association of rs699947 SNP with the susceptibility of DR [Citation12,Citation13,Citation33]. A recent meta-analysis demonstrated that rs699947 might be implicated in the emergence and progression of DR [Citation22]. Numerous studies have discovered an association between various genetic polymorphisms and DR, whereas others have failed to do so [Citation34]. This outcome discrepancy might be due to a sample size disparity and clinical or methodological heterogeneity, and sampling of allele inheritance but not genotype. The degree of heterogeneity is likely influenced by several factors, including ethnicity, environmental factors, and social relationships that govern the cross-mating between families. Also, it might be that these polymorphisms convey sex depending on where there are different proportions of males/females in various studies; the discrepancy occurs. Howover the two SNPs (rs699947 & rs2010963) among DR patinets revealed no association with gender, and since majority of included studies did not report the association of the genotypes of the rs699947 & rs2010963 SNPs with gender, we are unable to perform the further analysis. Only few studies found that sex may a risk factor for DR, but not polymorphisms [Citation35,Citation36]. Yet we drew in our account a conception; the two SNPs (rs699947 & rs2010963) among NPDR and PDR need to be studied further to identify the link between the two.
The findings suggest a moderate LD in two SNPs (rs2010963 and rs699947). The rs699947, as mentioned early in the study, it was observed to have no significant association with DR; however, the C allele shared with the G allele of rs2010963; both alleles are minor alleles (mutant) and contributed significantly >2 folds to DR progression. Thus, it is valuable to measure the LD, which confers a piece of more information if statistical genetics fails to find an association. LD occur when nonrandom gametic alleles are associated at various loci in a population [Citation37]. The haplotype has an effect that differs from genotypes on disease, and the locus is still of clinical importance even though there is no link between genotype and clinical condition [Citation38]. The VEGF gene SNPs located at the promoter region and 5’ UTR and
the presence of the CG haplotype association confer upregulation or downregulation of VEGF expression, eventually leads worsen normal vision. A recent study shows different haplotypes (CD) from rs699947 and rs35569394 polymorphisms have a non-significant association with DR. Simultaneously, their alleles C and D have strong LD [Citation33]. One explanation is differences in allele and genotype frequencies of SNPs on one side and, on the other, the differences between different studies. Also, the patterns of LD
are from each other have strong LD. The haplotype and LD are important for large-scale association-mapping studies [Citation39]. unpredictable; two loci near each other might have weak LD, whereas loci of different SNPs that are so far from each other have strong LD. The haplotype and LD are important for large-scale association mapping studies [Citation40].
Conclusion
The GG genotype of rs2010963 in the VEGF gene had a higher probability of developing either NPDR or PDR. No significant importance for rs699947 SNP with diabetic retinopathy. The findings suggest a moderate LD in two SNPs (rs2010963 and rs699947) and their CG haplotype associated with DR progression by 2 folds.
Abbreviations
CI: Confidence interval; DM: Diabetes mellitus DR: Diabetic retinopathy; HC: Healthy controls; HWE: Hardy–Weinberg equilibrium; MA: Microaneurysm; LD: Linkage disequilibrium; NDR: Non diabetic retinopathy;NPDR: Non-proliferative diabetic retinopathy; OR: Odds ratio; PDR: Proliferative diabetic retinopathy; SNPs: Single nucleotide polymorphisms; T-ARMS PCR: Tetra primer amplication refractory mutation system polymerase chain reaction; T1DM: Type 1 diabetes mellitus; T2DM: Type 2 diabetes mellitus; VEGF: Vascular endothelial growth factor; UTR: untranslated region.
Ethical approval
Ethical approval for this study was granted by the human ethics committee (IRB) at the University of Kufa ((347/2021). we are following the ethical standards of the Helsinki Declaration.
Acknowledgments
We would like to thank Dr. Giyath Al-Deen Thegil Neamah for his kind words and moral support.
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- abdul JAKK, Kais Abdul Jaleel Duaibel AK, Alnaji HA, et al. The role of MCP-1 in the developments of diabetic nephropathy in patients with type 2 diabetes mellitus patients. Int J Health Sci (Qassim). Internet]. 2022 May 7 cited 2022 Jul 26;6(S2):8158–8166. Available from:, https://sciencescholar.us/journal/index.php/ijhs/article/view/7049
- Akkaj D, Al-Janahi FAA, Naji AM, et al. Glu298Asp polymorphism in exon 7 of the eNOS gene in foot ulcer of adult patients with type 2 diabetes. AIP Conf Proc. Internet]. 2022 Jan 11 cited 2022 Jul 26;2386(1):020013. Available from:, https://aip.scitation.org/doi/abs/10.1063/5.0067092
- Ali Mezil BAA S. Complication of diabetes mellitus. Ann Rom Soc Cell Biol [Internet]. 2021 Mar 22 cited 2022 Oct 26;1546–1556. Available from : . https://www.annalsofrscb.ro/index.php/journal/article/view/1601
- Vaswani R, Shukla S, Acharya S. Pathophysiology of complication in diabetes mellitus. J Pharm Res Int. 2021 Dec 19;89–95. 10.9734/jpri/2021/v33i60A34459.
