ABSTRACT
Background
Regular assessments of disease activity are essential to prevent the progression of rheumatoid arthritis (RA).
Aim of the work
This study aimed to assess the utility of serum substance P (SP) level as a marker for RA activity and to establish the value of SP level for the diagnosis of subclinical RA activity.
Patients and Methods
The study included three groups each 30.
subject
Group 1 was with minimal or no disease, group 2 had moderate to severe disease activity and group 3 was healthy controls. RA patients were assessed using a Disease Activity Score-28 (DAS28) and power Doppler ultrasonography measurement of the synovitis total score. The serum substance P levels of study groups were assessed using ELISA technique.
Results
Statistically significant difference in serum SP level was detected in RA patients compared to controls (p ≤ 0.001) and between different disease activity groups (p ≤ 0.001). The best cut off SP value to distinguish patients in subclinical activity active disease patients was 555.5 pg/ml. This had a diagnostic sensitivity, specificity, and accuracy of 96.7%, 80% and 88.3 %, respectively.
Conclusion
Serum SP can be used as an indicator of RA disease activity even in those with subclinical activity.
Introduction
The autoimmune disorder rheumatoid arthritis (RA) is an autoimmune disease characterized by persistent synovitis, progressive destruction, and joint deformity [Citation1]. When compared to other autoimmune illnesses, which affect 5% to 7% of the global population, RA prevalence ranges from 0.3% to 1% [Citation2]. Women are more likely than men to have rheumatoid arthritis (3:1 ratio) [Citation3]. The T lymphocytes cells are the major cells responsible for causing synovitis and hence can be implicated in the pathophysiology of RA [Citation4]. Rheumatoid arthritis commonly impacts the smaller joints in the hands and feet. RA can also affect the musculoskeletal system, which includes tendons, muscles, bones, and less frequently extra-articular sites [Citation5].
Blood tests (anti-cyclic citrullinated peptide, rheumatoid factor, inflammatory markers), radiography, and ultrasonography all contribute to the diagnosis of RA. Early diagnosis is crucial because early action creates a ‘window of opportunity’. Currently, therapy aims to restrict disability and prevent structural damage in addition to relieving symptoms [Citation5]. The overall strategy for treating RA patients relies on the timely and prudent application of a variety of therapeutic approaches, which depends on early diagnosis and the early use of disease-modifying antirheumatic medications (DMARDs) with the goal of remission or low disease activity [Citation6].
Substance P (SP) is a neuropeptide containing 11 amino acids in its structure and is encoded by Tachykinin Precursor 1 (TAC1) gene on human chromosome 7 [Citation7]. It is widely present in both central and peripheral nervous systems [Citation8]. Also, SP is released from immune cells such as macrophages, lymphocytes, and dendritic cells [Citation9]. In addition to the role of SP in pain, the non-neuronal effects of SP include vasodilation, smooth muscle contraction, change in cardiovascular tone, blood cell production, pruritus, wound healing, cancer, etc. [Citation8,Citation10].
Asthma, inflammatory bowel disease, psoriasis, atopic dermatitis, fibromyalgia, sickle cell disease, and rheumatic disorders, such as RA, are among the pathological disorders where substance P levels are elevated [Citation11]. Since pro-inflammatory cytokines like IFN- α, TNF α and γ IL-1b are linked to the degeneration of cartilage and bone erosion in RA, the ability of SP to regulate the synthesis of pro-inflammatory cytokines in the synovium suggests that it played a key role in the development of RA [Citation12]. TNF is a well-known cytokine that is present in both the synovium and serum of RA patients. It is a strong inducer of proinflammatory cytokines and disturbs the physiological balance between pro- and anti-inflammatory mediators [Citation13]. A decrease in serum SP brought on by anti-TNF therapy is associated with a reduction in RA disease activity [Citation14]. Limited information is available about the variation of SP serum level in different stages of RA activity and its role as a diagnostic marker for RA. This study was aimed at investigating serum level of substance P as a marker of subclinical rheumatoid arthritis activity.