- Gadekallu TR, Khare N, Bhattacharya S, et al. Deep neural networks to predict diabetic retinopathy. J Ambient Intell Humaniz Comput. 2020;11:1–14.
- Ogurtsova K, da Rocha Fernandes JD, Huang Y, et al. IDF Diabetes Atlas: global estimates for the prevalence of diabetes for 2015 and 2040. Diabetes Res Clin Pract. 2017;128:40–50.
- Alnaji H, Alnuwaini MQ, Almulla AF. Low levels of postprandial C-peptide are clinically significant in non-proliferative diabetic retinopathy and diabetic macular oedema in Iraqi patients with type 2 diabetes. Art Int J Health Sci [Internet]. 2022 cited 2022 Jul 18; Available from. 10.53730/ijhs.v6nS1.6785 8258–8267
- Abdul Jaleel AK, Almulla AF, Alnaji HA, et al. Diabetes mellitus and non-proliferative diabetic retinopathy are accompanied by increase pro-inflammatory conditions indicated by a high blood-derived levels of monocyte chemoattractant protein-1 and interleukin-8. Iran J War Public Health [Internet]. 2022 cited 2022 Jul 25;14(2):203–210. Available from: http://ijwph.ir/article-1-1165-en.html
- Memon WR, Lal B, Sahto AA. Diabetic retinopathy. Prof Med J. 2017;24(2):234–238.
- Kavitha K. Incidence of retinopathy changes in new cases of diabetes mellitus type 2. 2013 cited 2022 Oct 26]; 2022 Oct 26: http://repository-tnmgrmu.ac.in/3144/
- Koutras A, Kotoula V, Fountzilas G. Prognostic and predictive role of vascular endothelial growth factor polymorphisms in breast cancer. Pharmacogenomics. 2015 Jan 1; 16(1):79–94.
- Qayyum S, Afzal M, Naveed A. Association of vascular endothelial growth factor A gene (VEGFA) polymorphisms, rs699947 and rs1570360, with diabetic retinopathy and altered VEGF. 2020 cited 2022 Jul 5]; 2022 Jul 5: https://www.researchsquare.com/article/rs-18816/latest.pdf
- Wijaya AR, Surudarma IW, Wihandani DM, et al. Polymorphisms of vascular endothelial growth factor—2578C/A rs699947 are risk factors for diabetic retinopathy in type-2 diabetes mellitus patients in Bali, Indonesia. Biomedicine (Taipei). Internet]. 2021 cited 2022 Jul 5;11(2):11. Available from.;():. /pmc/articles/PMC8824245/
- Alnaji HA, Hassan AH, Omran R. Genetic polymorphism of AKR1B1 with diabetic retinopathy. Int J Health Sci (Qassim). Internet]. 2022 May 25 cited 2022 Jul 26;6(S3):8310–8315. Available from:, https://sciencescholar.us/journal/index.php/ijhs/article/view/7898
- Organization WH. Classification of diabetes mellitus. 2019 cited 2022 Oct 22]; 2022 Oct 22: https://apps.who.int/iris/handle/10665/325182?locale-attribute=ar&utm_source=transaction&utm_medium=email
- Elfaki I, Mir R, Duhier FMA, et al. Clinical implications of MiR128, angiotensin I converting enzyme and vascular endothelial growth factor gene abnormalities and their association with T2D. Curr Issues Mol Biol. 2021, 43, 1859-1875 [Internet]. 2021 Nov 2 cited 2022 Jul 16;43(3):1859–1875. Available from. ;: . https://www.mdpi.com/1467-3045/43/3/130/htm
- Alsadawi AA, Duabel AKKA J, Alnaji HA. Hepatitis C and il-6 with 174G/C gene polymorphism in β-thalassemia. Int J Drug Delivery Technol. 2019;9(4):617–622.
- Wang H, Cheng JW, Zhu LS, et al. Meta-analysis of association between the −2578C/A polymorphism of the vascular endothelial growth factor and retinopathy in type 2 diabetes in Asians and caucasians. Ophthalmic Res. Internet]. 2014 cited 2022 Jul 4;52(1):1–8. Available from:
- Sellami N, Ben LL, Turki A, et al. Association of VEGFA variants with altered VEGF secretion and type 2 diabetes: a case-control study. Cytokine. 2018 Jun 1;106:29–34.
- Zhang S, Li Q, Yang M, et al. Relationship between the 5ʹUTR of vascular endothelial growth factor polymorphism and retinopathy of prematurity in Chinese premature newborns. Int J Clin Exp Pathol Internet]. 2017 cited 2022 Jul 4;10(12):11695. Available from.;:. /pmc/articles/PMC6966069/
- Al-Abdullah AA, Kozak I. Toward better evidence: meta-analysis of vascular endothelial growth factor (VEGF) polymorphisms in retinopathy of prematurity. Saudi J Ophthalmol Internet]. 2014 Oct 1 cited 2022 Jul 4;28(4):251. Available from:
- Hu L, Gong C, Chen X, et al. Associations between vascular endothelial growth factor gene polymorphisms and different types of diabetic retinopathy susceptibility: a systematic review and meta-analysis. J Diabetes Res. 2021;2021:1–12.