Patients and methods
Patients
A case–control study was carried on 60 rheumatoid arthritis patients fulfilled the criteria of the American College of Rheumatology/European League against Rheumatism (ACR/EULAR) 2010 [Citation15] attended to the outpatient clinics of the rheumatology & rehabilitation department at Mansoura University Hospital, and 30 healthy controls who attended to blood bank served as the control group. The study was conducted over a 12-month period, from November 2018 to October 2019. Both the patients and the controls were over the age of 18. Demographic data and clinical data including duration of illness, results of rheumatoid factor, c reactive protein and anticyclic citrullinated peptide laboratory tests were collected from patient’s files. Demographic data of the studied groups are presented in . According to the Disease Activity Score-28 (DAS28) score, rheumatoid arthritis patients were split into two groups based on their level of RA activity. Clinical evaluation of tender and swollen joints was performed. The DAS28 score, which incorporates the swollen joint count (SJC) and tender joint count, was used to measure the severity of the disease (TJC), the erythrocyte sedimentation rate (ESR) and the patient’s and physician’s global assessment of activity on the visual analogue scale (VAS).
Table 1. Demographic data of the studied groups.
Group 1 includes 30 cases who had minimal or no disease activity as determined by the DAS28 score (DAS28 ≤ 3.2). Group 2 consisted of 30 individuals with active disease (moderate and high disease activity); moderate disease activity (3.2 < DAS28 ≤ 5.1) and high disease activity (DAS28 > 5.1) [Citation16,Citation17]. The 30 healthy controls were assigned as group 3.
Laboratory tests
Serum Substance P levels were measured by
ELISA [Citation18]: For every subject in all the three groups, 5-ml venous blood samples were withdrawn under complete aseptic condition by nurses in outpatient clinics of rheumatology & rehabilitation department and the control samples were taken from blood bank. Then, serum was separated by centrifugation 2000–3000 RPM for 20 min and kept frozen in test tubes at −20c and this step was performed at (Immunological lab of medical microbiology and immunology department at faculty of medicine Mansoura University) until analysis of SP using ELISA kit (Shanghai Korain Biotech company, China) according to manufacture instructions.
Ultrasound imaging
Ultrasound examination of each patient was performed on the same day of sample collection by a sonographer trained in power Doppler US. The time required to scan a patient completely was 20 min using EDAN U 2 ultrasound device (Shenzhen, China) with linear array transducer (8–13.4 MHz) a frequency of 13 MHz was used. Power Doppler ultrasound was used to assess degree of synovitis and obtain synovitis total score by scanning of the most clinically affected 7 hand and foot joints (wrist, 2nd and 3rd metacarpophalangeal joint (MCP), 2nd and 3rd proximal interphalangeal joint (PIP), 2nd and fifth metatarsophalangeal joint (MTP) [Citation19]. Synovitis total score ranged from 0 to 39 and calculated by the equation described before [Citation20]. The scoring system had grades of 0–3 [Citation21].
The study was approved by the institutional review board at faculty of medicine Mansoura Univerisy. Informed consent was obtained from all patients included in the study.
Statistical analysis
Data were analyzed using SPSS program (Standard version 21). The normality of data was assessed using Kolmogorov–Smirnov test. Number and percentage were used to describe qualitative data. Continuous variables were presented (min-max), mean ± standard deviation (SD) for parametric data and median for non-parametric data. The two groups were compared with student t- test for parametric data and Mann Whitney test for non-parametric. More than two groups were compared by ANOVA test (parametric) and kruskal–Wallis test (non-parametric). Continuous parametric data were tested for correlation using Pearson correlation, whereas continuous non-parametric data were correlated using Spearman correlation. By using a receiver operating curve (ROC) sensitivity and specificity at different cutoff point were identified. Results were deemed significant for all of the aforementioned statistical tests when the p value was less than 0.05.
Results
The study enrolled 60 RA patients divided into 2 groups according to RA activity as determined by DAS28. And group 3 includes 30 healthy controls. Demographic data of the studied groups are presented in . No significant difference was detected between studied groups regarding age and sex.
All patients in group 1, 2 underwent assessment including clinical, laboratory, and radiographic assessments
Table 2. Clinical, laboratory, and radiographic data of patients
C reactive protein, ESR: Erythrocyte sedimentation rate, RF: Rheumatoid factor, ACCP: Anticyclic citrullinated peptide.
Regarding serum substance P level, there was a significant higher serum substance P level in group 1 and 2 compared to the healthy controls (Pa: ≤0.001 and Pb: ≤0.001 respectively). In addition, upon comparing patients in remission and low disease activity to patients in moderate and high disease activity regarding serum SP level (group 1 and group 2), there was a statistically significant difference between them (Pc value≤0.001) (.
Table 3. Clinical, laboratory, and radiographic data of patients.
Correlation between serum SP level in RA patient groups and clinical, laboratory parameters and synovitis total score was tested. Significant positive correlation between SP levels and synovitis total score, TJC, DAS28 and VAS was detected in group 1 and 2 patients. Moreover, significant positive correlation was detected between SP serum level and age (p = 0.046) in patients of group 1 only. In addition, serum SP level had significant positive correlation with SJC (p = 0.005) in group 2 patients only ().
Table 4. Clinical, laboratory, and radiographic data of patients.
C reactive protein. ESR: Erythrocyte sedimentation rate. RF: Rheumatoid factor. ACCP: Anticyclic citrullinated peptide.
r:Spearman correlation co-efficient. *statistically significant (if p < 0.05)
ROC curve analysis was applied to determine the best cutoff value of serum SP in diagnosing RA cases and in differentiating patients with active RA from those with subclinical activity. The best cutoff value of serum SP in diagnosing RA cases was 99.0 pg/ml and area under the curve (AUC) was 0.988. This had a diagnostic sensitivity, specificity and accuracy of 98.3%, 90% and 95.5%, respectively ( and .
Figure 1. Receiver Operating characteristics (ROC) curve of substance P in differentiating cases from control group.
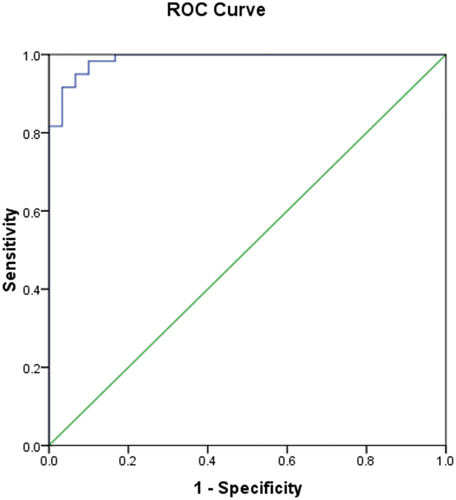
Table 5. Validity of substance P level (pg/ml) in diagnosing RA cases.
negative predictive value
The best cutoff value of serum SP in differentiating patients with active RA from those with subtle disease activity was 555.5 pg/ml. The AUC was 0.924. This had a diagnostic sensitivity, specificity and accuracy of 96.7%, 80% and 88.3% respectively ( and .
Figure 2. Receiver operating characteristics (ROC) curve of substance P for prediction of disease activity.
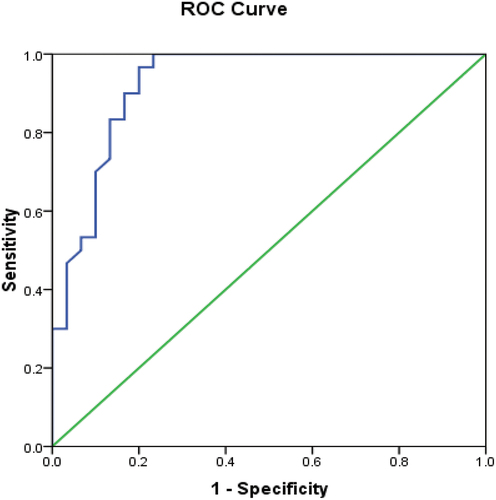
Table 6. Validity of serum substance P level (pg/ml) in differentiating active RA disease from subclinical activity.
negative predictive value
Discussion
Rheumatoid arthritis is an autoimmune, multisystemic inflammation disease that causes substantial functional decline over time, radiographic damage, recurrent job incapacity, and early mortality [Citation22]. Assessment of the illness’s activity is crucial for the best management of RA since rheumatoid flares and activity are linked to disease progression [Citation23,Citation24]. The primary goal of novel therapy approaches for RA, which include the currently available synthetic and biological DMARDs, is remission or at least low disease activity [Citation25]. Remission, however, may not always signify a total absence of inflammation. Power Doppler ultrasound is one of the more sensitive instruments that is needed to assess the course of the disease [Citation26]. These methods can be used to identify patients who are at risk of structural progression despite an apparent clinical response or to help with drug withdrawal decisions [Citation20]. Inflammatory and immunological modulators like Substance P are one of the tools that have been suggested [Citation14]. Inflammation, apoptosis, and the production of chemokines and pro-inflammatory cytokines are all significant aspects of how substance P controls the immune system [Citation7].