- Yang Q, Zhang Y, Zhang X, et al. Association of VEGF gene polymorphisms with susceptibility to diabetic retinopathy: a systematic review and meta-analysis. Hormone Metab Res. 2020 May 1; 52(5):264–279.
- Jin H, Jiang D, Ding Z, et al. Association of four gene polymorphisms in Chinese Guangxi population with diabetic retinopathy in type 2 diabetic patients. BMC Ophthalmol. 2021. Internet]. cited 2022 Jul 5;21:383. Available from;(1):.
- Fan XH, Wu QH, Li Y, et al. Association of polymorphisms in the vascular endothelial growth factor gene and its serum levels with diabetic retinopathy in Chinese patients with type 2 diabetes: a cross-sectional study. Chin Med J (Engl). 2014;127(4):651–657.
- Khan SZ, Ajmal N, Shaikh R. Diabetic retinopathy and vascular endothelial growth factor gene insertion/deletion polymorphism. Can J Diabetes. 2020 Apr 1; 44(3):287–291.
- Fattah RAA, Eltanamly RM, Nabih MH, et al. Vascular endothelial growth factor gene polymorphism is not associated with diabetic retinopathy in egyptian patients. Middle East Afr J Ophthalmol. Internet]. 2016 Jan 1 cited 2022 Jul 5;23(1):75. Available from ;:
- Shahin RMH, Abdelhakim MASE, Owid MESM, et al. A study of VEGF gene polymorphism in egyptian patients with diabetic retinopathy. Ophthalmic Genetics. Internet]. 2014 Dec 9 cited 2022 Dec 9;36(4):315–320.
- Wang H, Cheng JW, Zhu LS, et al. Meta-analysis of association between the −2578C/A polymorphism of the vascular endothelial growth factor and retinopathy in type 2 diabetes in Asians and Caucasians. Ophthalmic Res. 2014 cited 2022 Oct 28;52 1:1–8. Internet]: Available from: https://www.karger.com/Article/FullText/357110
- Lu Y, Ge Y, Shi Y, et al. Two polymorphisms (rs699947, rs2010963) in the VEGFA gene and diabetic retinopathy: an updated meta-analysis. BMC Ophthalmol. Internet]. 2013 Oct 16 cited 2022 Oct 28;13(1):1–9. Available from:, https://bmcophthalmol.biomedcentral.com/articles/10.1186/1471-2415-13-56
- Han L, Zhang L, Xing W, et al. The associations between VEGF gene polymorphisms and diabetic retinopathy susceptibility: a meta-analysis of 11 case-control studies. J Diabetes Res. 2014;2014:1–10.
- Qiu M, Xiong W, Liao H, et al. VEGF − 634G>C polymorphism and diabetic retinopathy risk: a meta-analysis. Gene. 2013 Apr 15; 518(2):310–315.
- Rabbind Singh A, Gupta R, Shukla M, et al. Association of VEGFA promoter polymorphisms rs699947 and rs35569394 with diabetic retinopathy among North-Central Indian subjects: a case-control study. Ophthalmic Genetics. 2021;43(1);80–87. Internet]. cited 2021 Jul 5; Available from
- Owyong M, Schwartz S. An update on the genetics of diabetic retinopathy. RETINA TODAY [Internet]. 2017 cited 2022 Oct 28]; 2022 Oct 28: https://assets.bmctoday.net/retinatoday/pdfs/0317RT_Cover_Scott.pdf
- Varma R, Macias GL, Torres M, et al. Biologic risk factors associated with diabetic retinopathy: the los angeles latino eye study. Ophthalmology. 2007 Jul 1; 114(7):1332–1340.
- Zhang X, Saaddine JB, Chou CF, et al. Prevalence of diabetic retinopathy in the United States, 2005-2008. JAMA Internet]. 2010 Aug 11 cited 2022 Dec 2;304(6):649–656. Available from:
- Qanbari S. On the extent of linkage disequilibrium in the genome of farm animals. Front Genet. 2020 Jan 17; 10:1304
- Yang X, Deng Y, Gu H, et al. Polymorphisms in the vascular endothelial growth factor gene and the risk of diabetic retinopathy in Chinese patients with type 2 diabetes. Mol Vis Internet]. 2011 cited 2022 Jul 4;17:3088. Available from: /pmc/articles/PMC3233387/
- Garcia JA, Lohmueller KE, Williams SM. Negative linkage disequilibrium between amino acid changing variants reveals interference among deleterious mutations in the human genome. PLoS Genet Internet]. 2021 Jul 28 cited 2022 Oct 26;17(7):e1009676. Available from:
- Wall JD, Pritchard JK. Haplotype blocks and linkage disequilibrium in the human genome. Nat Rev Genet. 2003 4:8 [Internet]. 2003 Aug 1 cited 2022 Aug 2;4(8):587–597. Available from: https://www.nature.com/articles/nrg1123