Our study was aiming at evaluation of the possible use of SP as a marker for RA activity. Serum substance P level was evaluated in age and sex matched groups including two groups of RA patients (low and active disease) and healthy control.
Our study confirmed the findings of numerous earlier findings [Citation27–29] by demonstrating that women are more likely than men to have RA. This can be related to the fact that females have strong immune systems and respond forcefully to vaccination and infection. Female hormones have also a significant role as estrogen and prolactin can stimulate the growth of B cell autoantibodies [Citation30].
Comparing serum SP in RA patients and healthy controls, significant higher serum substance P levels was found in RA patients (p ≤ 0.001). This result matches with previous studies [Citation31–33].
In our study, patients with remission and low disease activity (group 1) and patients with moderate and severe disease activity (group 2) had statistically different serum SP levels (p value ˂0.001). So even in patients who appear to be in remission, serum SP levels may be a sign of RA activity. Furthermore, our research revealed a significant positive correlation between serum SP levels and DAS28 score (p ≤ 0.001). These results are consistent with those previously obtained [Citation34]. On contrary, Lisowska et al. 2015 [Citation18] observed insignificant correlation between the DAS28 score and serum SP level.
In our study, serum SP and VAS were significantly correlated in groups 1 and 2 (p = 0.015 and ≤0.001, respectively). The VAS has been validated as a subjective tool for measuring both acute and ongoing pain [Citation35]. Additionally, for groups 1 and 2, a significant positive correlation between blood SP level and TJC was found (p values of 0.002 and ≤0.00, respectively). This contrasts with the finding of Origuchi et al. [Citation14] which showed an insignificant correlation between serum SP level and TJC. TJC has a multifactorial base and may not always indicate continuous inflammation (e.g. joint degeneration as in the case of secondary osteoarthritis or subluxation causing pain even if the RA inflammation is no longer present) [Citation36]. In comparison to clinical examination, power Doppler ultrasound is a contemporary imaging tool that allows for direct visualization of joint structures and is more effective at detecting synovitis and disease activity in RA [Citation37]. In order to differentiate between inflammatory and non-inflammatory arthritis, synovitis is evaluated using the synovitis total score [Citation38]. We found a significant positive correlation between synovitis total score and serum SP levels (P value≤0.001).
The best serum SP cutoff value for diagnosing RA cases and differentiating patients with active RA from those with subclinical activity was determined using ROC curve analysis. For diagnosis of RA cases, the optimum serum SP cutoff value was 99.0 pg/ml and AUC was 0.988. This exhibited 98.3% diagnostic sensitivity, 90% diagnostic specificity, and 95.5% diagnostic accuracy. The best cutoff value of serum SP in differentiating patients with active RA from those with subtle disease activity was 555.5 pg/ml, and the AUC was 0.924 with a diagnostic sensitivity, specificity and accuracy of 96.7%, 80% and 88.3%, respectively. In this regard, serum SP had higher sensitivity, specificity and accuracy when compared to other markers like serum calprotectin [Citation39].
To the best of our knowledge, this study is the first to specify a cutoff value for serum SP for RA diagnosis or activity assessment. In light of all prior findings, we recommend considering serum SP as a new candidate for RA diagnosis and activity assessment to increase the ability to recognize patients with subclinical disease activity and enable an earlier start to RA treatment. In addition, we advise future research to establish the function of SP in RA therapy monitoring and SP antagonist use.
Conclusion
Even in patients with subclinical activity, serum SP can be utilized as a marker of RA disease activity.
According to our knowledge, this study is the first study that defines a cut-off value of serum SP either to diagnose or to evaluate the activity of RA. According to all previous results, we recommended considering serum SP as a new diagnostic tool in RA to improve the ability of identifying patients in subclinical disease activity and this allows the earlier initiation of RA treatment. Also, we recommend further investigations to determine the role of SP in monitoring RA therapy and use of SP antagonists in RA treatment.
Conclusion
Serum SP can be used as a marker of RA disease activity even in those with subclinical activity.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Abbasifard M, Imani D, Bagheri-Hosseinabadi Z. PTPN22 gene polymorphism and susceptibility to rheumatoid arthritis (RA): updated systematic review and meta-analysis. J Gene Med. 2020 Sep;(9):e3204.
- Safiri S, Kolahi AA, Hoy D, et al. Global, regional and national burden of rheumatoid arthritis 1990-2017: a systematic analysis of the Global Burden of Disease study 2017. Ann Rheum Dis. 2019 Nov;78(11):1463–1471.
- Viatte S, Plant D, Raychaudhuri S. Genetics and epigenetics of rheumatoid arthritis. Nat Rev Rheumatol. 2013 Mar;9(3):141–153.
- Smolen JS, Aletaha D, Barton A, et al. Rheumatoid arthritis. Nat Rev Dis Primers. 2018 Feb 8;4(1):18001.
- Gulati M, Farah Z, Mouyis M. Clinical features of rheumatoid arthritis. Medicine. 2018;46(4):211–215.
- Arora S, Rafiq A, Jolly M. Management of rheumatoid arthritis: review of current guidelines. J Arthroscopy Joint Surg. 2016;3(2):45–50.
- Mashaghi A, Marmalidou A, Tehrani M, et al. Neuropeptide substance P and the immune response. Cell Mol Life Sci. 2016 Nov;73(22):4249–4264.
- Suvas S. Role of Substance P Neuropeptide in Inflammation, Wound Healing, and Tissue Homeostasis. J Immunol. 2017;199(5):1543–1552.
- Lambrecht BN, Germonpre PR, Everaert EG, et al. Endogenously produced substance P contributes to lymphocyte proliferation induced by dendritic cells and direct TCR ligation. Eur J Immunol. 1999;29:3815–3825.
- Gautam M, Prasoon P, Kumar R, et al. Role of neurokinin type 1 receptor in nociception at the periphery and the spinal level in the rat. Spinal Cord. 2016 Mar;54(3):172–182.
- Muñoz M, Coveñas R. Involvement of substance P and the NK-1 receptor in human pathology. Amino Acids. 2014 Jul;46(7):1727–1750.
- Rodriguez PL, Jiang S, Fu Y, et al. The proinflammatory peptide substance P promotes blood-brain barrier breaching by breast cancer cells through changes in microvascular endothelial cell tight junctions. Int J Cancer. 2014 Mar 1;134(5):1034–1044.
- Brennan FM, McInnes IB. Evidence that cytokines play a role in rheumatoid arthritis. J Clin Invest. 2008 Nov;118(11):3537–3545.
- Origuchi T, Iwamoto N, Kawashiri SY, et al. Reduction in serum levels of substance P in patients with rheumatoid arthritis by etanercept, a tumor necrosis factor inhibitor. Mod Rheumatol. 2011 Jun;21(3):244–250.
- Aletaha D, Neogi T, Silman AJ, et al. Rheumatoid arthritis classification criteria: an American College of Rheumatology/European League Against Rheumatism collaborative initiative. Arthritis Rheum. 2010 Sep;62(9):2569–2581.
- Canhão H, Rodrigues AM, Gregório MJ, et al. Common Evaluations of Disease Activity in Rheumatoid Arthritis Reach Discordant Classifications across Different Populations. Front Med. 2018 Mar 8;5:40. DOI:10.3389/fmed.2018.00040.
- Greenmyer JR, Stacy JM, Sahmoun AE, et al. DAS28-CRP Cutoffs for High Disease Activity and Remission are Lower Than DAS28-ESR in Rheumatoid Arthritis. ACR Open Rheumatol. 2020 Sep;2(9):507–511.
- Lisowska B, Lisowski A, Siewruk K. Substance P and Chronic Pain in Patients with Chronic Inflammation of Connective Tissue. PLoS ONE. 2015 Oct 7;10(10):e0139206.
- Kamel SR, Sadek HA, Mohamed FA, et al. Role of ultrasound disease activity score in assessing inflammatory disease activity in rheumatoid arthritis patients. Egypt Rheumatol. 2018;40(1):1–5.
- Hurnakova J, Hulejova H, Zavada J, et al. Serum Calprotectin Discriminates Subclinical Disease Activity from Ultrasound-Defined Remission in Patients with Rheumatoid Arthritis in Clinical Remission. PLoS ONE. 2016 Nov 10;11(11):e0165498.
- Krenn V, Morawietz L, Häupl T, et al. Grading of chronic synovitis–a histopathological grading system for molecular and diagnostic pathology. Pathol Res Pract. 2002;198(5):317–325.
- Lagrutta M, Alle G, Parodi RL, et al. Severe extra-articular manifestations of rheumatoid arthritis in absence of concomitant joint involvement following long-term spontaneous remission. A case report. Reumatol Clin. 2016 Jul-Aug;12(4):223–225.
- Markusse IM, Dirven L, Gerards AH, et al. Disease flares in rheumatoid arthritis are associated with joint damage progression and disability: 10-year results from the BeSt study. Arthritis Res Ther. 2015 Aug 31;17(1):232.
- Hammer HB, Fagerhol MK, Wien TN, et al. The soluble biomarker calprotectin (an S100 protein) is associated to ultrasonographic synovitis scores and is sensitive to change in patients with rheumatoid arthritis treated with adalimumab. Arthritis Res Ther. 2011;13(5):R178.
- Nagy G, van Vollenhoven RF. Sustained biologic-free and drug-free remission in rheumatoid arthritis, where are we now? Arthritis Res Ther. 2015 Aug 3;17(1):181.
- El-Serougy EM, Eesa NN, El-Azizi HM, et al. Power Doppler ultrasound in the evaluation of hand joints in rheumatoid arthritis patients in clinical remission: association with composite index scores and functional status. Egypt Rheumatol. 2019;41(1):7–10.
- Wu H, Liao W, Li Q, et al. Pathogenic role of tissue-resident memory T cells in autoimmune diseases. Autoimmun Rev. 2018 Sep;17(9):906–911.
- Santos-Moreno P, Castro CA, Villarreal L, et al. Prevalence of Sexual Disorders in Patients with Rheumatoid Arthritis and Associated Factors. Sex Med. 2020 Sep;8(3):510–516.
- Oweis AO, Alawneh KM, Alshelleh SA, et al. Renal dysfunction among rheumatoid arthritis patients: a retrospective cohort study. Ann Med Surg (Lond). 2020 Nov 4;60:280–284. DOI:10.1016/j.amsu.2020.11.011.
- Taneja V. Sex Hormones Determine Immune Response. Front Immunol. 2018 Aug 27;9:1931. DOI:10.3389/fimmu.2018.01931.
- Anichini M, Cesaretti S, Lepori M, et al. Substance P in the serum of patients with rheumatoid arthritis. Rev Rhum Engl Ed. 1997 Jan;64(1):18–21.
- Marshall KW, Chiu B, Inman RD. Substance P and arthritis: analysis of plasma and synovial fluid levels. Arthritis Rheum. 1990 Jan;33(1):87–90.
- Grimsholm O, Rantapää-Dahlqvist S, Forsgren S. Levels of gastrin-releasing peptide and substance P in synovial fluid and serum correlate with levels of cytokines in rheumatoid arthritis. Arthritis Res Ther. 2005;7(3):R416–26.
- Barbosa-Cobos RE, Lugo-Zamudio G, Flores-Estrada J, et al. Serum substance P: an indicator of disease activity and subclinical inflammation in rheumatoid arthritis. Clin Rheumatol. 2018 Apr;37(4):901–908.
- Delgado DA, Lambert BS, Boutris N, et al. Validation of Digital Visual Analog Scale Pain Scoring with a Traditional Paper-based Visual Analog Scale in Adults. J Am Acad Orthop Surg Glob Res Rev. 2018 Mar 23;2(3):e088.
- Younis AA, Jassim NA, Aliasghar A. Assessment of Disease Activity in Rheumatoid Arthritis: a Comparative Study of Clinical Evaluation with Ultrasonogra-phy. Iraqi Postgraduate Med J. 2016;15(2):202–210.
- Szkudlarek M, Wakefield RJ, Backhaus M, et al. The discriminatory capacity of ultrasound in rheumatoid arthritis: active vs inactive, early vs advanced, and more. Rheumatology (Oxford). 2012 Dec;51(Suppl 7):vii6–9.
- Slansky E, Li J, Häupl T, et al. Quantitative determination of the diagnostic accuracy of the synovitis score and its components. Histopathology. 2010 Sep;57(3):436–443.
- Adel N, William M, Al Swaff R, et al. Serum calprotectin level for diagnosis and detection of disease activity in rheumatoid arthritis. Int J Immunol. 2014;2(1):6–10.
